Abstract
The limiting component within the receptor-G protein-effector complex in airway smooth muscle (ASM) for β2-adrenergic receptor (β2-AR)-mediated relaxation is unknown. In cardiomyocytes, adenylyl cyclase (AC) is considered the “bottleneck” for β-AR signaling, and gene therapy trials are underway to increase inotropy by increasing cardiac AC expression. We hypothesized that increasing AC in ASM would increase relaxation from β-agonists, thereby providing a strategy for asthma therapy. Transgenic (TG) mice were generated with approximately two- to threefold overexpression of type 5 AC (AC5) in ASM. cAMP and airway relaxation in response to direct activation of AC by forskolin were increased in AC5-TG. Counter to our hypothesis, isoproterenol-mediated airway relaxation was significantly attenuated (∼50%) in AC5-TG, as was cAMP production, suggesting compensatory regulatory events limiting β2-AR signaling when AC expression is increased. In contrast, acetylcholine-mediated contraction was preserved. Gαi expression and ERK1/2 activation were markedly increased in AC5-TG (5- and 8-fold, respectively), and β-AR expression was decreased by ∼40%. Other G proteins, G protein-coupled receptor kinases, and β-arrestins were unaffected. β-agonist-mediated airway relaxation of AC5-TG was normalized to that of nontransgenic mice by pertussis toxin, implicating β2-AR coupling to the increased Gi as a mechanism of depressed agonist-promoted relaxation in these mice. The decrease in β2-AR may account for additional relaxation impairment, given that there is no enhancement over nontransgenic after pertussis toxin, despite AC5 overexpression. ERK1/2 inhibition had no effect on the phenotype. Thus perturbing the ratio of β2-AR to AC in ASM by increasing AC fails to improve (and actually decreases) β-agonist efficacy due to counterregulatory events.
Keywords: adenylyl cyclase, desensitization, asthma, gene therapy
the β2-adrenergic receptor (β2-AR) expressed on airway smooth muscle (ASM) acts to relax constricted muscle from most any stimulus, and β-agonists have become a mainstay in the acute treatment of bronchospasm and a chronic preventative treatment in asthma and chronic obstructive pulmonary disease (17). The smooth muscle relaxation effect of β2-AR is mediated through its coupling to the heterotrimer G protein Gs, with Gαs activating adenylyl cyclase (AC), which catalyzes the conversion of ATP to cAMP. Various mechanisms primarily centered on cAMP-dependent activation of protein kinase A, which phosphorylates multiple proteins within the contractile system, are responsible for the relaxation effect (7, 13). There have been a number of attempts to improve the clinical effectiveness of β-agonists, primarily through development of agonists with different structures that afford selectivity, potency, efficacy, and duration of action profiles (1) that would be favorable for treating obstructive airway disease. In considering how to best utilize this bronchodilating mechanism, it has become apparent that we do not understand the limiting factor, (i.e., the stoichiometry) at the initial steps of the pathway. Thus the physiologically or therapeutically optimal β2-AR-to-Gs-to-AC ratio in ASM is not known, and thus the potential benefits of opening the “bottleneck”, by pharmacological means or genetic therapy, to improve the clinical response, has not been realized. In the cardiomyocyte, it is thought that AC represents the limiting factor for β-AR signaling (16), and methods that increase AC protein in cardiomyocytes improve β1-AR signal transduction (5) and cardiac inotropy in animal models (6). In fact, a human clinical trial of gene therapy of AC6 in heart failure is currently underway (www.ClinicalTrials.gov, identifier NCT00787059). Our group has overexpressed the β2-AR in mice targeting ASM and found that, with marked overexpression, an augmentation of β-agonist-mediated relaxation is observed (11). The extent of the overexpression, though, is out of proportion to the somewhat modest gain-of-function. An additional complexity for the β2-AR subtype is its capacity to also couple to the inhibitory G protein Gi (3), which decreases AC activity (18) and also activates other pathways through Gi-mediated activation of mitogen activated protein kinase (3). Thus there is active competition between the Gs and Gi pathways that must be considered when contemplating pharmacological or gene therapy approaches to increase β2-AR expression. Indeed, since β2-AR coupling to Gi is triggered by a conformational change in the β2-AR protein from protein kinase A phosphorylation of the receptor (3, 20), there is the potential for any mechanism that inordinately increases cAMP to alter the optimal ratio of Gs and Gi signaling. To gain a better understanding of the stoichiometry of β2-AR to the AC effector, we have generated transgenic mice overexpressing AC5 (the ASM dominant AC isoform) in ASM by targeting expression via the SMP8 α-smooth muscle actin promoter. We hypothesized that AC is the limiting factor in β2-AR signaling to ASM relaxation, and thus modest increases in AC expression would result in enhanced β-agonist promoted bronchodilation, pointing toward novel therapeutic approaches. However, AC5 overexpression resulted in preeffector counterregulatory events that lead to a paradoxical decrease in β-agonist efficacy.
