Abstract
Increases in the epidermal growth factor receptor (EGFR) have been associated with the severity of airway thickening in chronic asthmatic subjects, and EGFR signaling is induced by asthma-related cytokines and inflammation. The goal of this study was to determine the role of EGFR signaling in a chronic allergic model of asthma and specifically in epithelial cells, which are increasingly recognized as playing an important role in asthma. EGFR activation was assessed in mice treated with intranasal house dust mite (HDM) for 3 wk. EGFR signaling was inhibited in mice treated with HDM for 6 wk, by using either the drug erlotinib or a genetic approach that utilizes transgenic mice expressing a mutant dominant negative epidermal growth factor receptor in the lung epithelium (EGFR-M mice). Airway hyperreactivity (AHR) was assessed by use of a flexiVent system after increasing doses of nebulized methacholine. Airway smooth muscle (ASM) thickening was measured by morphometric analysis. Sensitization to HDM (IgG and IgE), inflammatory cells, and goblet cell changes were also assessed. Increased EGFR activation was detected in HDM-treated mice, including in bronchiolar epithelial cells. In mice exposed to HDM for 6 wk, AHR and ASM thickening were reduced after erlotinib treatment and in EGFR-M mice. Sensitization to HDM and inflammatory cell counts were similar in all groups, except neutrophil counts, which were lower in the EGFR-M mice. Goblet cell metaplasia with HDM treatment was reduced by erlotinib, but not in EGFR-M transgenic mice. This study demonstrates that EGFR signaling, especially in the airway epithelium, plays an important role in mediating AHR and remodeling in a chronic allergic asthma model.
Keywords: allergic asthma, airway smooth muscle, airway hyperresponsiveness, house dust mite, pulmonary inflammation
asthma is a major public health problem and is now the most common chronic disease in children (22). Airway hyperreactivity (AHR) in asthmatic subjects is associated with features of both airway inflammation and remodeling (29). Asthmatic individuals have a more rapid decline in lung function with age that is believed to be due to airway remodeling (28). Airway smooth muscle (ASM) thickening is a major component of airway remodeling in people with asthma and likely contributes to the greater degree of contractile force seen in asthmatic airways (7). Although the exact timing of the initiation of airway remodeling is not clear, recent data suggest that it is an early event in the disease process (9). New therapeutic targets are urgently needed to prevent or reverse airway remodeling and declining lung function in asthmatic patients.
Early genetic linkage studies in patients identified a region on chromosome 7 that contains the epidermal growth factor receptor (EGFR) gene and was associated with AHR (4). More recently, CA repeat polymorphisms in intron 1 of the EGFR gene were also found to be associated with asthma (44). In addition, EGFR ligands are elevated in samples from asthma patients (1) and EGFR immunoreactivity is increased in ASM of asthmatic subjects and correlates with disease severity (34, 35, 45). Although these data suggest a potential role for EGFR in airway remodeling and dysfunction in asthma, EGFR signaling may also be important in epithelial repair following injury (2, 6, 35), and thus its role in asthma remains unclear.
Previous short-term studies in the ovalbumin (OVA)-induced allergic asthma model using EGFR inhibitors indicated a role for EGFR signaling in the early stages of the disease process (24, 39, 41, 43). However, asthma is a chronic disease with progressive changes in lung function over time (38) and so whether EGFR signaling contributes to chronic asthma is still uncertain. Based on prior studies with EGFR inhibitors and the association of EGFR with severe airway remodeling in chronic asthmatics (34, 35, 45), we hypothesized that EGFR signaling contributes to airway dysfunction and remodeling in chronic asthma. To test this hypothesis we used a chronic allergic asthma model of mice treated for 6 wk with house dust mite (HDM), an environmental aeroallergen that is relevant to human exposure, and erlotinib, which inhibits the tyrosine kinase activity of EGFR and is currently used to treat patients with lung cancer and pancreatic cancer (8, 20). We also performed immunostaining for phosphorylated EGFR in mice treated with HDM and detected strong staining in airway epithelial cells, as well as other cells types. Since the airway epithelium is increasingly recognized as playing an important role in asthma (2, 5, 13, 23) we also utilized lung epithelial-targeted EGFR mutant transgenic (EGFR-M) mice to examine the role of EGFR signaling in epithelial cells. Overall, our studies showed that inhibition of EGFR in a chronic allergic asthma model reduced AHR and ASM thickening and demonstrated an important role for EGFR signaling in distal epithelial cells.
METHODS AND MATERIALS
Animals and treatments.
