Abstract
Resveratrol, a dietary phytoalexin readily available in the diet, is reported to possess anti-tumorigenic properties in several cancers, including colorectal. However, the underlying mechanism(s) involved is not completely understood. In the present study, we investigated the effect of resveratrol treatment on gene modulation in human colorectal cancer cells and identified activating transcription factor 3 (ATF3) as the most highly induced gene after treatment. We confirmed that resveratrol up-regulates ATF3 expression, both at the mRNA and protein level, and showed resveratrol involvement in ATF3 transcriptional regulation. Analysis of the ATF3 promoter revealed the importance of early growth response-1 (Egr-1; located at −245 to −236) and Krüppel-like factor 4 (KLF4; located at −178 to −174) putative binding sites in resveratrol-mediated ATF3 transactivation. Specificity of these sites to the Egr-1 and KLF4 protein was confirmed by electrophoretic mobility shift and chromatin immunoprecipitation assays. Resveratrol increased Egr-1 and KLF4 expression, which preceded ATF3 expression, and further suggests Egr-1 and KLF4 involvement in resveratrol-mediated activity. We provide evidence for Egr-1 and KLF4 interaction in the presence of resveratrol, which may facilitate ATF3 transcriptional regulation by this compound. Furthermore, we demonstrate induction of apoptosis by resveratrol is mediated, in part, by increased ATF3 expression. Taken together, these results provide a novel mechanism by which resveratrol induces ATF3 expression and represent an additional explanation of how resveratrol exerts its anti-tumorigenic effects in human colorectal cancer cells.
Keywords: resveratrol, apoptosis, ATF3, Egr-1, and KLF4
Introduction
Current research suggests that various dietary phytochemicals function as chemopreventive and/or adjuvant chemotherapeutic agents, adding to the paradigm that a diet high in fruit and vegetable content confers protection against chronic disease (1). One such phytochemical is resveratrol (3, 4, 5′-trihydroxystilbene), a naturally occurring phytoalexin readily available in the diet and to which a plethora of health-promoting effects have been ascribed (2). Resveratrol has elicited much attention as a potential anti-cancer agent since the inhibitory effect of this compound on carcinogenic processes was first reported in 1997 (3). Subsequently, numerous studies have illustrated the anti-proliferative effect of resveratrol on cancer cells, which is believed attributable to induction of cell cycle arrest in the G1/S or G2/M phase and induction of apoptosis and related proteins (4, 5). More importantly, treatment with resveratrol inhibited tumorigenesis in vivo (6, 7). However, the underlying mechanism(s) involved in the anti-tumorigenic/carcinogenic activities of resveratrol remain poorly defined due to its capacity to modulate a multitude of signaling pathways.
Activating transcription factor 3 (ATF3), a member of the ATF/CREB family of bZIP transcription factors, is characterized as a stress-inducible or adaptive response gene (8). Much controversy exists as to the physiological role of ATF3 in tumorigenesis and is demonstrated to be a positive or negative modulator of tumor progression. Recently, a dichotomous role was reported for ATF3 in cancer development; the authors concluded its role as a tumor suppressor or oncogene is largely dependent on cellular context and extent of malignancy (9). Yet, several lines of evidence suggest that ATF3 may function as a tumor suppressor gene in colorectal carcinogenesis. First, ATF3 expression is markedly reduced in cancer tissues, including colon, when compared to normal adjacent tissue (10, 11). Secondly, ATF3 is reported to mediate or enhance induction of apoptosis by compounds demonstrated to have anti-tumor properties (12–16). Finally, ATF3 overexpression elicits a number of cellular responses, including induction of cell cycle arrest and inhibition of proliferation (17), induction of apoptosis in vitro and in vivo (12, 18–20), inhibition of invasion (21–23), and retardation of tumor formation in vivo (20, 22). Thus, we believe that ATF3 may play an anti-tumorigenic role in colorectal tumorigenesis.
In the present study, we examined the effect of resveratrol treatment on gene modulation in HCT-116 human colorectal cancer cells. We identified ATF3 as the most highly induced gene after treatment and sought to investigate the transcriptional mechanism and biological consequence of ATF3 expression in response to resveratrol. Here, we report that early growth response-1 (Egr-1) and Krüppel-like factor 4 (KLF4) mediate ATF3 transactivation by resveratrol. We show Egr-1 and KLF4 interaction in the presence of resveratrol, which may facilitate ATF3 transcriptional regulation by this compound. Furthermore, we demonstrate induction of apoptosis by resveratrol is mediated, at least in part, by ATF3.
Materials and Methods
Cell lines and reagents
All human cancer cell lines were purchased from American Type Culture Collection (Manassas, VA) unless otherwise stated; authentication occurred via short tandem repeat profiling, monitor of cell morphology, and karyotyping. SqCC/Y1 head and neck squamous cell carcinoma cell line was generously provided by Dr. Dong M. Shin (Emory University, Atlanta, GA) and characterized previously (24). Cell lines were maintained according to established protocol. Resveratrol was purchased from Alexis Biochemicals (San Diego, CA), 3, 3′-diindoylmethane (DIM) from Sigma Aldrich (St. Louis, MO), and cycloheximide from Fisher Scientific (Pittsburgh, PA). All chemicals were dissolved in dimethylsulfoxide (DMSO). ATF3, Egr-1, KLF4, and actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Construction of plasmids
For deletion analysis of the ATF3 promoter, pATF3 −1850/+34 (12) was serially deleted using the Erase-a-Base System (Promega, Madison, WI) according to manufacturer’s protocol. The pATF3 −514 del Egr-1 reporter construct was previously described (25). Putative binding sites of KLF4 within the −514 bp region of the promoter were deleted using Stratagene’s QuikChange II Site Directed Mutagenesis Kit (La Jolla, CA) with the following primers: del KLF4-A (F, 5′-CCCCCTCTCTTTCGGCCCCGCCTTGGCCCC-3′ and R, 5′-CGGGGCCGAAAGAGAGGGGGCACTGGTGATG-3′) and del KLF4-B (F, 5′-GGCCCCTCCTCCTTCCTCCGCTCCGTTCGG-3′ and R, 5′-CGGAGGAAGGAGGAGGGGCCAAGGCGGGGC-3′). pATF3 −514 del Egr-1 KLF4 reporter construct was generated using pATF3 −514 del Egr-1 and KLF4-A deletion primers as described. Deletions were confirmed by DNA sequencing. ATF3 (pCG-ATF3) expression vector was kindly provided by Dr. T. Hai (Ohio State University, Columbus, OH). KLF4 (pcDNA3.1/His/V5/KLF4) expression vector was generated using the primers F, 5′-CGAATTCTATGGCTGTCAGCGACGCG-3′ and R, 5′-CCCAAGCTTTTAAAAATGCCTCTTCATGTGTAAGGC-3′. Egr-1 (pcDNA3.1/NEO/Egr-1), Sp1 (pCMV-Sp1), and p53 (pcDNA3.1/myc/His/p53) expression vectors were previously described (26–28).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from HCT-116 cells treated with DMSO or resveratrol (50 μM) using 5 Prime Perfect RNA Cell/Tissue Kit (Gaithersburg, MD) and 1 μg of RNA was reverse transcribed using Verso cDNA Synthesis Kit (Thermo Fisher Scientific, Rockford, IL). PCR was performed as described (25) using ATF3 (F, 5′-GTTTGAGGATTTTGCTAACCTGAC-3′ and R, 5′-AGCTGCAATCTTATTTCTTTCTCGT-3′) and GADPH (F, 5′-TCAACGGATTTGGTCGTATT-3′ and R, 5′-CTGTGGTCATGAGTCCTTCC-3′) human gene specific primers.
