Abstract
Nitrite (NO2−) has been shown to limit injury to the heart, liver, and kidneys in various models of ischemia-reperfusion injury. Potential protective effects of systemic NO2− in limiting lung injury or enhancing repair have not been documented. We assessed the efficacy and mechanisms by which postexposure intraperitoneal injections of NO2− mitigate chlorine (Cl2)-induced lung injury in rats. Rats were exposed to Cl2 (400 ppm) for 30 min and returned to room air. NO2− (1 mg/kg) or saline was administered intraperitoneally at 10 min and 2, 4, and 6 h after exposure. Rats were killed at 6 or 24 h. Injury to airway and alveolar epithelia was assessed by quantitative morphology, protein concentrations, number of cells in bronchoalveolar lavage (BAL), and wet-to-dry lung weight ratio. Lipid peroxidation was assessed by measurement of lung F2-isoprostanes. Rats developed severe, but transient, hypoxemia. A significant increase of protein concentration, neutrophil numbers, airway epithelia in the BAL, and lung wet-to-dry weight ratio was evident at 6 h after Cl2 exposure. Quantitative morphology revealed extensive lung injury in the upper airways. Airway epithelial cells stained positive for terminal deoxynucleotidyl-mediated dUTP nick end labeling (TUNEL), but not caspase-3. Administration of NO2− resulted in lower BAL protein levels, significant reduction in the intensity of the TUNEL-positive cells, and normal lung wet-to-dry weight ratios. F2-isoprostane levels increased at 6 and 24 h after Cl2 exposure in NO2−- and saline-injected rats. This is the first demonstration that systemic NO2− administration mitigates airway and epithelial injury.
Keywords: pulmonary edema, apoptosis, necrosis, nitric oxide, airway injury
chlorine (Cl2) is the ninth-most abundantly produced chemical in the United States. It is transported mainly by rail to manufacturing plants and used to bleach pulp, sanitize waste, manufacture pharmaceuticals, and maintain swimming pools free of pathogens (11, 38). The accidental release of large amounts of Cl2 into the atmosphere as a result of train derailments has been shown to result in short- and long-term lung damage, resulting in significant morbidity and mortality (11, 30, 36).
The current treatment of Cl2-induced lung injury consists of alleviating hypoxemia by administration of supplemental oxygen and β2-agonists and, in severe cases, when patients develop acute respiratory distress syndrome or acute lung injury (ALI), mechanical ventilation (3, 11). Mice exposed to Cl2 (400 ppm for 30 min) develop increased respiratory system resistance and airway responsiveness to aerosolized methacholine (assessed by FlexiVent) up to 6 days after exposure. Decreased Na+-dependent alveolar fluid clearance across the distal lung epithelium was also present. These conditions were alleviated by postexposure administration of long-term β2-agonists (32). We have shown that exposure of rats and mice to Cl2 decreases ascorbate and GSH in the lung epithelial lining fluid and tissue and causes extensive injury to the airway and alveolar epithelia, leading to compromised gas exchange(22, 33). Rats pretreated with ascorbate, Desferal (deferoxamine mesylate USP), and N-acetylcysteine before exposure to Cl2 do not become hypoxic and have significantly lower levels of albumin in their bronchoalvelolar lavage (BAL) than rats treated with saline alone (22). In addition, postexposure administration of ascorbate and deferoxamine in mice exposed to lethal concentrations of Cl2 (600 ppm for 45 min) decreased mortality fourfold (39).
Recently, considerable attention has been focused on the ability of NO2− to prevent ischemia-reperfusion injury, which is mediated in part by reactive species generated during the reperfusion phase. It has been suggested that, during hypoxia, NO2− is reduced to nitric oxide (NO), thereby providing a source of NO at the ischemic tissue, which then protects against injury via its anti-inflammatory, antioxidant, and antiapoptotic effects (5, 7, 8, 13, 28, 31); additional salutatory effects of NO include inhibition of platelet and neutrophil adhesion to endothelial cells (20) and increased local blood flow by elevation of vessel cGMP levels. The mechanisms by which NO2− is reduced to NO remain under investigation, with oxygen-sensitive metalloproteins (e.g., hemoglobin, myoglobin, and xanthine oxidoreductase) proposed to play central roles (14). Therapeutic effects of NO2− in the lung have been demonstrated in the context of reversing hypoxic vasoconstriction and pulmonary arterial hypertension (14); however, potential beneficial effects of systemic administration in the alleviation of ALI have not been investigated.