METHODS
Transgenic mouse generation.
These studies were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore. The rat AC5 cDNA was cloned into the SMP8 α-smooth muscle actin promoter construct previously described (11) at Xho I sites. Subsequently, a Not I-digested fragment was gel purified and utilized for injection to generate transgenic founder mice (B6129F1 strain, Taconic, Hudson, NY) using standard techniques, as described previously (10, 11). Genomic screening from tail-clip DNA was carried out by PCR using primers of which one was from the AC5 gene (5′-CCTCAGTTAGCAGATACCAGCAGC-3′) and one from the SV40 portion of the transgene (5′-CCATTCATCAGTTCCATAGGTTGG-3′); thus there was no product from mice that failed to incorporate the transgene. mRNA expression was ascertained from cultured ASM cells using RT-PCR with oligo(dT) for the reverse transcription and the following rat-specific AC5 primers: 5′-GTGTTTATATCTGTGATCTACGCCT-3′ and 5′-CGTAGATGAGCTCGATGGC-3′. F0 mice that tested positive for the transgene were mated with nontransgenic (NTG) mice and F1 offspring tested for passage. Subsequent matings established five separate lines for initial screening, of which two had equivalent AC5 mRNA and protein expression. These lines were expanded for the physiological and cell-based studies, which were carried out with ∼60-day old F3-F8 mice.
Isolated airway physiology.
Sections of mouse trachea (5 mm) were rapidly excised after CO2 narcosis and studied in an isometric myograph system (Radnoti, Monrovia, CA) in a manner similar to that previously described (12, 19). The circulating bath consisted of Krebs buffer at 37°C aerated with 95% O2 and 5% CO2. Tracheal rings were set to a passive tension of 5 mN, and, after a 15-min stabilization period, the rings were dosed with various agents to evoke contraction or relaxation. These responses were continuously recorded, and changes in force were observed as early as 1 min after dosing and were stable at the 5-min point, which was considered the response to the drug at that concentration. Initial studies were to ascertain the contractile response to acetylcholine using 12 concentrations (10−8-10−3 M), with the data presented as the isometric force in milli-Newtons. After washout from these dose-response studies, a single concentration of acetylcholine that achieved contraction of 18 mN was maintained in the bath. Increasing doses of isoproterenol (8 concentrations, 10−9 to 3 × 10−6 M) were then added to ascertain the relaxation response, which was normalized to the maximal force and presented as a percentage. Tracheas were also exposed to forskolin at a concentration of 0.1 μM. In some studies, mice were treated with pertussis toxin (PTX) by an intraperitoneal injection of 100 μg/kg 18 h before trachea extraction and ring studies. In other experiments, rings in the bath were exposed to 10 μM of the extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor U0126 for 1 h before study. cAMP was measured as previously described (19) by incubating tracheas in Krebs buffer with 100 μM isobutylmethylxanthine and 10 μM isoproterenol or 100 μM forskolin for 15 min at 37°C.
Intact mouse lung physiology.