Western blot analysis
Cells were grown to 60–80% confluence in 60 mm plates, serum starved overnight, and treated with resveratrol as indicated in serum-free media. Protein lysates were isolated in RIPA buffer containing 1 mM PMSF, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 0.1 nM Na3VO4, and 25 mM NaF. Total protein was subjected to Western blot analysis as previously described (25).
De novo protein synthesis
HCT-116 cells were grown to 60–80% confluence in 60 mm plates and pretreated with cycloheximide (10 mg/mL) for 30 min in serum-free media. After pretreatment, DMSO or resveratrol (50 μM) was added directly to the media and incubated for 24 h. Total RNA was isolated and reverse transcribed. Real-time RT-PCR was performed according to Fast SYBR Green Master Mix protocol (Applied Biosystems, Carlsbad, CA) and analyzed using MyIQ Single Color Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA).
Transfection using luciferase reporter system
Transient transfections were performed using LipofectAMINE (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. HCT-116 cells were seeded in 12-well plates at a concentration of 2.0×105 cells per well. The next day, plasmid mixtures containing ATF3 promoter (0.5 μg) and pRL-null vector (0.05 μg) were cotransfected for 5 h in serum-free media. For cotransfection experiments, 0.25 μg of the ATF3 promoter and 0.25 μg of expression vector were cotransfected with 0.05 μg pRL-null vector. For competition assays, plasmid mixtures were prepared as indicated in Fig. 5A. After transfection, cells were treated with DMSO or resveratrol (50 μM) in serum-free media for 24 h. Cells were harvested in 1X passive lysis buffer and luciferase activity was measured and normalized to pRL-null luciferase activity using DualGlo Luciferase Assay Kit (Promega, Madison, WI).
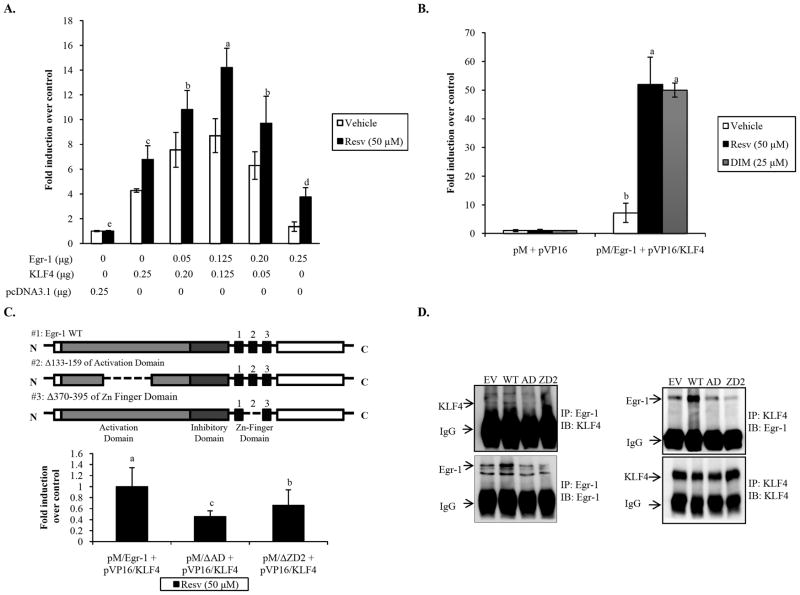
Fig. 5. Egr-1 and KLF4 interaction facilitates ATF3 activation by resveratrol.
(A) The pATF3 −514/+34 reporter construct (0.25 μg) was cotransfected with empty, Egr-1, and KLF4 expression vectors as indicated in the presence of pRL-null vector (0.05 μg). Cells were treated with vehicle or Resv in serum-free media for 24 h. The y-axis shows fold induction relative to pcDNA3.1 control. The results are the means ± SD of three replicates Data analyzed using Tukey’s multiple comparison test; means with same letters indicate no significance (p<0.05). (B) Mammalian two hybrid system was performed as described in Materials and Methods. HCT-116 cells were cotransfected with pM and pVP16 empty vectors (0.2 μg each) and pM/Egr-1 and pVP16/KLF4 vectors (0.2 μg each) in the presence of pG5luc vector (0.2 μg) and pRL-null vector (0.06 μg). Cells were treated with vehicle, Resv, or DIM for 24 h in serum-free media. Promoter activity was measured as a ratio of firefly luciferase signal/renilla luciferase signal and results are expressed as the means ± SD of three replicates. The y-axis shows fold induction over control vectors. Data analyzed using Tukey’s multiple comparison test; means with same letters indicate no significance (p<0.05). (C) Upper panel, Schematic diagram of Egr-1 protein structure and locations used to generate internal deletions. Lower panel, HCT-116 cells were transfected with pM/Egr-1 internal deletion constructs, pVP16/KLF4 vector, and pG5luc vector. Cells were treated with vehicle or Resv in serum-free media for 24 h. Values are normalized to vehicle treatment. The y-axis represents fold induction relative to pM/Egr-1 and pVP16/KLF4 control. Data analyzed using Tukey’s multiple comparison test; means with same letters indicate no significance (p<0.05). (D) HCT-116 cells were transfected with empty, Egr-1 (WT or deletion), or cotransfected with KLF4 expression vectors as indicated and grown to 60–80% confluence. Cells were serum starved overnight, treated with Resv (50 μM) for 2 h, and harvested as described. The cell extracts were immunoprecipitated (IP) and immunoblotted (IB) with antibody against Egr-1and KLF4.
RNA interference
Egr-1 sense and antisense oligonucleotides were previously described (25). ATF3 and KLF4 siRNA were purchased from Santa Cruz Biotechnology; control siRNA was purchased from Ambion (Austin, TX). HCT-116 cells were transfected with either 100 nM (ATF3 and KLF4) or 200 nM (Egr-1) of each construct using TransIT-TKO transfection reagent (Mirus, Madison, WI). After transfection for 24 h, cells were serum starved overnight and treated as indicated. Total protein was subjected to Western blot analysis as described.
Electrophoretic mobility shift assay (EMSA)
HCT-116 cells were grown to 80% confluence. After overnight serum starvation, cells were treated with resveratrol (50 μM) for 24 h. Cells were then washed with PBS and nuclear extracts were prepared using Nuclear Extract Kit (Active Motif, Carlsbad, CA) according to protocol. Double-stranded oligonucleotides corresponding to the Egr-1 (F, 5′-GTGAGCGAGGGCGGGG-3′) and KLF4 (F, 5′-TCCACCCCTTCCACCCCT-3′) binding sites were synthesized and end-labeled with biotin (Operon, Huntsville, AL). Mutant oligonucleotides of Egr-1 (F, 5′-GTGAGCGAAAACGGGG-3′) and KLF4 (F, 5′-TCCAAAACTTCCAAAACT-3′) were also synthesized. To ensure specific binding of Egr-1 and KLF4, recombinant proteins were generated using TNT Quick Coupled Transcription/Translation System (Promega). EMSA was performed as previously described (29).