Protocols for animal use were approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital. Wild-type (WT) mice were given HDM (50 μg; Greer Laboratories) or saline (Controls) intranasally, three times a week for 3 or 6 wk. WT mice were also given erlotinib (100 mg/kg, OSI Pharmaceuticals, Melville, NY) by gavage six times a week for 6 wk, during the HDM treatment period. Control animals were given the same volume of the gavage vehicle (0.5% wt/vol methylcellulose; 130–180 μl). To assess the role of EGFR signaling in the epithelium, transgenic mice expressing a dominant negative mutant EGFR under the control of the epithelial surfactant protein-C gene promoter (17), referred to as EGFR-M mice, were given intranasal HDM or saline three times a week for 6 wk.
AHR assessments.
AHR to methacholine were assessed in mice treated for 6 wk with HDM, 48 h after the last treatment as previously described (27). Briefly, AHR was measured utilizing a flexiVent system (SCIREQ, Montreal, QC, Canada) after ultrasonic nebulization of 1× PBS (baseline) and in response to increasing doses of methacholine (12.5, 25, and 50 mg/ml; acetyl-β-methylcholine chloride, Sigma) to assess AHR.
Allergic sensitization and inflammation.
Bronchoalveolar lavage fluid (BALF) was collected after AHR studies, and inflammatory cells were collected by low-speed centrifugation. Total and HDM-specific IgG and IgE were measured in undiluted BALF by ELISA. ELISAs were performed using kits from BD Biosciences and following the manufacturer's instructions. For measurement of HDM-specific IgE and IgG levels, wells were coated with 0.01% HDM overnight and then the ELISA was performed. Cells from the BALF were resuspended in red blood cell lysis buffer (Sigma), neutralized, and collected again by centrifugation. Cells were resuspended and counted with a hemocytometer to obtain total cell counts. Cell suspension was also centrifuged onto slides by use of a cytospin (Shandon) and cells were stained by use of a Kwik-Diff kit (Thermo Scientific). The number of macrophages, lymphocytes, eosinophils, and neutrophils were counted in a total of 300 cells per slide. The percentage of each cell type was determined.
Histology and immunohistochemistry.
After AHR assessments and collection of BALF, lungs were inflation fixed and paraffin embedded, and 5-μm sections were cut (27). Immunohistochemical staining was performed with the following antibodies: phosphorylated-EGFR [p-EGFR (Tyr1173) 1:62.5 dilution; Cell Signaling], α-smooth muscle actin (α-SMA; 1:20,000 dilution; Clone 1A4 Sigma), and chloride channel, calcium activated, family member 3 (CLCA3; 1:12,500 dilution; Abcam). Sections were also stained with hematoxylin-eosin. As well as CLCA3 (alias gob-5) immunostaining to detect mucus-producing goblet cells (30), Alcian blue and periodic acid-Schiff histochemical staining were also performed. Pictures of the p-EGFR immunostaining of mice treated with saline (control) or HDM for 3 wk were taken using a Zeiss Axioplan 2 microscope and camera. For each animal four to five pictures of fields (×10 objective) were taken and the number of p-EGFR-positive cells were analyzed via Metamorph software (v6.2; Universal Imaging/Molecular Devices). Cells that stained positive by immunostaining for p-EGFR were counted in the epithelium of terminal bronchioles and expressed relative to the length of the epithelium (mm). Other cells that stained positive for p-EGFR in the field were also counted and expressed relative to the area of the field (mm2). To determine changes in goblet cells in the groups treated for 6 wk, pictures were taken of all the airways (100–600 μm in diameter) in three sections from the lung of each mouse. Goblet cells were identified by CLCA3-positive staining and counted. The length of the basement membrane of each airway was measured (mm) with Metamorph, and goblet cell counts for that airway were corrected to this length (31). Both p-EGFR-positive cells and goblet cells were counted by an observer blinded to the identity of the slides.
Western blot analysis.
Western blot analysis was performed on lung homogenates, and antibodies were used to detect CLCA3 (1:2,000; Abcam) and pan-actin (1:20,000; C4 Seven Hills Bioreagents). Secondary antibodies were either goat anti-rabbit or goat anti-mouse (1:10,000; Calbiochem). Chemiluminescence was detected by using ECL Plus (GE Healthcare), and images were obtained by using an LAS4000 imaging system and quantified with MultiGauge software (Fujifilm).
ASM measurement.
To assess changes in ASM in bronchioles the cross-sectional area of ASM and internal perimeter of the airway were measured (25, 27). Measurements were performed by an observer blinded to the identity of the slides. The square root of ASM area was corrected to internal perimeter, as previously described (25, 27).