Mice were sedated with tribromoethanol, a tracheotomy was performed, and then mice were intubated with an 18-g blunt needle. They were ventilated, and airway resistance was measured as previously described (4) using the Scireq system (Scientific Respiratory Equipment, Quebec, Canada). The ventilatory rate was set at 150 breaths/min, with a tidal volume of 10 ml/kg (∼250 μl/breath) and a positive and expiratory pressure of 2.5 cmH2O. Resistance measurements were taken every 1 min and averaged over 10 min. After stabilization, they were challenged by aerosolized methacholine at doses from 2 to 16 mg/ml until airway resistance increased sixfold over baseline. During this stable constrictive period, mice were then administered aerosolized fenoterol at a dose of 0.5 mg/ml delivered over 10 s.
ASM cell culture.
Primary mouse ASM cultures were established as described (11). Briefly, tracheas were excised and cut in a longitudinal fashion. Cross sections of 2–3 mm were placed intima side down in culture dishes and maintained in Dulbecco's modified Eagle's medium with 20% calf serum and 100 μg/ml gentamycin and 0.5 μg/ml amphotericin. Explants were kept at 37°C, 5% CO2, 95% air, for 3 days and then maintained in growth media, which was the same as above except with 50% less calf serum, gentamycin, and amphotericin. The outgrowing cells that adhered under these conditions were >95% ASM and after confluency were passaged at 1:4 and maintained in growth media. Studies were performed with cells at 90% confluency, from passages 2–8. In some studies, cells were treated with 1 μg/ml PTX in the culture medium.
Western blots, radioligand binding.
Western blots were carried out in a standard fashion using 20 μg of ASM cell lysates, as described (10). Primary antibodies were as follows: phosphodiesterase type 4 (PDE4; 1:500; FabGennix, Frisco, TX), Gαi1/2 (1:1,000; Millipore, Billerica, MA), Gαq (1:1,000; CalBiochem, San Diego, CA), Gαs (1:200; Santa Cruz, Santa Cruz, CA), AC5 (1:200, Santa Cruz), ERK1/2 (1:1,000, Cell Signaling Technology, Danvers, MA), phosphorylated ERK1/2 (pERK1/2; 1:1,000; Cell Signaling Technology), G protein-coupled receptor kinase 2 (GRK2; 1:200; Santa Cruz), GRK5 (1:1,000; Santa Cruz), β-arrestin 1 (β-arr1; 1:500; Novus Biologicals, Littleton, CO), β-arr2 (1:500; Novus Biologicals), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:200; Santa Cruz), and Na+-K+-ATPase (1:200; Santa Cruz). Detection was by the ECL Advanced Western Blotting kit (GE Healthcare, Piscataway, NJ), and images were acquired directly from the membrane using a Fuji LAS-3000 charge-coupled device camera (Fujifilm Medical Systems, Stamford, CT). β-AR expression on ASM membrane preparations was determined using quantitative 125I-cyanopindolol radioligand binding, as described (8), with nonspecific binding defined by coincubations with 10 μM alprenolol.
Statistical analysis.
Dose-response data were fit by iterative least squares techniques to sigmoid curves using PRISM (GraphPad, La Jolla, CA), where the minimal and maximal responses, and the concentration of agent resulting in 50% of the response (EC50) were derived. Data from Western blots were acquired using ImageQuant software (Fuji). Results from all studies were compared by paired or unpaired t-tests, with significance imparted when P < 0.05. Data are presented as means ± SE.
RESULTS
Transgenic expression of AC5.