Chromatin immunoprecipitation (ChIP)
HCT-116 cells were grown to 80% confluence and treated with DMSO or resveratrol (50 μM). After 24 h, cells were fixed with 1% formaldehyde at 37°C for 10 min and ChIP was performed as previously described (29). The region between −298 and −114 bp of the human ATF3 promoter was amplified by real-time and RT-PCR using F, 5′-CGGCTCCGGTCCTGATATGG-3′ and R, 5′-AGAACCGGCCGAACGGAGCG-3′. The 184 bp product was resolved on 2% agarose gel and visualized under UV light.
Mammalian two hybrid system assay
The pM/Egr-1 and pVP16/KLF4 vectors for mammalian two hybrid were generated. PCR fragments were amplified from pcDNA3.1/NEO/Egr-1 and pcDNA3.1/His/V5/KLF4 using Egr-1 (F, 5′-TTGAATTCGCCGCGGCCAAGGCCGAG-3′ and R, 5′-TTAAGCTTGCAAATTTCAATTGTCC-3′) and KLF4 (F, 5′-CGAATTCTATGGCTGTCAGCGACGCG-3′ and R, 5′-CCAAAGCTTTTAAAAATGCCTCTTCATGTGTAAGGC-3′)-specific primers, respectively. After PCR, fragments were cloned into pCR2.1/TOPO vector (Invitrogen), digested with EcoRI and HindIII restriction enzymes, and cloned into pM or pVP16 vectors. Deletion clones of pM/Egr-1 were generated as described using the primers: del AD (F, 5′-CATCACCTATGGCCTAGTGAGCATGACCAACCCAC-3′ and R, 5′-TCACTAGGCCATAGGTGATGGGGGGCAGTCGAGTG-3′) and del ZD2 (F, 5′-GCCCTCCCAGTTCGCCTGCGACATCTGTGGAAGAA-3′ and R, 5′-CGCAGGCGAACTGGAAGGGCTTCTGGCCTGTGTGG-3′). Mammalian two hybrid assay was performed according to Matchmaker Mammalian Assay Kit 2 protocol (Clontech, Mountain View, CA). Transfection occurred as described using plasmid mixtures containing 0.2 μg of pM/Egr-1 or deletion construct, 0.2 μg of pVP16/KLF4, 0.2 μg pG5Luc, and 0.06 μg of pRL-null vector.
Immunoprecipitation of Egr-1 and KLF4
For immunoprecipitation of Egr-1 (WT and deletions, generated as described above) and KLF4, each expression vector was transfected into HCT-116 cells as indicated in Fig. 5D and grown to confluence. Cells were then serum starved overnight and treated with resveratrol (50 μM) for 2 h. After washing with ice-cold PBS, cells were harvested in RIPA buffer containing inhibitors and mixed at 4°C for 15 min. Cell suspensions were centrifuged and protein concentrations were measured. Immunoprecipitation was performed as previously described (12).
Caspase 3/7 enzymatic activity
Enzyme activity of caspase 3/7 was analyzed by Apo-ONE Homogenous Caspase-Glo 4/7 Assay (Promega) according to manufacturer’s protocol. The cells were harvested in RIPA buffer containing protease and phophatase inhibitors. The same volume of caspase-Glo 3/7 reagent was added to cell lysates (30 μg protein) in 96-well plates and incubated at room temperature in the dark for 1 h. Luminescence was measured using FLX800 microplate reader (BioTek, Winooski, VT).
Statistical analysis
SAS for Windows (v9.2) (SAS Institute, Inc., Cary, NC) statistical analysis software was used. For multiple group comparisons, analysis of variance with Tukey’s multiple comparison test was used to compare mean values. The Student’s t test was used to analyze differences between samples. Results were considered statistically significant at p<0.05*, p<0.01**, and p<0.001***.
Results
Resveratrol increases ATF3 expression in cancer cells
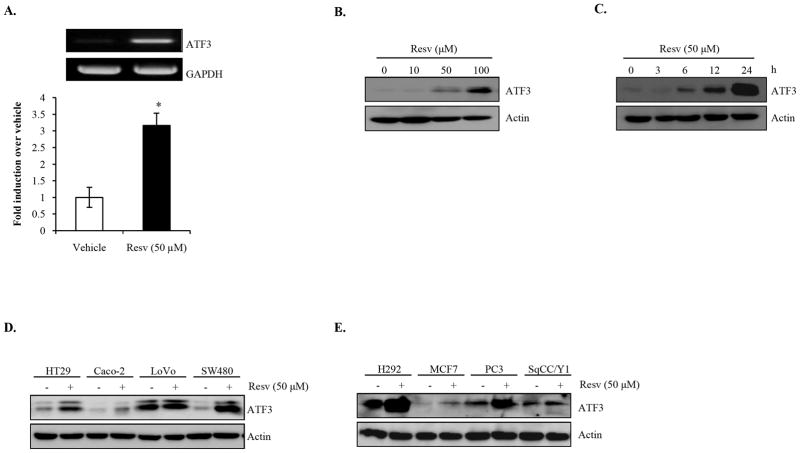
The mechanism(s) underlying the anti-tumorigenic properties of resveratrol in colorectal cancer remain mostly unclear. To investigate how resveratrol alters gene expression in colorectal cancer cells, microarray analysis was performed using HCT-116 cells treated with resveratrol (50 μM and 100 μM). Among the genes up-regulated by the treatment, ATF3 was identified as most highly induced (Table S1 and S2). We confirmed resveratrol-induced ATF3 transcript using RT-PCR (Fig. 1A). We next determined whether resveratrol increases ATF3 expression at the protein level. As shown in Fig. 1B and 1C, resveratrol increased ATF3 in a concentration- and time-dependent manner in HCT-116 cells. Furthermore, increased ATF3 expression was observed in other colorectal (Fig. 1D) and non-colorectal (Fig. 1E) cancer cells after treatment; however, we did not observe ATF3 induction in LoVo and SqCC/Y1 cells. Absence of ATF3 induction by resveratrol in these cells is most likely due to high endogenous expression of ATF3 (LoVo) or the lack of proper signaling pathway(s) needed for ATF3 increase by resveratrol (SqCC/Y1). Because resveratrol increased ATF3 expression at the mRNA and protein level, we sought to characterize ATF3 as a molecular target of this compound at the transcription level.
Fig. 1. Resveratrol increased ATF3 expression in cancer cells.