Statistical analysis.
Data analyses were performed by use of the Prism 4 software (GraphPad Software). Unpaired t-tests were used to make statistical comparisons, and P < 0.05 was considered statistically significant. Values reported are means ± SE.
RESULTS
EGFR activation in HDM-induced asthma model.
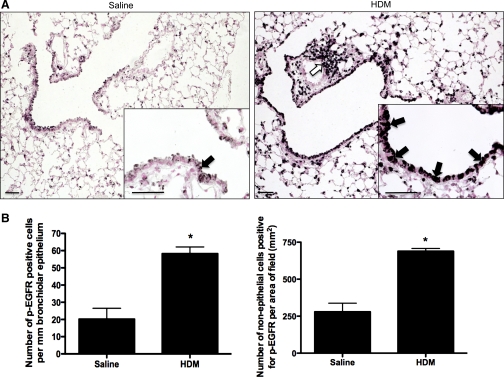
Immunostaining for p-EGFR detected increases in EGFR activation in mice treated with HDM for 3 wk, including increased p-EGFR staining in bronchiolar epithelial cells (3-fold; P < 0.05), as well as other cells (2.5-fold; P < 0.05) (Fig. 1, A and B).
Fig. 1.
EGF receptor (EGFR) activation in lungs of mice treated with house dust mites (HDM). A: immunostaining was performed by using a phosphorylated EGFR (Tyr1173)-specific antibody on mice treated for 3 wk with intranasal HDM (n = 5) or the same volume of saline (saline; n = 4). Three lung sections per animal were examined; pictures shown are representative. Weak staining for phosphorylated EGFR was detected in occasional bronchial epithelial cells (arrow) and scattered cells in the alveolar regions in the control mice. In HDM-treated mice staining for phosphorylated EGFR was increased, particularly in bronchial (see high-power inset, solid arrows) and alveolar epithelial cells and in inflammatory cells around vessels (open arrow) and airways. Bar = 100 μm. B: cells staining positively for phosphophorylated EGFR were counted in the bronchiolar epithelium and normalized to the length of the epithelium. Cells staining in other areas were also counted and normalized to the area of the picture field. *Significantly different from Saline, P < 0.05.
Inflammation in chronic allergic asthma model with EGFR inhibition.
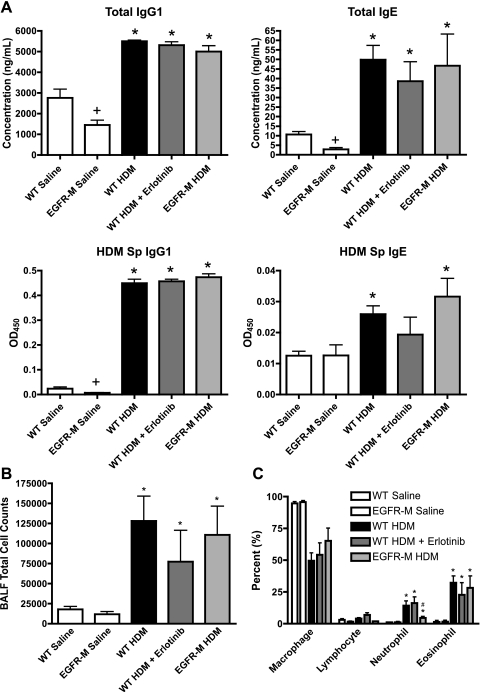
Total IgG1 and IgE were similarly increased in all mouse groups treated with HDM for 6 wk (Fig. 2A). Total IgG1 and IgE levels were lower in BALF from saline-treated EGFR-M mice compared with saline-treated WT mice. However, in response to HDM, IgG1 and IgE levels in EGFR-M mice increased and were similar to HDM-treated WT mice. HDM specific IgG1 increased similarly in all HDM-treated groups (Fig. 2A). HDM-specific IgE increased in HDM-treated WT and EGFR-M groups but did not reach significance in the HDM erlotinib-treated group, although total IgE was increased in this group (Fig. 2A). Total inflammatory cell counts in BALF (Fig. 2B), as well as eosinophils (Fig. 2C), were increased similarly in all HDM-treated groups. Neutrophils were increased in all HDM-treated groups, but to a much lesser extent in the EGFR-M mice (Fig. 2C).
Fig. 2.