Here, we demonstrate that rats exposed to Cl2 (400 ppm for 30 min) and returned to room air develop severe lung injury. Intraperitoneal injections of NO2− after Cl2 exposure decreased BAL protein concentration, pulmonary edema, and severe lung airway injury assessed by quantitative morphology. To our knowledge, this is the first demonstration that systemic administration of small concentrations of NO2−, which is used for the treatment of cyanide poisoning in adults (2), mitigates ALI in vivo. Thus the results of this study complement previous findings documenting beneficial effects of NO2− in preventing injury following ischemia-reperfusion and highlight its potential as a novel therapeutic agent in ALI.
MATERIALS AND METHODS
Animals.
Pathogen-free Sprague-Dawley male rats (200–250 g; Harlan Laboratories, Indianapolis IN) were used for these studies. All procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Exposure to Cl2.
Rats were placed inside a cylindrical glass chamber and exposed to 400 ppm Cl2 for 30 min, as previously described (22). At the end of the exposure, they were returned to room air, injected intraperitoneally with saline containing NO2− (1 mg/kg body wt, 200–200 μl of saline per injection; Fisher Chemicals, Pittsburgh, PA) at 10 min and 2, 4, and 6 h, and killed 6 or 24 h after exposure. Peripheral oxygen saturations were measured with a MouseOx Small Animal Oxymeter (STARR Life Sciences, Allison Park, PA) connected to a personal computer equipped with analysis software. The sensor was placed on the rat tail. Respiratory rates were measured by observation.
Lung histology and morphology.
After the animal was killed and the chest was opened, the lungs were filled in situ very slowly with 10% formalin at a constant pressure of 25 cmH2O (∼8 ml); they were then removed en bloc and immersed in 10% formalin for 48 h. The left lung was trisected horizontally, and sections from its middle were processed for paraffin sectioning and staining with hematoxylin and eosin (12). Images were recorded with an Olympus BX41 microscope with a Q-Color 3 digital camera using QCapture software (QImaging, Surrey, BC, Canada) and examined by two independent blinded reviewers, who scored them using the following criteria: 0 = normal epithelium, consisting of intact ciliated and nonciliated cells; 1 = loss of cilia, presence of inflammatory infiltrates, <10% of epithelium exfoliated; 2 = >10% but <50% of epithelium exfoliated; 3 = >50% but less than 75% of epithelium exfoliated; 4 = >75% of epithelium exfoliated.
Assessment of apoptosis/necrosis by TUNEL.
Paraffin-embedded lung tissues were deparaffinized with xylene and washed and rehydrated with ethanol, fixed with 4% formaldehyde in PBS, and processed for terminal deoxynucleotidyl (TdT)-mediated dUTP nick end labeling (TUNEL) using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI). Accordingly, they were permeabilized with proteinase K solution and incubated in a nucleotide mixture containing fluorescein-12-dUTP and TdT for 60 min according to the manufacturer's instructions. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Green fluorescence (indicative of TUNEL) was visualized with an inverted high-resolution digital microscope (Eclipse TE-2000U, Nikon Instruments, Melville, NY). Average intensity of green fluorescence was determined by MetaMorph software (Universal Imaging, Downingtown, PA).
Measurements of isoprostanes and lung weights.
Immediately after the lungs were perfused with PBS for removal of blood, the right lung was removed and frozen with liquid nitrogen. Phospholipids were extracted and subjected to alkaline hydrolysis in the presence of butylated hydroxytoluene to release F2-isoprostanes, which were quantified by GC/NICI-MS, as previously described (25). The left lung was blotted, weighed (for determination of wet weight), placed in an oven at 70°C for 7 days, and then weighed again for determination of the dry weight.
Plasma NO2− measurement.