(A) HCT-116 cells were treated with vehicle or resveratrol (Resv) in serum-free media for 24h. RT-PCR was performed using ATF3 and GAPDH human primers, the latter served as a loading control. Representative gel pictures (upper panel) and gel densitometry (lower panel) of three independent experiments are shown. Values are expressed as fold induction relative to vehicle-treated cells adjusted to GAPDH. p< 0.05* and p< 0.01**. (B-E) Cancer cells were seeded and grown to 60–80% confluence, serum starved overnight, and treated with Resv as indicated in serum-free media. Protein lysates were harvested and subjected to Western blot analysis using ATF3 and actin antibodies. (B) HCT-116 cells were treated with 0, 10, 50, and 100 μM Resv for 24 h. (C) HCT-116 cells were incubated with Resv for the indicated times. (D) Colorectal (HT-29, Caco-2, LoVo, and SW480) and (E) non-colorectal (NCI-H292 lung, MCF7 breast, PC3 prostate, and SpCC/Y1 head and neck) cancer cells were treated with vehicle or Resv for 24 h.
Egr-1 and KLF4 involved in resveratrol-induced ATF3 expression
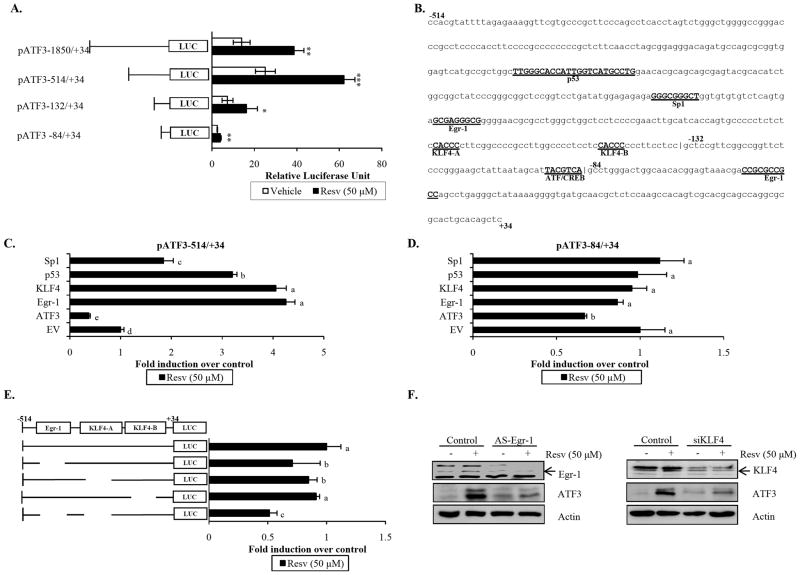
To identify the transcriptional binding site responsible for resveratrol-mediated ATF3 expression, HCT-116 cells were transfected with different promoter constructs spanning the −1850 to +34 promoter region (Fig. 2A). Resveratrol treatment resulted in a significant increase in ATF3 promoter activity for all reporter constructs; however, the greatest induction was observed within the −514 and +34 bp region, suggesting that a major response element(s) may be present. This region was further analyzed using three independent transcription factor search engines (Genomatix-MatInspector, TFSearch, and Transcription Element Search System), and five possible factors were commonly identified: p53, Sp1, Egr-1, KLF4, and ATF/CREB (Fig. 2B).
Fig. 2. Egr-1 and KLF4 are involved in resveratrol induction of ATF3.
(A) Each indicated construct of the ATF3 promoter (0.5 μg) and pRL-null vector (0.05 μg) were transiently transfected into HCT-116 cells and treated with vehicle or Resv for 24 h. The promoter activity was measured as a ratio of firefly luciferase signal/renilla luciferase signal. The x-axis shows relative luciferase unit of each construct. The results are the means ± SD of three replicates. p<0.05*, p<0.01** and p<0.001***, based on Student’s t test. (B) Nucleotide sequence of the −514 to +34 regions of the ATF3 promoter. Predicted binding sites of identified transcription factors are capitalized and underlined with name located underneath. (C and D) Empty vector (pCDNA3.1/NEO) or the indicated expression vector (0.25 μg each) was cotransfected with pATF3 −514/+34 (C) or pATF3 −84/+34 (D) and pRL-null vector (0.05 μg) into HCT-116 cells. Cells were then treated with vehicle or Resv in serum-free media for 24 h. The x-axis shows fold induction relative to pcDNA3.1 control. Data analyzed using Tukey’s multiple comparison test; means with same letters indicate no significance (p<0.05). (E) pATF3 −514/+34 or its internal deletion clones (0.5 μg each) were transfected into HCT-116 cells followed by treatment with vehicle or Resv in serum-free media for 24 h. Values were normalized to vehicle treatment. The x-axis represents fold induction relative to pATF3 −514/+34 control. Data analyzed using Tukey’s multiple comparison test; means with same letters indicate no significance (p<0.05). (F) HCT-116 cells were transfected with Egr-1 sense/antisense oligonucleotides or control/KLF4 siRNA using TransIT-TKO transfection reagent. Cells were serum starved overnight and treated with vehicle or Resv for 6 h (KLF4) or 24 h (Egr-1). Protein lysates were harvested and subjected to Western blot analysis for Egr-1 or KLF4, ATF3, and Actin.
To investigate the role of identified cis-acting elements in resveratrol-mediated transcriptional regulation of ATF3, cotransfection experiments were performed utilizing expression vectors of the aforementioned factors and ATF3 promoter constructs as indicated (Fig. 2C and 2D). Expression of each vector, with the exception of ATF3, resulted in increased pATF3 −514/+34 luciferase activity after resveratrol treatment compared to pcDNA3.1 empty vector control (Fig. 2C). Interestingly, cells overexpressing KLF4 and Egr-1 showed the greatest increase in resveratrol-induced ATF3 promoter activity, resulting in a 4.1- and 4.3-fold induction, respectively. A similar pattern was observed for the −132 to +34 bp region (Fig. S1); however, cotransfection of these expression vectors with pATF3 −84/+34 reporter construct had no effect on promoter activity after treatment (Fig. 2D). These results identify putative resveratrol response-elements located within the −514 to −84 bp region of the ATF3 promoter and suggest that both Egr-1 and KLF4 may play a role in ATF3 regulation by resveratrol.
To clarify the importance of Egr-1 and KLF4 in resveratrol-mediated activation of ATF3, internal deletion clones lacking the Egr-1 and KLF4 binding sites were generated and transfected into HCT-116 cells. Deletion of Egr-1 or KLF4-A binding site markedly reduced ATF3 promoter activity in response to resveratrol treatment when compared to wild type (Fig. 2E). Deletion of the KLF4-B binding site did not change resveratrol-induced transactivation. Because deletion of binding sites corresponding to either Egr-1 or KLF4-A resulted in significant decrease in resveratrol-induced promoter activity, a deletion clone lacking both these sites was generated. Resveratrol-induced activity was dramatically reduced in cells transfected with the double deletion promoter construct compared to wild type (Fig. 2E). Moreover, suppression of either endogenous Egr-1 or KLF4 decreased ATF3 expression in the presence of resveratrol (Fig. 2F), confirming both Egr-1 and KLF4 contribute to resveratrol-induced ATF3 expression.