Allergic sensitization and inflammatory response to chronic HDM is unaltered with EGFR inhibition. A: sensitization was assessed by measurement of total IgG1, total IgE, and HDM-specific (HDM Sp) IgG1 and IgE in bronchoalveolar lavage fluid (BALF) from mice treated with intranasal HDM or saline for 6 wk. HDM-treated groups also included wild-type (WT) mice, erlotinib-treated WT mice, and transgenic mice expressing a mutant dominant negative epidermal growth factor receptor in the lung epithelium (EGFR-M). Increases in total IgG1, total IgE, and HDM-specific IgG1 were similar in all HDM-treated groups. HDM-specific IgE was increased in HDM-treated WT mice and EGFR-M mice, but not the erlotinib-treated group; *P < 0.05 vs. saline control group; +P < 0.05 vs. WT saline. Data were derived from 5–13 animals per group. B: total numbers of cells were counted in BALF collected from mice treated with HDM or saline for 6 wk. Cell counts were not different between saline-treated WT mice and EGFR-M mice. HDM-treated groups all showed similar increases in total cell counts, indicating that EGFR inhibition does not alter inflammatory cell influx overall. *P < 0.05 vs. saline control group. Data were derived from 5–13 animals per group. C: cytospins, differential cell stains, and counts were performed on HDM and saline-treated groups to determine percent changes in inflammatory cell types. Macrophages were reduced similarly in all HDM-treated groups as the percentage of neutrophils and eosinophils increased; *P < 0.05 vs. saline control group; #P < 0.05 vs. WT HDM. Data were derived from 4–13 animals per group. EGFR inhibition did not alter BALF differential inflammatory cell counts with chronic HDM treatment, although increases in neutrophils in the EGFR-M mice were not as high in the other HDM-treated groups.
AHR and ASM thickening in chronic allergic asthma model are attenuated by EGFR inhibition.
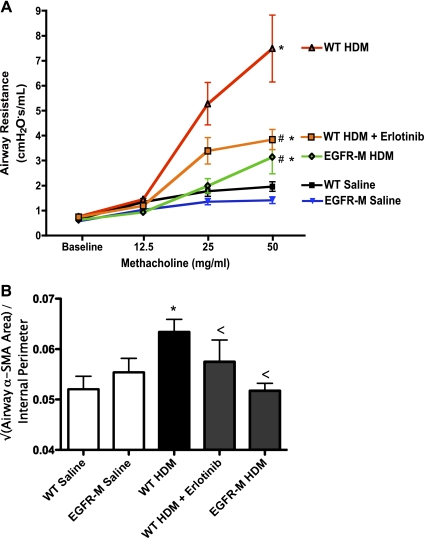
All HDM-treated groups demonstrated increased AHR to methacholine relative to controls (Fig. 3A). However, AHR was lower in both the erlotinib-treated and the EGFR-M groups relative to HDM-treated WT mice. No difference in AHR was detected in saline-treated EGFR-M mice compared with saline-treated WT controls. ASM area was measured, following immunostaining for smooth muscle α-actin (see Supplemental Fig. S1 online; the online version of this article contains supplemental data), and corrected to the internal perimeter of the airway (Fig. 3B). No difference in ASM area was detected between saline-treated WT and EGFR-M mice so the data from these two groups were pooled. WT mice treated with HDM showed increased ASM area relative to saline-treated controls, whereas ASM area in HDM-treated WT mice that received erlotinib and HDM-treated EGFR-M mice were not significantly different from saline-treated controls.
Fig. 3.
EGFR inhibition reduces airway hyperreactivity and airway smooth muscle thickening induced by chronic HDM treatment. A: airway resistance was measured at baseline and in response to increasing doses of nebulized methacholine in anesthetized mice placed on a flexiVent system. Mice were treated with saline (control) or HDM for 6 wk before assessment of lung mechanics. Saline control groups included WT mice and EGFR-M mice. HDM-treated mice all showed increased airway resistance with 50 mg/ml methacholine. However, increases in airway resistance in response to methacholine were lower in mice treated with erlotinib and also EGFR-M mice treated with HDM; *P < 0.05 vs. saline control group; #P < 0.05 vs. WT HDM. Data were derived from 5–13 animals per group. B: airway smooth muscle area was measured following HDM or saline treatment for 6 wk. See Supplemental Fig. S1 online for representative images of the α-smooth muscle actin immunostaining. HDM-treated WT mice had increased airway smooth muscle area. Erlotinib treated and EGFR-M mice treated with HDM failed to show significant increases in airway smooth muscle area; *P < 0.05 vs. saline control group; <P = not significantly different from saline control. Data were derived from 6–10 animals per group.
Goblet cell metaplasia in chronic allergic asthma model is attenuated by EGFR inhibition.