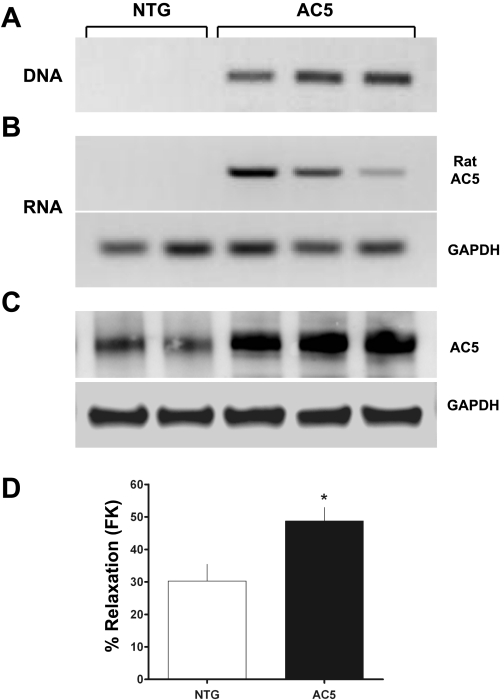
Using the SMP8 α-smooth muscle actin promoter, transgenic mice were generated using methods that we have previously described (see methods). As shown in Fig. 1, the transgene was incorporated into the genome of founder progeny as assessed by a specific PCR utilizing primers from the SV40 portion of the transgene and the rat AC5 using tail-clip genomic DNA as a template (A), and rat AC5 mRNA was expressed only in ASM cells cultured from transgene-positive mice (B). AC5 protein expression, as determined by Western blots, was increased in these ASM cells approximately two- to threefold over NTG cells (C). In addition, direct activation of AC by forskolin was found to result in enhanced relaxation from acetylcholine contraction of the AC5 airways compared with those from NTG mice (D), indicating functional overexpression of the transgene. The transgene was transmitted to progeny in a manner consistent with Mendelian inheritance. Taken together, these genomic, mRNA and protein expression, and functional results revealed successful generation of AC5 transgenics. Life span, body weight, and reproduction were unaffected in AC5 transgenics, and there were no gross respiratory signs noted. Of the multiple lines established from the founders, several had similar levels of AC5 overexpression, and two (denoted here AC5–1 and AC5–2) were chosen for additional studies.
Fig. 1.
Generation of transgenic mice overexpressing type 5 adenylyl cyclase (AC5). A: genomic PCR for the transgene using a primer from rat AC5 and one from SV40 of the transgene. B: mRNA expression from RT-PCR using rat-specific AC5 primers shows rat AC5 mRNA expression in airway smooth muscle (ASM) exclusively from mice that express the SMP8-AC5 transgene. C: AC5 protein expression in mice expressing the SMP8-AC5 transgene is increased twofold in ASM compared with that in nontransgenic (NTG) mice. Shown are results from two NTG controls and three different lines (F2) of AC5 transgenics. D: forskolin (FK)-mediated airway relaxation from acetylcholine (ACh)-constricted rings is greater in AC5 mice. Values are means ± SE. Results are from experiments with 4 NTG and 4 AC5 transgenic mouse tracheas. *P < 0.05 vs. NTG.
Paradoxical decrease in β-agonist-mediated ASM relaxation in AC5 transgenics.
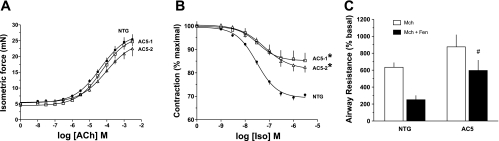
Having established overexpression of AC5 and the expected enhanced relaxation outcome from direct stimulation by forskolin in the screening studies, we next established the phenotypes of AC5 transgenics in response to β-agonist. Rings were first contracted with acetylcholine, and the AC5 transgenics were found to have equivalent contractile responses to the NTG (Fig. 2A). After washout, rings were contracted continuously with a fixed concentration of acetylcholine that achieved equivalent force (∼18 mN) for each ring, and then increasing doses of isoproterenol were added to the bath. The results from these β-agonist-mediated relaxation experiments were in marked contrast to our original hypothesis. Not only did AC5 transgenics fail to display a more robust relaxation, they consistently had less relaxation in response to β-agonist compared with NTG rings (Fig. 2B). In NTG, maximal relaxation amounted to 30 ± 1.5%, while, in the two AC5-transgenic lines, maximal relaxation was 18 ± 2.2 and 15 ± 3.3% (P < 0.01 vs. NTG), respectively. The potency for isoproterenol was comparable for the three lines (−log[EC50] = 7.51 ± 0.05, 7.45 ± 0.03, and 7.63 ± 0.06, respectively). Additional experiments were performed with isoproterenol in the presence of the specific β1-AR antagonist CGP-20712A (0.1 μM), so as to confirm that the relaxation phenotype was observed from β2-AR subtype stimulation. In these experiments, NTG relaxation was 24 ± 2.8%, while relaxation of AC5–1 was 12 ± 2.9% (P < 0.05, n = 4 mice of each genotype). Thus the phenotype of depressed relaxation in the AC5 transgenics was observed under conditions of selective β2-AR activation. Additional studies were also performed to examine lung function in the intact, intubated mouse. As shown in Fig. 2C, β2-agonist (fenoterol)-mediated decreases in airway resistance were depressed in AC5 mice compared with NTG, consistent with the results in the ex vivo isolated airway studies.