Egr-1 and KLF4 bind to the ATF3 promoter
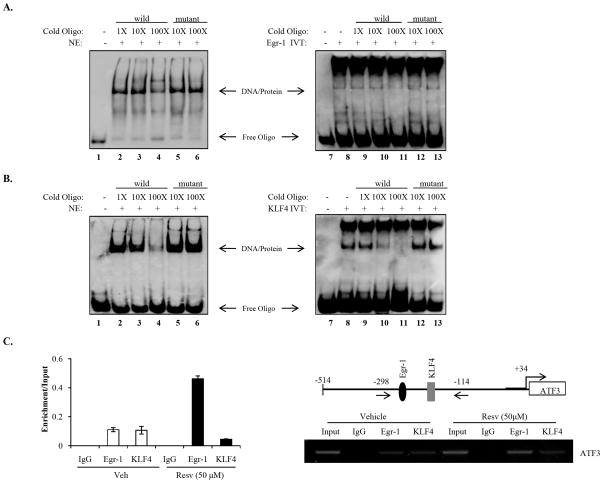
To determine whether Egr-1 and KLF4 bind to the ATF3 promoter, EMSA was performed using biotin-labeled oligonucleotides containing 1–2 copies of the corresponding promoter binding site. First, we examined the effect of resveratrol on promoter binding by these factors using nuclear extracts prepared as described in Materials and Methods. Preincubation of resveratrol-treated nuclear extracts with Egr-1 and KLF4 oligonucleotides resulted in DNA/protein complex formation that was competed out with addition of 10- and 100X molar excess of unlabeled wild type oligonucleotides (Fig. 3A and 3B, left, lanes 3–4), whereas preincubation with unlabeled mutant oligonucleotides had no affect on DNA/protein complex formation (Fig. 3A and 3B, left, lanes 5–6). This suggests that both Egr-1 and KLF4 are able to bind to the ATF3 promoter. Secondly, we verified the specificity of the binding sites for Egr-1 and KLF4 using in vitro translated proteins mixed with their respective oligonucleotides. As shown in Fig. 3A and 3B, both Egr-1 and KLF4 bind to their specific binding sites as evidenced by the formation of shift bands that were competed out by 10- and 100X molar excesses of unlabeled oligonucleotides (Fig. 3A and 3B, right, lanes 10–11). Addition of mutant oligonucleotides did not affect binding of Egr-1 or KLF4 to the ATF3 promoter (Fig. 3A and 3B, right, lanes 12–13). Finally, a ChIP assay was performed to further confirm that Egr-1 and KLF4 bind to the ATF3 promoter after resveratrol treatment. As shown in Fig. 3C, immunoprecipitation of the chromatin/protein complex with Egr-1 and KLF4 resulted in the enrichment of these proteins at the ATF3 promoter (left) and the visualization of a 184 bp band of the amplified promoter (right). In conjunction with previous results shown in Fig. 2, these data suggest that both Egr-1 and KLF4 bind to the ATF3 promoter and activate transcription in the presence of resveratrol. However, Egr-1 may contribute more to resveratrol-induced ATF3 expression, since resveratrol enhances Egr-1 binding capacity to the ATF3 promoter.
Fig. 3. Egr-1 and KLF4 bind to the ATF3 promoter.
(A and B) Gel shift assays were performed using nuclear extracts (NE) from Resv (50 μM)-treated HCT-116 cells for 24 h or in vitro translated (IVT) proteins as described in Materials and Methods. Competitions were done in the presence of 10- and 100X excess of unlabeled oligonucleotides (lanes 3–4 and 10–11). Specificity of the DNA/protein complex was confirmed by the absence of competition with an excess of unlabeled mutated oligonucleotide (lanes 5–6 and 12–13). A, Egr-1 and B, KLF4. Arrows, DNA/protein complexes. (C) HCT-116 cells were treated with vehicle or Resv for 24 h. The chromatin/protein complexes were cross-linked by formaldehyde treatment, and chromatin pellets were extracted and sonicated. The associated Egr-1 and KLF4 DNA was isolated as described. The sequence of the human ATF3 promoter (−298/−114) was amplified by PCR primer pairs (arrows). The input represents PCR products obtained from 1% aliquots of chromatin pellets before immunoprecipitation. Left, ChIP real-time RT-PCR. The x-axis shows enrichment relative to input. The results are the means ± SD of three experiments. Right, RT-PCR; representative gel picture of three experiments is shown.
De novo synthesis is required to increase ATF3 expression
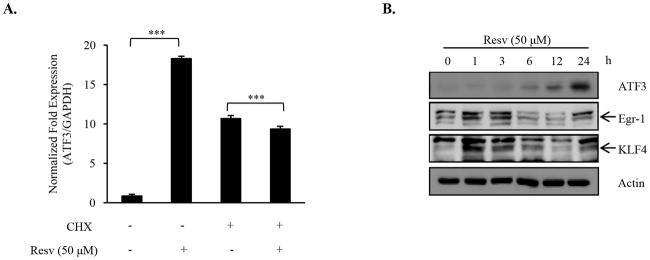
We then examined requirement of de novo protein synthesis for resveratrol-mediated ATF3 expression because Egr-1 and KLF4 appear to be required for induction of ATF3. HCT-116 cells were treated with cycloheximide and resveratrol (50 μM). In the presence of cycloheximide, resveratrol was unable to increase ATF3 expression, suggesting that ATF3 increase by resveratrol is dependent on de novo protein synthesis (Fig. 4A). We next determined whether resveratrol could alter expression of Egr-1 and KLF4. Resveratrol treatment increased Egr-1 and KLF4 mRNA transcript (Fig. S2) and protein expression (Fig. 4B). It should be noted that induction of both Egr-1 and KLF4, which occurred at approximately 1 h of treatment and continued until 3 h, preceded that of ATF3, which began after 6 h. Together, these results are compatible with the notion that observed increase in ATF3 expression by resveratrol requires synthesis of Egr-1 and KLF4.
Fig. 4. de novo protein synthesis of Egr-1 and KLF4 necessary for resveratrol-mediated ATF3 activation.
(A) HCT-116 cells were pretreated with cycloheximide (CHX, 10 mg/mL) for 30 mins in serum-free media followed by treatment with Resv for 24 h. Values are normalized fold induction relative to GAPDH expression. The data are representative of three independent experiments. (B and C) HCT-116 cells were serum starved overnight and treated with Resv as indicated. Protein lysates were harvested and subjected to Western blot analysis using ATF3, Egr-1, KLF4, and Actin antibodies. (B) HCT-116 cells were treated with 0, 10, 50, and 100 μM Resv for 24 h. (C) HCT-116 cells were incubated with Resv for the indicated times.
Egr-1 and KLF4 interaction facilitates resveratrol-mediated ATF3 induction
Because both Egr-1 and KLF4 are involved in resveratrol-mediated activity, we examined whether these factors coordinate or compete in ATF3 expression. HCT-116 cells were cotransfected with pATF3 −514/+34 and expression vectors and treated as described. As observed previously, both Egr-1 and KLF4 increased ATF3 promoter activity in the presence of resveratrol (Fig. 5A). Moreover, co-expression of these factors resulted in enhanced transactivation of ATF3 at both the basal level and after resveratrol treatment.