Blood was collected via cardiac puncture into 1-ml syringes containing 1.5% (wt/vol) sodium citrate, 5 mM N-ethylmaleimide, and 100 μM diethylenetriaminepentaacetic acid. The contents of the syringe were mixed and then centrifuged at 2,000 g for 90 s at 25°C, and plasma was collected and further stabilized with 1 mM N-ethylmaleimide and 100 μM diethylenetriaminepentaacetic acid. NO2− concentrations were measured by a reductive (I3−) chemiluminescence method, as previously described (21), and verified by acid-sulfanilamide-dependent scavenging.
Statistical analysis.
Data were analyzed by one-way ANOVA followed by the Tukey-Kramer multiple-comparisons test. P < 0.05 was considered significant.
RESULTS
Physiological variables.
All rats were alive 24 h after Cl2 exposure. Upon return to room air, they exhibited evidence of respiratory distress, with marked flaring of the nostrils during inspiration, depressed respiratory rate, and hypoxemia (Fig. 1). These variables returned to normal by 24 h after exposure. Intraperitoneal injections of NO2− did not accelerate the rate of recovery of these variables compared with saline (Fig. 1). Exposure to Cl2 increased the BAL protein at 6 and 24 h after exposure (Fig. 2), although the value was significantly less at 24 h than at 6 h; injection of NO2− resulted in significantly lower BAL proteins at 6 and 24 h. Similarly, in rats exposed to Cl2, lung wet-to-dry weight ratios were significantly higher than normal at 6 h after exposure; however, the lung wet-to-dry weight ratios of Cl2-exposed rats injected with NO2− were considerably lower than those receiving saline and not different from air controls (Fig. 2). To clear the lungs of blood (which could potentially confound the interpretation of our results), lungs were perfused via the pulmonary artery in situ with normal saline until they were clear of blood. Of course, it is possible that perfusion of the lungs may have increased their extravascular lung water. However, our values for control wet-to-dry weight ratios are similar to those we reported previously for nonperfused rat lungs (22) and to those reported by others (4), and lungs from all groups were perfused using identical conditions. These findings indicate that injections of NO2− prevented Cl2-induced injury to the alveolar epithelium and lung microvasculature. Injection of NO2− did not increase levels of NO2− in the rat plasma at 6 h after exposure: 4.9 ± 0.9 and 3.7 ± 1 (SE) pmol/mg protein in rats injected with saline (n = 5) and NO2− (n = 6), respectively (P = 0.4).
Fig. 1.
Breathing frequencies and peripheral oxygen saturations. Rats were exposed to 400 ppm Cl2 for 30 min, returned to room air, and killed 6 h (A and C) or 24 h (B and D) after exposure. All rats received intraperitoneal injections of NO2− (1 mg/kg body wt) or an equivalent amount of saline (50 μl) at 0.1, 2, 4, and 6 h after exposure. Values are means ± SE. A and B: breath rates (determined by observation). C and D: peripheral oxygen saturations. In A and C, n = 8 air + saline and air + NO2−, n = 7 Cl2 + saline, n = 9 Cl2 + NO2−; in B and D, n = 3 air + saline and air + NO2−, n = 6 Cl2 + saline and Cl2 + NO2−. Breathing frequencies and oxygen saturations for Cl2 + saline and Cl2 + NO2− at all time points, except 24 h after exposure, are significantly different from their corresponding control values (1-way ANOVA followed by Tukey-Kramer multiple-comparisons test).
Fig. 2.
NO2− decreases protein levels in bronchoalveolar lavage (BAL) and bloodless lung wet-to-dry weight ratios. Rats were exposed to 400 ppm Cl2 for 30 min, returned to room air, and killed 6 or 24 h after exposure. All rats received intraperitoneal injections of NO2− (1 mg/kg body wt) or an equivalent amount of saline (200–250 μl) at 0.1, 2, 4, and 6 h after exposure. At 6 or 24 h after exposure, they were killed and their lungs were lavaged with 8 ml of normal saline, which was instilled and withdrawn slowly 3 times; BAL samples were spun at 300 g for 10 min at 4°C to pellet cells (22). A: protein concentrations in cell-free BAL measured using the bicinchoninic acid (BCA) Protein Assay Reagent Kit (Pierce, Rockford, IL) and as previously described (17). B: blood-free lung wet-to-dry weight (W/D) ratios. Values are means ± SE; n = 8 air + saline, n = 2 air + NO2−, n = 7 Cl2 + saline, n = 10 Cl2 + NO2− at 6 h; n = 3 air + NO2−, n = 6 Cl2 + saline and Cl2 + NO2− at 24 h; for wet-to-dry lung weights, n = 4 for each group. *P < 0.05 vs. corresponding air value (ANOVA followed by Tukey's t-test for multiple comparisons). #P < 0.05 vs. corresponding saline value (2-tailed t-test). ^P < 0.05 vs. corresponding saline value (1-tailed t-test).