Fig. 2.
Paradoxical consequences of AC5 overexpression in airway relaxation responses to β-agonist. A: isolated airway contractile responses to ACh are not different between the two AC5 mouse lines and NTG mice. Rings were set to 5 mN passive force before ACh exposure. B: isolated airway relaxation responses to isoproterenol (Iso) are decreased in the two AC5 mouse lines compared with NTG mice. Rings were contracted with a continuous dose of ACh (typically 0.3 mM) that resulted in 18 mN total force before Iso exposure. Values are means ± SE. Results from A and B are from 8 NTG, 4 AC5–1, and 4 AC5–2 mice. *P < 0.01 vs. NTG. C: β-agonist-promoted decreases in airway resistance are depressed in AC5 mice. Mice were studied using the intact, intubated method, and the indicated agonists were given by inhaled aerosols. Values are means ± SE. Results are from 5 NTG and 6 AC5 transgenic mice. #P < 0.05 vs. NTG. Mch, methacholine; Fen, fenoterol.
Mechanisms of counterregulation in AC5 transgenics.
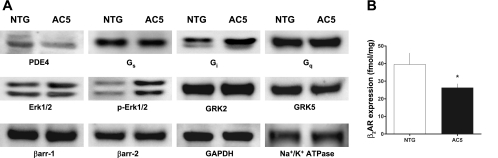
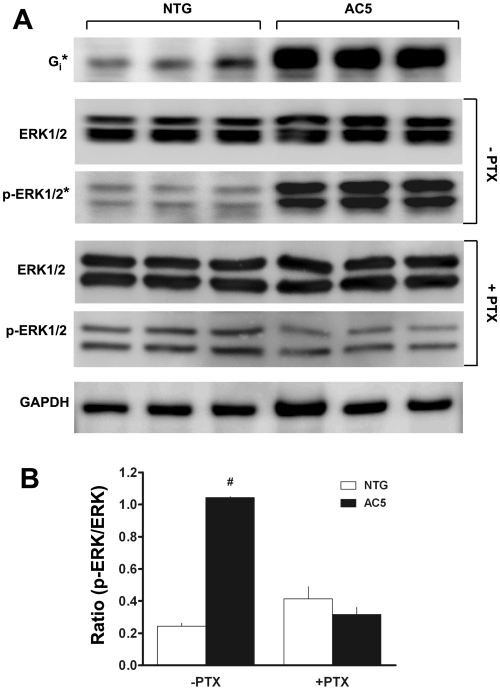
Given these unexpected results observed in both AC5 mouse lines, we screened by Western blots AC5-transgenic ASM for expression of various components of the β2-AR and related signal transduction systems to ascertain changes that might lead to the diminished response. As shown in Fig. 3, Gαs, Gαq, GRK2, GRK5, β-arr1 and β-arr2 expression were unchanged. However, PDE4 expression was decreased, while Gαi and pERK1/2 were increased in the AC5-transgenic ASM cells compared with NTG. The decreased PDE4 expression was accompanied by a decrease in cAMP PDE activity (data not shown); however, this event would not explain a decrease in β2-AR function. The increased Gαi expression was further explored in additional studies (Fig. 4A), where Gαi was found to be increased by fivefold (P < 0.001). One functional consequence of β2-AR-Gi coupling is phosphorylation of ERK1/2 by a Giβγ/β-arr-dependent pathway (3, 14). While the expression of the nonphosphorylated form of ERK1/2 was not different between NTG and AC5 transgenics, the extent of phosphorylation was increased by 8.5-fold in the transgenic ASM cells (Fig. 4A, P < 0.001). To confirm the link between the increased Gαi and enhanced activation of ERK1/2, cultured ASM cells from NTG and AC5 mice were treated with PTX and extracts blotted for total and pERK1/2. As shown in Fig. 4A, the enhanced pERK1/2 in the AC5 ASM cells was no longer observed under these conditions. The ratio of pERK1/2 to total ERK1/2 expression, which was markedly elevated in the AC5 ASM cells in the absence of PTX, was normalized to that of NTG when cells were treated with PTX (Fig. 4B).