Previous studies have shown interaction between two Zn finger transcription factors (30) and that this interaction facilitates transcriptional activation (31). To investigate the potential for Egr-1and KLF4 interaction, mammalian two hybrid assay was performed using pM/Egr-1 and pVP16/KLF4 vectors. Egr-1 and KLF4 bind to each other at basal level and this interaction is enhanced after resveratrol treatment (7.2- vs. 52-fold increase; Fig. 5B). Increased Egr-1 and KLF4 interaction was also observed after DIM treatment. DIM increases ATF3 expression (13). To exclude the potential for direct binding of pVP16/KLF4 to the DNA binding domain of the pM empty vector, the vectors were cotransfected and mammalian two hybrid assay was performed; no interaction was detected, allowing one to infer the interaction of Egr-1 and KLF4 is genuine (Fig. S3).
We next sought to tentatively identify the region that may facilitate this interaction and generated pM/Egr-1 deletion constructs lacking (1) 133–159 aa of the activation domain and (2) 370–395 aa of the Zn finger domain (Fig. 5C, upper panel). Both the partial deletion of the activation domain and deletion of the second Zn finger domain of Egr-1 resulted in decreased interaction with KLF4 (Fig. 5C, lower panel). We confirmed mammalian two hybrid assay by Egr-1 and KLF4 immunoprecipitation experiments (Fig. 5D). Egr-1 and KLF4 are co-immunoprecipitated with either antibody, whereas Egr-1 deletion clones had diminished binding capacity to KLF4. Together, these data suggest that Egr-1 and KLF4 physically interact, which is increased by resveratrol, and that the Egr-1 activation or Zn finger domain may mediate this interaction.
Knockdown of ATF3 suppresses resveratrol-induced apoptosis
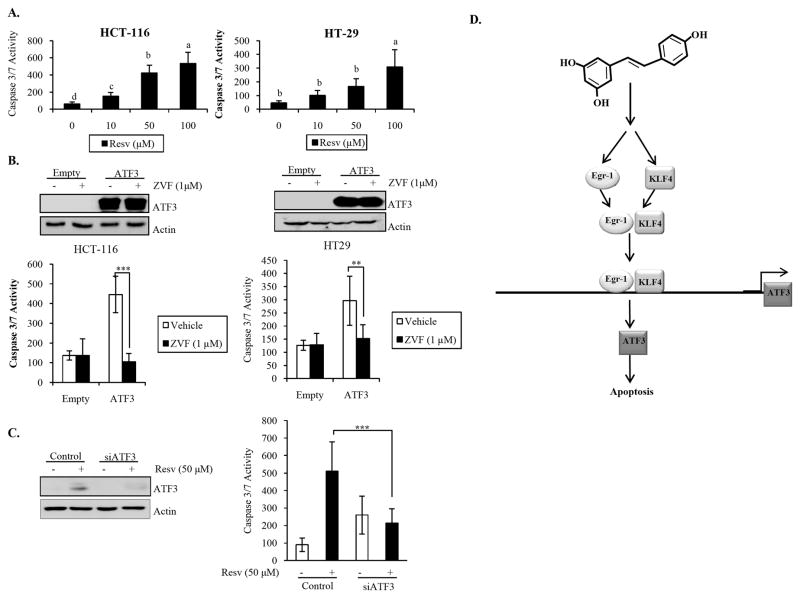
We have identified ATF3 as a molecular target of resveratrol; however, the biological consequence(s) of ATF3 induction by resveratrol in colorectal tumorigenesis is unknown. As described in the introduction, pharmaceuticals and phytochemicals’ increase of ATF3 mediated or enhanced apoptosis by these compounds (12–16). To determine if this is true for resveratrol, we measured caspase 3/7 enzymatic activity for resveratrol-treated HCT-116 and HT29 colorectal cancer cells. As depicted in Fig. 6A, resveratrol treatment increased apoptosis in a dose-dependent manner. Furthermore, ATF3 overexpression in these cells also increased apoptosis, which depend on caspase activation (Fig. 6B). Together, these data suggest that both resveratrol and ATF3 expression induce apoptosis in our model system. Next, to investigate whether resveratrol-induced apoptosis is mediated by ATF3, ATF3 expression was knocked down by siRNA followed by resveratrol treatment for 24 h. As depicted in Fig. 6C, knockdown of ATF3 significantly abrogated caspase 3/7 enzymatic activity by resveratrol. This suggests that ATF3 plays a role in resveratrol-induced apoptosis.
Fig. 6. Relevance of ATF3 in resveratrol-induced apoptosis.
Caspase 3/7 enzymatic activity was measured as described in Materials and Methods. (A) HCT-116 and HT29 cells were treated with 0, 10, 50, and 100 μM Resv for 24 h. Data analyzed using Tukey’s multiple comparison test; means with same letters indicate no significance (p<0.05). (B) HCT-116 and HT29 cells were transfected with empty or ATF3expression vector and grown to 60–80% confluence. Cells were serum starved overnight and treated with the pan caspase inhibitor z-vad-fmk ( ZVF, 1 μM) for 3 h. ATF3 overexpression was validated (upper panel). (C) HCT-116 cells were transfected with control/ATF3 siRNA using TransIT-TKO transfection reagent and treated with Resv 24 h. Validation of ATF3 knockdown is indicated (upper panel). p<0.01** and p<0.001***, based on Student’s t test. (D) Schematic diagram of resveratrol’s action in colorectal cancer cells: Egr-1 and KLF4 are involved in the induction of ATF3 by resveratrol. Resveratrol increases the expression of both Egr-1 and KLF4; this in turn results in the interaction of Egr-1 with KLF4, potentially facilitated by the activation or Zn finger domain of Egr-1, leading to promoter binding and transactivation of ATF3. Increased ATF3 expression results in increase of apoptosis in colorectal cancer cells.
Discussion
Resveratrol, a dietary phytoalexin readily available in the diet, has garnered much attention as a potential chemopreventive and/or chemotherapeutic agent. Numerous studies, utilizing in vitro and in vivo model systems, have illustrated resveratrol’s capacity to inhibit the stages of carcinogenesis (initiation, promotion, and progression) and modulate a multitude of signaling pathways associated with cellular growth and division, apoptosis, angiogenesis, and metastasis (32). However, the underlying molecular mechanism(s) involved in the anti-tumorigenic/carcinogenic activities of resveratrol, especially in colorectal cancer, are complex and remain poorly defined. The present study sought to investigate the effect of resveratrol on gene modulation in HCT-116 human colorectal cancer cells in order to identify a novel target that may mediate the anti-cancer activities of this compound and determine the mechanism involved in its regulation. Our data showed ATF3 was indeed up-regulated at both the mRNA and protein (Fig. 1) level by the compound.
Recently, a dichotomous role in cancer development was reported for ATF3; the authors of that study demonstrated both a tumor suppressive (early stage tumorigenesis) and oncogenic (late stage tumorigenesis) role in breast cancer cell lines derived from similar genetic backgrounds with varying degrees of malignancy (9). Similarly, a duality of function was demonstrated for ATF3 in cancer studies of the prostate (19, 33) and of the colon (22, 34). Yet, as described in the introduction, several lines of evidence suggest ATF3 behaves as a negative regulator of tumorigenesis. ATF3 expression is induced by several compounds demonstrated to possess anti-tumorigenic properties (12–16). The results presented here add to the list of compounds that induce ATF3 with mechanistic data.