Morphological assessment of airway and distal lung injury.
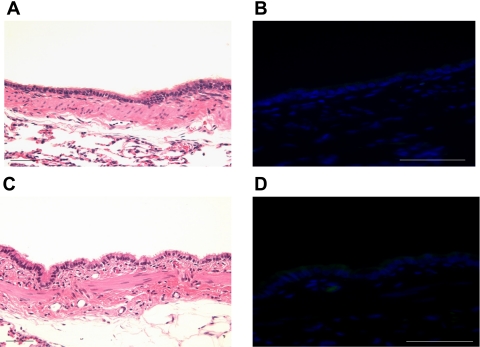
Rats exposed to air, injected with saline or NO2−, and killed 6 or 24 h later had normal airway lung morphology, as shown by the presence of ciliated cells, intact epithelial surfaces, and thin interstitial spaces, without interstitial cells or edema (Fig. 3). All sections were TUNEL-negative (Fig. 3B). In contrast, airways of saline-injected rats exposed to Cl2 and killed 6 h later exhibited significant injury to their epithelial surfaces: in most sections, the epithelium had completely separated from its basement membrane, and the interstitial space was infiltrated by inflammatory cells (Fig. 4A). These sloughed cells were TUNEL-positive (Fig. 4B). On the other hand, airways of rats exposed to Cl2 and injected with NO2− exhibited diminished injury, as shown by significantly fewer sloughed epithelia (Fig. 4C). On the basis of the criteria outlined in materials and methods, the average airway injury scores at 6 h after exposure were as follows (means ± SE, n = 3): 0 ± 0 for air + saline, 0 ± 0 for air + NO2−, 3.3 ± 0.1 for Cl2 + saline, 2.73 ± 0.1 for Cl2 + NO2− [P < 0.05 compared with Cl2 + saline, by ANOVA followed by Tukey-Kramer multiple-comparisons test (GraphPAD InStat Software)]. Significantly less green fluorescence was seen in the airway of Cl2-exposed rats injected with NO2− than in those treated with saline (Figs. 4D and 5), although in both cases, levels of green fluorescence were significantly higher than in the airways of rats exposed to room air (Fig. 5).
Fig. 3.
Proximal airways of rats exposed to air and injected with NO2−. A and C: hematoxylin-and-eosin-stained sections of rat proximal airways injected with saline (A) or NO2− (C). Note normal lung architecture with prominent cilia. B and D: adjacent sections processed for terminal deoxynucleotidyl-mediated dUTP nick end labeling (TUNEL) using the DeadEnd Fluorometric TUNEL System and imaged with a fluorescent microscope for detection of FITC (green color). Note absence of green fluorescence, indicating absence of apoptosis/necrosis. Approximately 5 sections were examined from each rat (n = 3) with similar results. Scale bar, 50 μm.
Fig. 4.
Exposure to Cl2 causes extensive injury to proximal lung airway epithelia. Rats were exposed to Cl2 (400 ppm for 30 min), returned to room air, injected with saline (A and B) or NO2− (C and D), and killed 6 h after exposure. A and C: hematoxylin-and-eosin-stained sections of proximal airway. Note sloughed airway epithelium and injury to the interstitial space (A; saline) and relatively less sloughing of airway epithelium (C; NO2−). B and D: adjacent sections processed for TUNEL using the DeadEnd Fluorometric TUNEL system and imaged with a fluorescence microscope for detection of FITC (green color) and 4′,6-diamidino-2-phenylindole (blue fluorescence) to identify nuclei. Sloughed portion of the epithelium in A stained positive, as indicated by considerable green fluorescence (B). Very little green fluorescence is seen in D. Approximately 5 sections were examined from each rat (n = 3) with similar results. Scale bar, 50 μm.