Fig. 3.
Protein expression screening from ASM cells derived from NTG and AC5 mice. A: Western blots probing for the indicated proteins indicate decreased phosphodiesterase type 4 (PDE4) and increased Gi and phosphorylated ERK1/2 (pERK1/2) in ASM from the AC5 mice. GAPDH and Na+-K+-ATPase represent intracellular and membrane-bound control proteins, respectively. Results are representative of experiments performed with 3 NTG and 4 AC5 transgenic mouse ASM cell lines. GRK2 and GRK5, G protein-coupled receptor kinases 2 and 5, respectively; β-arr, β-arrestin. B: 125I-cyanopindolol radioligand binding indicates decreased β2-adrenergic receptor (β2-AR) expression in AC5 ASM cells. *P < 0.05 vs. NTG. Values are means ± SE. Results are from experiments performed from 3 plates of ASM cells from NTG and 3 from AC5 transgenic mice.
Fig. 4.
AC5 transgenic ASM have increased expression of Gαi2 and pERK1/2. A: shown are Western blots using antibodies to the indicated proteins. Enhanced chemiluminescence output was captured on a charge-coupled device camera directly from the membranes and quantitated using ImageQuant software (see methods). *P < 0.001 AC5 vs. NTG. B: ratio of pERK1/2 to total ERK1/2 is enhanced in AC5 ASM cells and is sensitive to pertussis toxin (PTX). Values are means ± SE. Results are from 3 NTG and 3 AC5 transgenic ASM cell lines. #P < 0.01 vs. (−PTX) NTG.
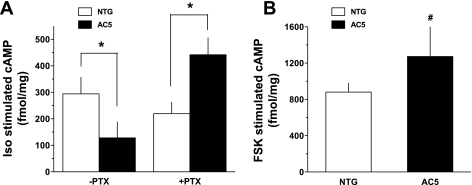
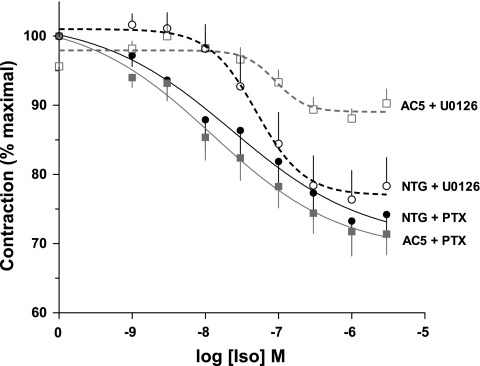
Expression of β2-AR on ASM was ascertained by quantitative 125I-cyanopindolol radioligand binding, where a ∼40% decrease in expression was found with the AC5 transgenics compared with NTG (Fig. 3B). Taken together, these results suggested that the depressed β-agonist responsiveness in intact airways observed with the AC5 transgenics could be due to decreased β-agonist-promoted cAMP production from decreased β2-AR expression and/or increased Gαi. A decrease in isoproterenol-stimulated cAMP in AC5-transgenic trachea was indeed found (Fig. 5A) and amounted to a ∼50% decrease compared with NTGs, which is what might be expected from a similar decrease in functional airway relaxation (Fig. 2B). In contrast to findings with β-agonist, forskolin stimulated cAMP was increased in the AC5 tracheas (Fig. 5B). Of the changes in signal transduction proteins that might lead to the observed paradoxical cellular (cAMP) and physiological (relaxation) consequences of AC5 overexpression, the increase in Gαi was intriguing, since β2-AR-Gi coupling has been demonstrated, although primarily in cell-based or reconstituted systems (3, 18). We considered that an increase in Gαi would be consistent with enhanced relaxation response to forskolin, but a diminished relaxation response to isoproterenol, since the β2-AR-mediated signaling would include enhanced coupling to Gαi, which would inhibit AC. To establish this interaction, mice were treated with PTX and studied 18 h later for tracheal cAMP production and relaxation. The results from agonist-stimulated cAMP accumulation from these tracheas compared with those under the same conditions without PTX are shown in Fig. 5A. PTX reversed the phenotype, with AC5-transgenic tracheas having enhanced cAMP accumulation compared with NTG. The effect of PTX on airway relaxation is shown in Fig. 6. The impairment of agonist-mediated relaxation of AC5 airways was no longer apparent after PTX, being virtually identical to NTG mice. In contrast, inhibition of ERK1/2 activation with the MEK inhibitor U0126 had no effect on the differential β-agonist-mediated relaxation observed between NTG and AC5-transgenic airways (Fig. 6).