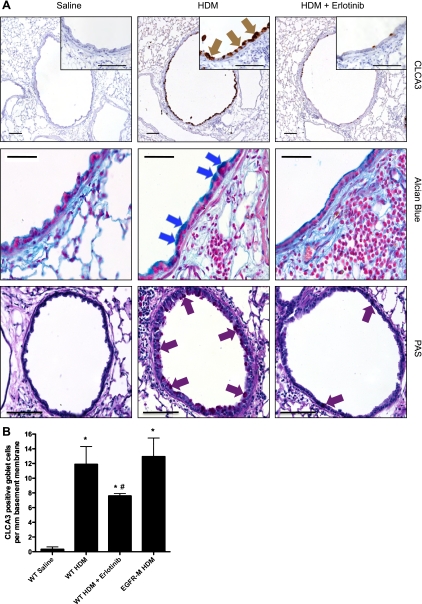
Immunostaining for CLCA3 and Alcian blue staining detected abundant goblet cells in the airways of all groups treated with HDM for 6 wk, particularly in the large conducting airways (Fig. 4A). Only occasional goblet cells were observed in the saline-treated mice. Immunostaining and Western blot analysis of CLCA3 demonstrated that goblet cell induction was lower in HDM mice that received erlotinib, but similar in HDM-treated EGFR-M mice, compared with HDM-treated WT controls (Fig. 4B; see Supplemental Fig. S2 online).
Fig. 4.
EGFR tyrosine kinase inhibitor attenuates goblet cell metaplasia in HDM-induced lung disease. A: goblet cells were detected in lung sections from mice treated with HDM or saline for 6 wk. Top: immunohistochemical staining for CLCA3 in goblet cells (see high-power inset, brown arrows). Middle: Alcian blue histochemical staining that detects mucopolysaccharides in goblet cells (blue arrows). Increases in goblet cell markers CLCA3 and Alcian blue staining with chronic HDM treatment were reduced by treatment with the EGFR inhibitor erlotinib. Bottom: periodic acid-Schiff histochemical staining (red arrows). Bar = 100 μm. B: goblet cells identified by CLCA3 immunostaining were counted in 100- to 600-μm airways and normalized to the length of the basement membrane (mm). Data are from 5–7 animals per group. *P < 0.05 vs. WT saline control group; #P < 0.05 vs. WT HDM.
DISCUSSION
In this study, we detected increases in phosphorylated EGFR in bronchiolar epithelial cells of mice treated with the aeroallergen HDM. Treatment with erlotinib, an EGFR inhibitor, reduced AHR, ASM thickening and goblet cell metaplasia in mice exposed to chronic HDM. Increases in AHR and ASM with HDM exposure were especially attenuated in mice expressing a mutant form of EGFR in epithelial cells. Hence, both pharmacological and genetic inhibition of EGFR signaling attenuated allergen-induced increases in AHR and ASM. Although increases in ASM in mice treated with HDM for 6 wk were not severe, they did correlate very well with increases in AHR and also reductions in AHR with EGFR inhibition. Whether reductions in AHR with EGFR inhibition are solely due to the prevention of ASM thickening is not clear because AHR can also occur in association with inflammation prior to airway remodeling. The converse is also true, and previous studies by our group using transforming growth factor-α (TGF-α transgenic mice on an Egr-1-null background), in which EGFR signaling is chronically increased, showed that severe AHR occurred in conjunction with ASM thickening but in the absence of detectable inflammation (27).
Previous studies have examined the role of EGFR in short-term asthma models using pharmacological inhibitors. AG-1478, an early EGFR inhibitor, has been used in mice challenged with OVA, leukotriene, IL-13, and monocyte chemotactic protein-1 for 2–3 days (41, 43). AG-1478 treatment reduced AHR, goblet cell metaplasia, airway collagen deposition, and eosinophil influx. In OVA-treated rats, AG-1478 also reduced ASM and epithelial cell hyperplasia as well as goblet cells after three challenges over 12 days (41). Another study used the oral EGFR inhibitor gefitinib, which also inhibited goblet cell metaplasia and AHR in mice after two OVA challenges (over 5 days following intraperitoneal sensitization to OVA), as well as attenuating eosinophil recruitment and proinflammatory cytokine levels (IL-4 and -13) (24). Takeyama and colleagues (39) showed that another EGFR inhibitor, BIBX1522, prevented goblet cell induction by tumor necrosis factor-α (TNF-α) and EGFR ligands, as well as OVA in rats. For this study, TNF-α and EGFR ligands were given over 1–3 days before the mice were euthanized and studied. The OVA study was also short-term with three OVA challenges performed over 6 days. This study also reported increases in EGFR in airway epithelial cells with TNF-α treatment that was coupled to increased mucus production. In our study, erlotinib treatment also reduced goblet cell increases in HDM-exposed mice, consistent with the previous studies identifying an important role for EGFR signaling in epithelial cell hyperplasia and goblet cell metaplasia both in vitro and in vivo (2, 3, 32, 39, 40, 42). EGFR signaling has been suggested to be important for epithelial repair following injury (2, 6, 35). However, a recent in vitro study reported that the EGFR inhibitor AG-1478 reduced HDM-triggered decreases in epithelial resistance and improved restoration of epithelial junctions (21). Similarly, EGFR inhibition increased epithelial barrier recovery upon electroporation-induced injury (21).