Fig. 5.
Quantification of TUNEL in lung airways of saline- and NO2−-injected animals exposed to Cl2. Rats were exposed to Cl2 (400 ppm for 30 min), returned to room air, and killed 6 h after exposure. They received injections of NO2− or saline. Average intensity of green fluorescence levels in main bronchus images (see Fig. 4) was calculated using Metamorph imaging software by 2 different investigators who were blinded regarding the groups of the rats. Five images were obtained for each rat, which were averaged so that each rat was weighted equally. Values are means ± SE; n = 3 rats in each group. *P < 0.001 vs. corresponding control value. #P < 0.001 vs. corresponding saline value in the same group.
To determine whether positive findings for TUNEL resulted from apoptotic or necrotic cells, we immunostained lung tissues with an antibody against the cleaved form of caspase-3 (catalog no. 9661, Cell Signaling Technology, Beverly, MA) at 4°C overnight and subsequently with a FITC-labeled secondary antibody (Sigma-Aldrich, St. Louis, MO). No significant amount of caspase-3 staining was observed in lung tissues of rats exposed to Cl2 and injected with saline or NO2− (data not shown). The results of these studies indicate that most of the TUNEL staining was the result of necrosis of airway epithelial cells.
Focal areas of alveolar consolidation and inflammation were also observed, and some contained cellular exudates (data not shown), as previously reported. Alveolar injury, although present, was less extensive than airway injury, in agreement with our previous findings (22). However, the increased amount of protein in the BAL, as well as injury to pulmonary surfactant and ion transport of distal lung epithelia of rats and mice exposed to 400 ppm Cl2 (22, 33), indicate that, at these concentrations, Cl2 or its reactive intermediates damage airway and distal lung epithelia.
There was considerable variability in the extent of injury in the airways of rats 24 h after exposure to Cl2. Focal areas of extensive injury, with denuded airway epithelia and extensive injury to the underlying structures, were present in all sections. However, there were numerous areas where the appearance of the airway epithelium was consistent with the presence of repair (Fig. 6C). These sections were TUNEL-negative.
Fig. 6.
Airway sections of rats exposed to Cl2 and killed 24 h after exposure. Rats were exposed to 400 ppm Cl2 for 30 min, returned to room air, injected with saline (A and B) or NO2− (C and D), and killed 24 h after exposure. A and C: hematoxylin-and-eosin-stained sections of proximal airways. Note absence of epithelium in saline-injected rat (A) and presence of pseudostratified epithelium, indicating onset of repair, in NO2−-injected rat. Only background levels of green fluorescence were seen in the adjoining sections (B and D) processed for TUNEL, indicating the absence of necrotic/apoptotic cells. Approximately 5 sections were examined from each rat (n = 3) with similar results. Scale bar, 50 μm.
Mechanistic insights: number of cells in BAL and F2-isoprostanes.
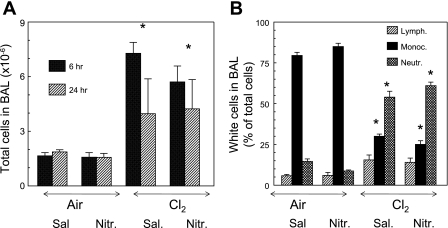
A possible mechanism by which NO2− may diminish lung injury is to decrease the influx of inflammatory cells in the BAL and lung tissue. As shown in Fig. 7, exposure to Cl2 resulted in a significant increase in the number of cells in the BAL at 6 and 24 h after exposure mainly due to the influx of neutrophils. Injection of NO2− did not decrease neutrophils in the BAL. Similarly, lung tissue myeloperoxidase activity [measured as previously described (1)] of saline- and NO2−-injected rats exposed to Cl2 was elevated to the same extent (data not shown). These data indicate that protective effects of NO2− are not due to decreased levels of inflammatory cells in the lung tissue or BAL.
Fig. 7.