Fig. 5.
Effect of AC5 overexpression on cAMP responsiveness. A: Iso-stimulated cAMP is depressed in AC5 transgenic mouse trachea compared with NTG. After mice were treated with PTX, cAMP accumulation was greater in the AC5 transgenic tracheas compared with NTG. Values are means ± SE. Results are from 5 NTG and 6 AC5 transgenic mice for the −PTX condition, and 4 NTG and 4 AC5 transgenic mice for the +PTX condition. *P < 0.01 vs. NTG under the same PTX condition. B: the cAMP response to forskolin (FSK) is greater in tracheas from AC5 transgenic mice (n = 4) compared with NTG mice (n = 4). Values are means ± SE. #P < 0.05 vs. NTG.
Fig. 6.
Effects of disruption of β2-AR-Gi coupling and ERK1/2 activation on the AC5-transgenic airway relaxation phenotype. Mice were treated by intraperitoneal injection of PTX to disrupt β2-AR-Gi interaction, or tracheal rings treated with the MEK inhibitor U0126 to inhibit ERK1/2 activation. PTX treatment normalized the AC5-transgenic relaxation response to that of NTG. The impaired relaxation of the AC5-transgenic airways compared with NTG was not affected by the MEK inhibitor. Values are means ± SE. Results are from 4 mice in each of the 4 groups.
DISCUSSION
In the present work, we investigated the relevance of the stoichiometric ratio of receptor (β2-AR) and its cognate effector AC in physiological signaling of ASM. The basis for these studies was to ascertain whether pharmacological or genetic-based therapies aimed at increasing AC5 in ASM might improve β-agonist-mediated relaxation, thereby providing a novel avenue for improved treatment of obstructive airway disease. Our laboratory had previously found that transgenic overexpression of β2-AR on ASM was minimally effective and required marked overexpression, resulting in augmentation of relaxation that was not in proportion to overexpression (11). This suggested that receptor expression was not the “bottleneck” in this signal transduction system in ASM. In analogy to findings in cardiomyocytes, where AC expression has been shown to limit β-AR function (5), we proceeded to generate transgenic mice with ASM-targeted overexpression of AC5. There were no overt deleterious effects of this overexpression in terms of life span, reproduction, or respiratory signs. In initial studies, we found that direct activation of AC by the diterpene forskolin resulted in increased cAMP production and airway relaxation in the AC5 transgenics compared with NTG, indicating functional overexpression of AC5 in ASM. Paradoxically, and counter to our original hypothesis, AC5 transgenics had decreased relaxation in response to β-agonists. This effect was not due to an artifact from altered contraction, as acetylcholine-mediated contraction was not affected in the transgenics. Furthermore, cAMP production by β-agonist was also decreased in the transgenics, indicating that, while the AC5 transgene was functional, the signaling of the β2-AR via AC5 at the cellular and physiological levels was depressed. This implied that events had occurred that acted to attenuate (actually, overcompensate) the potential effects of the increased AC5 component.