For this study we generated a chronic model by treating with the allergen HDM for 6 wk. Although this model may not necessarily reflect a lifetime of disease, it did allow us to examine the role of EGFR in a more chronic model than that used in previous studies. Two approaches were utilized to assess the role of EGFR: a pharmacological inhibitor, erlotinib, that is approved for treatment of patients with lung and pancreatic cancer (8), and a genetic approach, using transgenic mice expressing a mutant EGFR receptor (17). This transgenic mouse model allowed us to specifically examine the role of EGFR signaling in distal epithelial cells, since the mice express a truncated EGFR mutant transgene targeted to epithelial cells in bronchioles and type II cells. The mutant receptor can still bind EGFR ligands but lacks the intracellular signaling domain (17) and so fails to induce intracellular signaling and may also form dimers with WT EGFR and inhibit their signaling. The human SP-C promoter region used to target the mutant EGFR to the lungs of the transgenic mice drives gene expression both in type II alveolar epithelial cells and in bronchiolar epithelial cells, but not epithelial cells in the large conducting airways in adult mice (10–12, 21, 37). Determining the role of EGFR signaling in epithelial cells in vivo was an important goal of our study as an emerging body of information suggests that epithelial cells play a major role in airway dysfunction (2, 5, 13, 23), both as a respondent to and mediator/modulator of allergic and inflammatory stimuli. AHR and ASM thickening were very attenuated in the EGFR mutant mice, indicating an important role for EGFR signaling in epithelial cells in this chronic asthma model. EGFR signaling through other cell types could also be playing a role, since increased AHR was still detected in the HDM-treated EGFR-M mice although only at the highest dose of methacholine. EGFR-M mice showed smaller increases in neutrophils (in BALF) with HDM treatment, and saline-treated control EGFR-M mice had lower total IgG1 and IgE levels compared with controls. EGFR can regulate production of the neutrophil chemoattractant IL-8 by epithelial cells in response to respiratory syncytial virus infection, cigarette smoke, bacterial LPS/TACE activation, and reactive oxygen species (14, 32, 33, 36). Despite smaller increases in neutrophil influx in HDM-treated EGFR-M mice, eosinophil influx was similar, as was total IgG1, IgE and HDM-specific IgG1. In the studies mentioned earlier using the OVA model, EGFR inhibitor treatment was also associated with significant reductions in inflammatory cell numbers, including eosinophils (24, 43). These results are different from our study where we did not detect any major changes in eosinophils with EGFR inhibition in the chronic HDM model. This may be due to differences between the role of EGFR signaling in the OVA model or to the fact that the OVA studies were of shorter duration (3–12 days) than our study, which was 6 wk long. Another possible source of differences in the inflammatory cell counts is that inflammatory cell influx can be different depending on the timing of the BALF collection after the last allergen treatment and we only performed BALF cell counts at one time point. In addition, different mouse strains (BP2 and BALB/c) as well as rats were used in prior studies (24, 39, 41, 43), whereas our studies were all performed in FVB/N strain mice since that was the genetic background for the EGFR-M transgenic mice.
Inflammatory pathways can lead to activation of EGFR signaling (2, 6). Production and release of EGFR ligands can be triggered by factors known to play important roles in the etiology of asthma, including allergens, proinflammatory cytokines, environmental tobacco smoke, and virus infection (26, 43), supporting the concept that the EGFR pathway may act downstream of inflammatory pathways. Consistent with this, this study shows increases in EGFR phosphorylation in mice treated with HDM, including in bronchial epithelial cells. Transgenic mice in which the EGFR pathway was activated by chronic overexpression of TGF-α develop pulmonary fibrosis (15, 16, 18, 19). However, TGF-α transgenic mice null for the transcription factor Egr-1 also develop severe ASM and AHR, which interestingly occurs in the absence of any inflammatory changes (27).