Injections of NO2− in Cl2-exposed rats do not decrease the number of inflammatory cells in BAL. Rats were exposed to 400 ppm Cl2 for 30 min, returned to room air, and killed 6 or 24 h after exposure. All rats received intraperitoneal injections of NO2− (1 mg/kg body wt in 50 μl of normal saline) or an equivalent amount of saline at 0.1, 2, 4, and 6 h after exposure. Values are means ± SE; n = 8 animals in 6-h group and 3 animals in 24-h group. *P < 0.05 vs. corresponding air values.
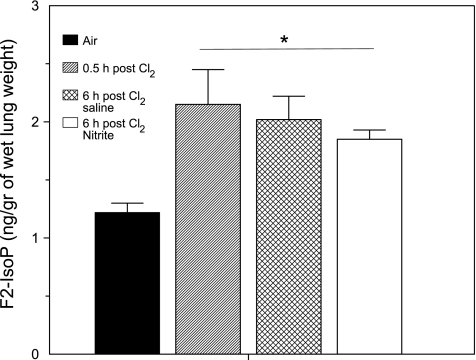
In our next series of experiments, we measured levels of F2-isoprostanes, a group of prostaglandin F2-like compounds derived from the nonenzymatic oxidation of arachidonic acid (25) in lung tissues of rats at 6 and 24 h after Cl2 exposure. As shown in Fig. 8, exposure to Cl2 increased F2-isoprostanes in lung tissue by 2.5-fold, and values remained elevated at 6 h after exposure. Administration of NO2− did not affect the F2-isoprostane levels, indicating that its protective effect was not mediated by preventing lipid peroxidation.
Fig. 8.
Exposure to Cl2 increases F2-isoprostane (F2-IsoP) levels in lung tissues. Rats were exposed to 400 ppm Cl2 for 30 min, returned to room air, and killed 6 h after exposure. All rats received intraperitoneal injections of NO2− (1 mg/kg body wt in 50 μl of normal saline) or an equivalent amount of saline at 0.1, 2, 4, and 6 h after exposure. Values are means ± SE; n = 8 in each group. *P < 0.05 vs. corresponding air values.
DISCUSSION
Cl2 gas exposure results in significant postexposure toxicity to the lungs, leading to ALI and acute respiratory distress syndrome. The risk for Cl2 exposure in a mass-casualty situation, therefore, has led to an interest in developing targeted therapeutics that can be administered after exposure to limit lung injury. With this goal in mind, we tested the potential of NO2−, which has been the subject of recent interest as a therapy to replenish NO bioactivity in diseases characterized by ischemia and inflammation.
The Cl2 exposure regimens used in this study were chosen to approximate levels encountered in the vicinity of industrial accidents. For example, Weill et al. (37) reported 400 ppm Cl2 within 75 yards of accidental spills of Cl2 from rail cars. Ten of the exposed individuals were hospitalized with pulmonary edema. In another report, 23 of 418 patients exposed to Cl2 during a chemical spill were hospitalized with pulmonary edema (11). Our previous data indicate that exposure of rats and mice to 400 ppm Cl2 for 30 min results in extensive injury to alveolar epithelial compartments, as evidenced by arterial hypoxemia, increased concentrations of protein in the BAL, compromised alveolar fluid clearance and surfactant function, and decreased levels of low-molecular-weight scavengers in the BAL and lung tissues (22, 33, 34, 38).
Data presented in the present study document the presence of significant neutrophilia and extensive necrosis of airway epithelia and inflammation and edema, as indicated by increased levels of neutrophils and protein in the BAL. Interestingly, as shown in Fig. 2, at 24 h after exposure, BAL protein levels decreased from their peak values, suggestive of tissue repair. Similarly, the hypoxemia that is prevalent immediately after Cl2 gas exposure recovers close to baseline levels by 6 h. Previous measurements of arterial Po2 in rats exposed to 400 ppm Cl2 for 30 min and returned to room air for 24 h were consistent with the presence of arterial hypoxemia, corresponding to an oxygen saturation of ∼90%, similar to that found in this study (22), within the experimental error of the measurements. Importantly, however, and despite improvement in indexes of oxygenation, lung permeability, and inflammation within 24 h of exposure, emerging data show that long-term pulmonary dysfunction occurs (observed over several days after Cl2 exposure), as evidenced by the development of reactive airway syndrome (23, 35) and decreased pulmonary function (19, 29). This further underscores the need for a better understanding of the mechanisms that mediate post-Cl2 exposure lung injury and development of targeted postexposure therapies that can prevent secondary injury and/or facilitate repair.