To elucidate the molecular mechanism by which resveratrol induced ATF3 expression, the promoter region spanning −1850 to +34 bp was assessed by luciferase assay in response to resveratrol. We found that resveratrol transactivated the ATF3 promoter and identified putative response-elements located within the −514 to −84 bp region (Fig. 2A). Cotransfection experiments suggested involvement of the transcription factors Egr-1 and KLF4 (Fig. 2C). Egr-1 and KLF4 participation in resveratrol-mediated activation of ATF3 was confirmed by deletion (Fig. 2E) and knockdown (Fig. 2F) experiments. Interestingly, simultaneous deletion of both Egr-1 and KLF4-A binding sites from the ATF3 promoter dramatically reduced transactivation by resveratrol, indicating that the combination of these two factors is important for ATF3 transcriptional regulation by resveratrol. Yet, we cannot exclude the involvement of other cofactors, such as p53 and Sp1 (Fig. 2C), in the compound’s mediated activity. ATF3 regulation by p53 is documented in the literature (35), which is increased by resveratrol (4) and suggests a potential role for p53 in resveratrol-mediated ATF3 activation. Furthermore, Sp1 was recently demonstrated to regulate ATF3 expression in colon cancer cells (36); however, we did not observe Sp1 induction by resveratrol (data not shown). Here, we focused on Egr-1 and KLF4 because both increased resveratrol-mediated ATF3 activation and were modulated by the compound.
Egr-1 and KLF4 belong to a family of immediate early response genes whose expression is transiently induced in response to various environmental stimuli (37, 38). Each encodes a transcription factor containing three carboxyl C2H2 type Zn finger motifs that coordinate the expression of genes associated with cell proliferation, differentiation, and apoptosis (38–40). In addition, Egr-1 and KLF4 are suggested to act as master regulatory proteins involved in cell fate decisions (41, 42). Nonetheless, as with ATF3, much controversy exists as to the role of Egr-1 and KLF4 in cancer development and their biological function appears largely context dependent. Several studies have demonstrated that Egr-1 and KLF4 expression facilitates tumor progression in vivo (43–45); however, there is ample evidence supporting a tumor suppressive role for both transcription factors (29, 46–51).
Our data indicates that Egr-1 and KLF4 are able to bind to the ATF3 promoter (Fig. 3) and that their biosynthesis is necessary for resveratrol-mediated activity (Fig. 4). Moreover, these transcription factors cooperate in ATF3 transactivation (Fig. 5A). From this, two inferences can be made: (1) synergism between Egr-1 and KLF4 promote ATF3 activation, which is enhanced by resveratrol, and (2) interaction between the two facilitates transcriptional regulation of ATF3 by the compound. Indeed, the results ascertained by mammalian two hybrid assay and immunoprecipitation experiments corroborate Egr-1 and KLF4 interaction (Fig. 5B and 5D). Furthermore, resveratrol and DIM increased Egr-1 and KLF4 interaction. These results imply that a similar mechanism may contribute to induction of ATF3 expression by these and possibly other phytochemicals that increase ATF3 expression.
Current research suggests the importance of C2H2 type Zn finger domains in protein-protein interaction (52). Consistently, we found the Zn finger domain of Egr-1 as a possible region responsible for interaction with KLF4. Interestingly, our data also identified the activation domain of Egr-1 as responsible for mediating the interaction with KLF4 (Fig. 5C and 5D). To our knowledge, this is the first report identifying the involvement of the activation domain in protein-protein interaction of two Zn finger transcription factors. Thus, the activation domain contributes not only to the initiation of transcription but also contribute to protein-protein interactions with other transcription factors; interaction via the activation domain or the Zn finger domain leads to enhanced compound-induced transcription.
Results identify ATF3 as a molecular target of resveratrol whose regulation is mediated by Egr-1 and KLF4 interaction. However, the biological consequence(s) of ATF3 induction by the compound is unknown in colorectal tumorigenesis. To answer this question, we examined induction of apoptosis by resveratrol and ATF3 in colorectal cancer cells because, as described, ATF3 mediated or enhanced apoptosis by compounds with known anti-tumor activities. As depicted in Fig. 6A and 6B, resveratrol and ATF3 overexpression increased caspase 3/7 enzymatic activity in HCT-116 and HT29 cancer cells. Furthermore, knockdown of ATF3 expression resulted in reduced apoptosis by resveratrol and suggests that ATF3 plays a role in resveratrol-induced apoptosis. However, further study is required to determine whether ATF3 contributes to resveratrol anti-cancer effects in vivo.
In conclusion, we found the stress-inducible and/or adaptive response gene ATF3 as most highly induced gene after resveratrol treatment in human colorectal cancer cells. Here, we report for the first time the involvement of resveratrol in transcriptional regulation of ATF3 and that this regulation is mediated by the C2H2 type Zn finger transcription factors Egr-1 and KLF4. We demonstrate that Egr-1 and KLF4 interact with each other in the presence of resveratrol, which may facilitate ATF3 transcriptional regulation by this compound. Furthermore, increased ATF3 expression by resveratrol facilitates induction of apoptosis by the compound (Fig. 6D).
Supplementary Material
Acknowledgments
We thank Misty Bailey for her critical reading of this manuscript. We also thank Dr. Luis Miguel Lembcke for his technical assistance.
Grant Support: American Cancer Society grant CNE-111611, NIH grant RO1CA108975, The University of Tennessee Center of Excellence in Livestock Diseases and Human Health (S.J. Baek), and in part by NIEHS/NIH intramural research program (T.E. Eling).