In conclusion, this study shows that in adult mice inhalation of the allergen HDM increases EGFR activation. Our data demonstrate an important role for EGFR signaling, particularly in epithelial cells, in mediating the AHR and remodeling that develop as a result of chronic allergen treatment.
GRANTS
This research was funded by National Institutes of Health Grants U19A170235 (G. K. Khurana Hershey), U19AI070489 (M. J. Holtzman), HL097135 (T. D. Le Cras and G. K. Khurana Hershey), HL095580 (J. A. Whitsett), HL086598 (W. D. Hardie and T. R. Korfhagen), and AHA 740069N (T. D. Le Cras).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Kenneth Iwata at OSI Pharmaceuticals for helping facilitate the provision of erlotinib for these studies.
REFERENCES
- 1. Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med 157: 1907–1912, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J 32: 1068–1081, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 59: 992–996, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, Faux JA, Ryan GF, le Souef PN, Lathrop GM, Musk AW, Cookson WO. A genome-wide search for quantitative trait loci underlying asthma. Nature 383: 247–250, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Davies DE, Holgate ST. Asthma: the importance of epithelial mesenchymal communication in pathogenesis. Inflammation and the airway epithelium in asthma. Int J Biochem Cell Biol 34: 1520–1526, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Davies DE, Polosa R, Puddicombe SM, Richter A, Holgate ST. The epidermal growth factor receptor and its ligand family: their potential role in repair and remodelling in asthma. Allergy 54: 771–783, 1999 [PubMed] [Google Scholar]
- 7. Dulin NO, Fernandes DJ, Dowell M, Bellam S, McConville J, Lakser O, Mitchell R, Camoretti-Mercado B, Kogut P, Solway J. What evidence implicates airway smooth muscle in the cause of BHR? Clin Rev Allergy Immunol 24: 73–84, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Erlotinib (Tarceva) for advanced non-small cell lung cancer. Med Lett Drugs Ther 47: 25–26, 2005 [PubMed] [Google Scholar]
- 9. Fedorov IA, Wilson SJ, Davies DE, Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax 60: 389–394, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, Alger LA, Tuder RM, Voelkel NF. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest 103: 1509–1515, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR. Human SP-C gene sequences that confer lung epithelium-specific expression in transgenic mice. Am J Physiol Lung Cell Mol Physiol 278: L933–L945, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Glasser SW, Korfhagen TR, Wert SE, Bruno MD, McWilliams KM, Vorbroker DK, Whitsett JA. Genetic element from human surfactant protein SP-C gene confers bronchiolar-alveolar cell specificity in transgenic mice. Am J Physiol Lung Cell Mol Physiol 261: L349–L356, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Hamilton LM, Davies DE, Wilson SJ, Kimber I, Dearman RJ, Holgate ST. The bronchial epithelium in asthma—much more than a passive barrier. Monaldi Arch Chest Dis 56: 48–54, 2001 [PubMed] [Google Scholar]
- 14. Hamilton LM, Torres-Lozano C, Puddicombe SM, Richter A, Kimber I, Dearman RJ, Vrugt B, Aalbers R, Holgate ST, Djukanovic R, Wilson SJ, Davies DE. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy 33: 233–240, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hardie WD, Bruno MD, Huelsman KM, Iwamoto HS, Carrigan PE, Leikauf GD, Whitsett JA, Korfhagen TR. Postnatal lung function and morphology in transgenic mice expressing transforming growth factor-alpha. Am J Pathol 151: 1075–1083, 1997 [PMC free article] [PubMed] [Google Scholar]
- 16. Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, Whitsett JA, Korfhagen TR. EGFR- tyrosine kinase inhibitors diminish transforming growth factor-α induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L1217–L1225, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hardie WD, Kerlakian CB, Bruno MD, Huelsman KM, Wert SE, Glasser SW, Whitsett JA, Korfhagen TR. Reversal of lung lesions in transgenic transforming growth factor alpha mice by expression of mutant epidermal growth factor receptor. Am J Respir Cell Mol Biol 15: 499–508, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, Nichols W, Whitsett JA, Leikauf GD. Genomic profile of matrix and vasculature remodeling in TGF-alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol 37: 309–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hardie WD, Piljan-Gentle A, Dunlavy MR, Ikegami M, Korfhagen TR. Dose-dependent lung remodeling in transgenic mice expressing transforming growth factor-α. Am J Physiol Lung Cell Mol Physiol 281: L1088–L1094, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hegymegi-Barakonyi B, Eros D, Szantai-Kis C, Breza N, Banhegyi P, Szabo GV, Varkondi E, Petak I, Orfi L, Keri G. Tyrosine kinase inhibitors — small molecular weight compounds inhibiting EGFR. Curr Opin Mol Ther 11: 308–321, 2009 [PubMed] [Google Scholar]