Our data show significant bronchiolar deepithelialization and loss of basement membrane, with accumulation of TUNEL-positive cells and debris, within 6 h after Cl2 exposure. Paralleling this are increased levels of F2-isoprostanes, stable markers of lipid peroxidation (25). Interestingly, however, lung F2-isoprostane levels were generated during Cl2 exposure and did not increase further after Cl2 exposure. At first glance, these data may suggest that propagating lipid peroxidation reactions do not continue beyond the initial exposure period and that Cl2 itself and/or its hydrolysis product HOCl is likely the mediator of arachidonic acid oxidation. On the other hand, it is also possible that constant levels of F2-isoprostanes may reflect the presence of equilibrium between their rates of production and breakdown by the platelet-activating factor acetylhydrolase. HOCl is a potent oxidant and will react with multiple biological molecules, including amines, to produce chloramines (k ∼105 M−1·s−1, where k is the apparent second order rate constant) (26). It is likely that the biological effects of HOCl could be at least partially attributed to effects of the chloramines (24, 27), which are relatively long-lasting, and selective chlorinating species that remain after the cessation of Cl2 exposure. Previously, we showed that that chloramines produced by the reaction of HOCl with glycine or taurine inhibit the activity of epithelial sodium channels (33).
NO2−-mediated protection against inflammatory tissue injury is thought to occur primarily by its reduction to NO by reduced hemoglobin in hypoxic red blood cells, which in turn could affect ALI by several mechanisms, including modulation of leukocyte adhesion and infiltration, inhibition of endothelial/epithelial cell permeability, prevention of cell death, and exertion of antioxidant effects (5, 7, 8, 13, 28, 31). As shown in Fig. 1, rats exposed to Cl2 showed signs of moderate-to-severe hypoxemia immediately upon return to room air, in agreement with our previous findings (22). However, oxygen saturations improved significantly during the next 6 h, reaching ∼90%. For this reason, we decided to initiate NO2− injections as soon as possible upon return of Cl2-exposed rats to room air. Administration of NO2− after Cl2 exposure, during the period that rats experienced moderate-to-severe hypoxemia, prevented pulmonary edema, indicated by inhibition of protein accumulation in BAL and maintenance of lung wet-to-dry weight ratios. Interestingly, NO2− did not affect BAL inflammatory cell accumulation, nor did NO2− alter F2-isoprostane levels. NO2− did, however, inhibit TUNEL-positive cells collectively, suggesting that NO2− protected against lung injury by modulating permeability and cell death, consistent with findings from a study of in vivo ischemia-reperfusion of heart and liver (8).
The protective effects of NO2− were also evident in histological analysis of bronchial airways 6 h after exposure. Since NO2− was administered after exposure, it is not surprising that only partial (∼30%) protection was observed (Fig. 6), with airways showing a phenotype intermediate to control animals and animals exposed to Cl2 alone. Specifically, Cl2 exposure resulted in a complete loss of epithelia and basement membrane, whereas in the NO2−-treated group, basement membrane, together with partial epithelium, was seen. The temporal and molecular mechanisms that regulate post-Cl2 toxicity and repair are not well defined. The sites at which systemically administered NO2− acts to mitigate lung injury are not clear and require further investigation; without this information, it is difficult to assess more detailed molecular mechanisms of NO2− action. At relatively high doses, myeloperoxidase may catalyze the reaction on NO2− and hydrogen peroxide to form reactive nitrogen species (including nitrogen dioxide) (9, 10), which have the capacity to cause extensive injury to alveolar and airway targets (6, 15, 16). In addition, high concentrations of NO2− in the plasma are likely to result in significant levels of methemoglobin, which will interfere with gas exchange. Different studies have reported that beneficial effects of NO2− supplementation in heart, liver, and brain ischemia-reperfusion injury are U-shaped, with protection being seen in the range of 48–480 nmol [∼1.6–16 nmol/g mouse body wt (with the assumption that mouse body weight is 30 g)] (8, 18). In this study, we used a dose toward the higher end of this range (250 μg nitrite/250-g rat, ∼21 nmol/g body wt) and observed no increases in inflammatory injury, which would be observed with increased reactive nitrogen species, nor was hypoxemia made worse, suggesting that safe doses of NO2− can be administered.