References
- 1.Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 3.Jang M, Cai L, Udeani GO, et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 4.Harikumar KB, Aggarwal B. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 5.Fan E, Jiang S, Zhang L, Bai Y. Molecular mechanism of apoptosis induction by resveratrol, a natural cancer chemopreventive agent. Int J Vitam Nutr Res. 2008;78(1):3–8. doi: 10.1024/0300-9831.78.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Bishayee A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical Trials. Cancer Prev Res. 2009;2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Jin Y, Hofseth AB, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res. 2010;3(4):549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401(2):559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin X, DeWille JW, Hai T. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene. 2008;27(15):2118–2127. doi: 10.1038/sj.onc.1210861. [DOI] [PubMed] [Google Scholar]
- 10.Yan C, Boyd DD. ATF3 regulates the stability of p53: a link to cancer. Cell Cycle. 2006;5(9):926–929. doi: 10.4161/cc.5.9.2714. [DOI] [PubMed] [Google Scholar]
- 11.Bottone FG, Martinez JM, Collins JB, Afshari CA, Eling TE. Gene modulation by the cyclooxygenase inhibitor, sulindac sulfide, in human colorectal carcinoma cells. J Biol Chem. 2003;278(28):25790–25801. doi: 10.1074/jbc.M301002200. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi K, Lee S-H, Kim J-S, et al. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase–independent pathway. Cancer Res. 2006;66(4):2376–2384. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- 13.Lee S-H, Kim J-S, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005;328(1):63–69. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- 14.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4(2):233–241. [PubMed] [Google Scholar]
- 15.Mashima T, Udagawa S, Tsuruo T. Involvement of transcriptional repressor ATF3 in acceleration of caspase protease activation during DNA damaging agent-induced apoptosis. J Cell Physiol. 2001;188(3):352–358. doi: 10.1002/jcp.1130. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Mora-Jensen H, Weniger MA, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106(7):2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan F, Jin S, Amundson SA, et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21(49):7488–7496. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 18.Turchi L, Aberdam E, Mazure N, et al. Hif-2alpha mediates UV-induced apoptosis through a novel ATF3-dependent death pathway. Cell Death Differ. 2008;15(9):1472–1480. doi: 10.1038/cdd.2008.74. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J Biol Chem. 2008;283(44):29795–29801. doi: 10.1074/jbc.M802515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu D, Wolfgang C, Hai T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem. 2006;281(15):10473–10481. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- 21.Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 cells. Mol Cancer Res. 2004;2(7):403–416. [PubMed] [Google Scholar]
- 22.Bottone FG, Moon Y, Kim JS, et al. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3) Mol Cancer Ther. 2005;4(5):693–703. doi: 10.1158/1535-7163.MCT-04-0337. [DOI] [PubMed] [Google Scholar]
- 23.Yan C, Wang H, Boyd DD. ATF3 represses 72-kDa type IV collagenase (MMP-2) expression by antagonizing p53-dependent trans-activation of the collagenase promoter. J Biol Chem. 2002;277(13):10804–10812. doi: 10.1074/jbc.M112069200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Chen Z, Khuri FR, Shin DM. Induction of cell cycle arrest and apoptosis by a combined treatment with 13-cis-retinoic acid, interferon-α2a, and α-tocopherol in squamous cell carcinoma of the head and neck. Head Neck. 2007;29(4):351–361. doi: 10.1002/hed.20525. [DOI] [PubMed] [Google Scholar]
- 25.Cho K-N, Sukhthankar M, Lee S-H, Yoon J-H, Baek SJ. Green tea catechin (-)-epicatechin gallate induces tumour suppressor protein ATF3 via EGR-1 activation. Eur J Cancer. 2007;43(16):2404–2412. doi: 10.1016/j.ejca.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek SJ, Kim J-S, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-β superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. 2004;279(8):6883–6892. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- 27.Baek SJ, Horowitz JM, Eling TE. Molecular Cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. J Biol Chem. 2001;276(36):33384–33392. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 28.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23(3):425–432. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 29.Lee S-H, Bahn JH, Choi CK, et al. ESE-1/EGR-1 pathway plays a role in tolfenamic acid-induced apoptosis in colorectal cancer cells. Mol Cancer Ther. 2008;7(12):3739–3750. doi: 10.1158/1535-7163.MCT-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JS, Galvin KM, Shi Y. Evidence for physical interaction between the zinc-finger transcription factors YY1 and Sp1. Proc Natl Acad Sci USA. 1993;90(13):6145–6149. doi: 10.1073/pnas.90.13.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merika M, Orkin S. Functional synergy and physical interactions of the erythroid transcription factor GATA-1 with the Kruppel family proteins Sp1 and EKLF. Mol Cell Biol. 1995;15(5):2437–2447. doi: 10.1128/mcb.15.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kundu JK, Surh Y-J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269(2):243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 33.Bandyopadhyay S, Wang Y, Zhan R, et al. The tumor metastasis suppressor gene Drg-1 down-regulates the expression of activating transcription factor 3 in prostate cancer. Cancer Res. 2006;66(24):11983–11990. doi: 10.1158/0008-5472.CAN-06-0943. [DOI] [PubMed] [Google Scholar]
- 34.Ishiguro T, Nagawa H, Naito M, Tsuruo T. Inhibitory effect of ATF3 antisense oligonucleotide on ectopic growth of HT29 human colon cancer cells. Jpn J Cancer Res. 2000;91:833–836. doi: 10.1111/j.1349-7006.2000.tb01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Gao C, Kawauchi J, et al. Transcriptional activation of the human stress-inducible transcriptional repressor ATF3 gene promoter by p53. Biochem Biophys Res Commun. 2002;297(5):1302–1310. doi: 10.1016/s0006-291x(02)02382-3. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AJ, Chueh AC, Tögel L, et al. Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an SP1/SP3-activated transcriptional program involving immediate-early gene induction. Cancer Res. 2010;70(2):609–620. doi: 10.1158/0008-5472.CAN-09-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193(3):287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Geiman DE, Shields JM, et al. The Gut-enriched Krüppel-like factor (Krüppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275(24):18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Rangnekar V, Adamson E, Mercola D. Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther. 1998;5(1):3–28. [PubMed] [Google Scholar]
- 40.Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278(4):2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan S-F, Fujita T, Lu J, et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6(12):1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Q, Hong Y, Zhan Q, Shen Y, Liu Z. Role for Kruppel-like factor 4 in determining the outcome of p53 response to DNA damage. Cancer Res. 2009;69(21):8284–8292. doi: 10.1158/0008-5472.CAN-09-1345. [DOI] [PubMed] [Google Scholar]
- 43.Baron V, De Gregorio G, Krones-Herzig A, et al. Inhibition of Egr-1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene. 2003;22(27):4194–4204. doi: 10.1038/sj.onc.1206560. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y-J, Wu C-Y, Chang C-C, et al. Nuclear Kruppel-like factor 4 expression is associated with human skim squamous cell carcinoma progression and metastasis. Cancer Biol Ther. 2008;7(5):777–782. doi: 10.4161/cbt.7.5.5768. [DOI] [PubMed] [Google Scholar]
- 45.Pandya AY, Talley LI, Frost AR, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10(8):2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 46.Choi BH, Kim CG, Bae Y-S, et al. p21Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: Role of early growth response-1 expression. Cancer Res. 2008;68(5):1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 47.Park SE, Lee SW, Hossain MA, et al. A chenodeoxycholic derivative, HS-1200, induces apoptosis and cell cycle modulation via Egr-1 gene expression control on human hepatoma cells. Cancer Lett. 2008;270(1):77–86. doi: 10.1016/j.canlet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 48.Zagurovskaya M, Shareef MM, Das A, et al. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene. 2009;28(8):1121–1131. doi: 10.1038/onc.2008.461. [DOI] [PubMed] [Google Scholar]
- 49.Ghaleb AM, McConnell BB, Nandan MO, et al. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67(15):7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu W, Hofstetter WL, Li H, et al. Putative Tumor-suppressive function of Krüppel-like factor 4 in primary lung carcinoma. Clin Cancer Res. 2009;15(18):5688–5695. doi: 10.1158/1078-0432.CCR-09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27KIP1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008;68(12):4631–4639. doi: 10.1158/0008-5472.CAN-07-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brayer K, Segal D. Keep your fingers off my dna: protein–protein interactions mediated by c2h2 zinc finger domains. Cell Biochem Biophys. 2008;50(3):111–131. doi: 10.1007/s12013-008-9008-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.