- 21. Heijink IH, van Oosterhout A, Kapus A. EGFR signaling contributes to house dust mite-induced epithelial barrier dysfunction. Eur Respir J 36: 1016–1026, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Hesselmar B, Aberg B, Eriksson B, Aberg N. Asthma in children: prevalence, treatment, and sensitization. Pediatr Allergy Immunol 11: 74–79, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 29, Suppl 2: 90–95, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Hur GY, Lee SY, Lee SH, Kim SJ, Lee KJ, Jung JY, Lee EJ, Kang EH, Jung KH, Lee SY, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. Potential use of an anticancer drug gefinitib, an EGFR inhibitor, on allergic airway inflammation. Exp Mol Med 39: 367–375, 2007 [DOI] [PubMed] [Google Scholar]
- 25. James AL, Hogg JC, Dunn LA, Pare PD. The use of the internal perimeter to compare airway size and to calculate smooth muscle shortening. Am Rev Respir Dis 138: 136–139, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Kim S, Shim JJ, Burgel PR, Ueki IF, Dao-Pick T, Tam DC, Nadel JA. IL-13-induced Clara cell secretory protein expression in airway epithelium: role of EGFR signaling pathway. Am J Physiol Lung Cell Mol Physiol 283: L67–L75, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kramer EL, Mushaben EM, Pastura PA, Acciani TH, Deutsch GH, Khurana Hershey GK, Korfhagen TR, Hardie WD, Whitsett JA, Le Cras TD. Early growth response-1 suppresses epidermal growth factor receptor-mediated airway hyperresponsiveness and lung remodeling in mice. Am J Respir Cell Mol Biol 41: 415–425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 339: 1194–1200, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Laprise C, Laviolette M, Boutet M, Boulet LP. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodelling. Eur Respir J 14: 63–73, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Leverkoehne I, Gruber AD. The murine mCLCA3 (alias gob-5) protein is located in the mucin granule membranes of intestinal, respiratory, and uterine goblet cells. J Histochem Cytochem 50: 829–838, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Makela MJ, Kanehiro A, Dakhama A, Borish L, Joetham A, Tripp R, Anderson L, Gelfand EW. The failure of interleukin-10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am J Respir Crit Care Med 165: 824–831, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Monick MM, Cameron K, Staber J, Powers LS, Yarovinsky TO, Koland JG, Hunninghake GW. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J Biol Chem 280: 2147–2158, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol 292: L1289–L1296, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Polosa R, Puddicombe SM, Krishna MT, Tuck AB, Howarth PH, Holgate ST, Davies DE. Expression of c-erbB receptors and ligands in the bronchial epithelium of asthmatic subjects. J Allergy Clin Immunol 109: 75–81, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 14: 1362–1374, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Richter A, O'Donnell RA, Powell RM, Sanders MW, Holgate ST, Djukanovic R, Davies DE. Autocrine ligands for the epidermal growth factor receptor mediate interleukin-8 release from bronchial epithelial cells in response to cigarette smoke. Am J Respir Cell Mol Biol 27: 85–90, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Simpson DS, Mason-Richie NA, Gettler CA, Wikenheiser-Brokamp KA. Retinoblastoma family proteins have distinct functions in pulmonary epithelial cells in vivo critical for suppressing cell growth and tumorigenesis. Cancer Res 69: 8733–8741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int 56: 331–340, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA 96: 3081–3086, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, Kroschel P, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 280: L165–L172, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Tamaoka M, Hassan M, McGovern T, Ramos-Barbon D, Jo T, Yoshizawa Y, Tolloczko B, Hamid Q, Martin JG. The epidermal growth factor receptor mediates allergic airway remodelling in the rat. Eur Respir J 32: 1213–1223, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, Spoor MS, You Y, Brody SL, Holtzman MJ. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 116: 309–321, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vargaftig BB, Singer M. Leukotrienes mediate part of Ova-induced lung effects in mice via EGFR. Am J Physiol Lung Cell Mol Physiol 285: L808–L818, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Wang X, Saito J, Ishida T, Munakata M. Polymorphism of egfr Intron1 is associated with susceptibility and severity of asthma. J Asthma 43: 711–715, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Yamanaka Y, Hayashi K, Komurasaki T, Morimoto S, Ogihara T, Sobue K. EGF family ligand-dependent phenotypic modulation of smooth muscle cells through EGF receptor. Biochem Biophys Res Commun 281: 373–377, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.