In summary, we present evidence documenting that exposure of rats to Cl2, in concentrations likely to be encountered in the vicinity of industrial accidents, causes extensive injury to airway and alveolar epithelia that is at least partially reversed by the postexposure administration of small quantities of NO2−. Thus our data are in agreement with previous reports documenting the efficacy of NO2− to reduce systemic injury following oxidant stress and point to its potential importance as a new therapeutic agent in inhaled oxidant injury to the lungs.
GRANTS
This work was supported by National Institutes of Health Grants 5U54 ES-017218, 5U01 ES-015676, and GM-42056.
DISCLOSURES
P. P. Patel is a coinventor of a NIH-UAB patent for use of nitrite salts for cardiovascular conditions. No other conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels. J Immunol 169: 2111–2119, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol 32: 368–375, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Bell DG. Management of acute respiratory distress syndrome (ARDS) following chlorine exposure (Abstract). Am J Respir Crit Care Med 176: A314, 2008 [Google Scholar]
- 4. Comellas AP, Pesce LM, Azzam Z, Saldias FJ, Sznajder JI. Scorpion venom decreases lung liquid clearance in rats. Am J Respir Crit Care Med 167: 1064–1067, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis IC, Zhu S, Sampson JB, Crow JP, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic Biol Med 33: 1703–1713, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res 75: 327–338, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115: 1232–1240, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem 271: 19199–19208, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391: 393–397, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Evans RB. Chlorine: state of the art. Lung 183: 151–167, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Fanucchi MV, Day KC, Clay CC, Plopper CG. Increased vulnerability of neonatal rats and mice to 1-nitronaphthalene-induced pulmonary injury. Toxicol Appl Pharmacol 201: 53–65, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 36: 707–717, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol 291: H2026–H2035, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hickman-Davis JM, Lindsey JR, Matalon S. Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis. Infect Immun 69: 6401–6410, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hickman-Davis JM, Nicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during Mycoplasma infection. Am J Respir Crit Care Med 173: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hickman-Davis JM, Wang Z, Fierro-Perez GA, Chess PR, Page GP, Matalon S, Notter RH. Surfactant dysfunction in SP-A−/− and iNOS−/− mice with Mycoplasma infection. Am J Respir Cell Mol Biol 36: 103–113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke 37: 2744–2750, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kennedy SM, Enarson DA, Janssen RG, Chan-Yeung M. Lung health consequences of reported accidental chlorine gas exposures among pulpmill workers. Am Rev Respir Dis 143: 74–79, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest 117: 2583–2591, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168: 568–574, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, Winterbourn CC. IκB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-κB activity by glycine chloramine through methionine oxidation. Biochem J 396: 71–78, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milne GL, Yin H, Brooks JD, Sanchez S, Jackson RL, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol 433: 113–126, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated protein oxidation: how important are chloramine transfer reactions and protein tertiary structure? Biochemistry 46: 9853–9864, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Peskin AV, Midwinter RG, Harwood DT, Winterbourn CC. Chlorine transfer between glycine, taurine, and histamine: reaction rates and impact on cellular reactivity. Free Radic Biol Med 37: 1622–1630, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Rubbo H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch Biochem Biophys 324: 15–25, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Salisbury DA, Enarson DA, Chan-Yeung M, Kennedy SM. First-aid reports of acute chlorine gassing among pulpmill workers as predictors of lung health consequences. Am J Ind Med 20: 71–81, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Sexton JD, Pronchik DJ. Chlorine inhalation: the big picture. J Toxicol Clin Toxicol 36: 87–93, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Post exposure administration of a β2-agonist decreases chlorine induced airway hyper-reactivity in mice. Am J Respir Cell Mol Biol. 2010. September 20 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 285: 9716–9728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 299: L289–L300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res 9: 61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van SD, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27: 1–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am Rev Respir Dis 99: 374–379, 1969 [PubMed] [Google Scholar]
- 38. Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 7: 278–283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration post chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol. In press. 2010 Dec 3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]