To investigate the mechanism of this attenuation, expression of multiple members of the signaling cascade, and those of associated proteins that may alter relaxation by β2-AR functional regulation or competitive antagonism, was examined. Increases in the GRKs or β-arr, for example, might result in enhanced short-term β2-AR desensitization, leading to a depressed isoproterenol-mediated relaxation. Similarly, a decrease in Gαs or an increase in PDE might be an expected consequence of attempts to enhance signaling in a situation in which there is plasticity that works toward a homeostatic set point. However, of these proteins, only PDE4 expression was altered, and it was actually decreased, which, if anything, would result in enhanced β2-AR function. On the other hand, Gαi expression was increased, and, in confirmation of this increase, ERK1/2 phosphorylation was enhanced. β2-AR have been documented to couple not only to Gs, but also to Gi, which has the potential to inhibit AC in an agonist-dependent manner (18, 20). Our results are consistent with this process, since β2-AR-mediated cAMP generation and relaxation are decreased in the transgenics, but nonreceptor-mediated (forskolin) cAMP and relaxation are enhanced. Furthermore, on exposure to PTX, which ADP-ribosylates Gi and ablates its coupling to β2-AR, the loss-of-function phenotypes of agonist-promoted cAMP accumulation and ASM relaxation were no longer apparent in the AC5 mice. This establishes that the increase in Gi is one component of the counterregulation.
ERK1/2 activation was increased in AC5 ASM, and this kinase is known to phosphorylate GRK2, but this event decreases GRK2 function (15) and would lead to enhanced β-agonist relaxation. ERK1/2 also phosphorylates β-arr1, but this leads to a loss of function (9) and again would be expected to enhance β2-AR function. Other potential effects of marked enhancement of activity of this ubiquitous mitogen-activated protein kinase in ASM relaxation are not readily predictable. Nevertheless, we showed that the increased Gi is responsible for the enhanced phosphorylation of ERK1/2 in AC5 ASM, since PTX treatment resulted in a loss of this phenotype. However, the enhanced pERK1/2 in the AC5 mice does not appear to contribute to the agonist-mediated ASM relaxation phenotype, as MEK (ERK) inhibition had no effect on the depressed response of the AC5 ASM.
We also noted a decrease in β2-AR expression in the AC5 transgenics, which is consistent with the fact that cAMP analogs evoke β2-AR downregulation (2). PTX treatment resulted in a normalization of the AC5-transgenic airway relaxation response to β-agonist, indicating that, when the β2-AR-Gi coupling component is ablated, the impaired β2-AR function is no longer present. These results suggest that enhanced β2-AR-coupled Gi inhibition of AC functions as a primary mechanism of the depressed β-agonist relaxation in the AC5-transgenic mice. Transgenic overexpression of Gαi on ASM would confirm the cause-and-effect relationship proposed here. And indeed, our laboratory has recently generated Gαi2 overexpressing transgenic mice and found decreased relaxation in response to β-agonist (10). So, the increase in Gαi found in the AC5 transgenics is one mechanism of the depressed β-agonist-mediated relaxation based on the results from the PTX experiments and the previous results from the Gαi2-overexpressing transgenics. Interestingly, PTX treatment in the AC5 transgenics resulted in relaxation that was equivalent to, but not greater than, that in NTG mice. One might expect that, with ablation of the Gi-inhibitory effect, overexpression of AC5 would lead to enhanced β2-AR-mediated relaxation over NTG, similar to what we noted with direct activation of AC with forskolin. The lack of an enhancement over the NTG response with PTX treatment is likely due to the ∼40% reduction in β2-AR expression on AC5-transgenic ASM compared with NTG.
Collectively, our results suggest that the degree of β2-AR signaling in ASM may have a homeostatic set point (or range), and, when perturbed outside of the boundaries, mechanisms are evoked to return signaling toward being inside the range. We find that perturbing the relationship between β2-AR and AC5 in ASM in an attempt to enhance β-agonist efficacy by increasing AC5 expression results in counterregulatory events that act to completely abrogate the effort. It is intriguing to note that there are multiple mechanisms within the proximal portion of the β2-AR pathway, whereby counterregulation might occur, such as a decrease in Gs or an increase in GRK expression. However, this particular perturbation (increased AC) appears to promote a specific set of events, increased Gi and decreased β2-AR, which not only depress β2-AR signaling, but overcompensate such that signaling is less than NTG. In contrast to cardiomyocyte β-AR signaling, which is enhanced by overexpressing AC, and gene therapy trials that are underway, we conclude that an analogous approach with ASM for improving bronchodilation would not be beneficial.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grants HL045967 and HL104119.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
ACKNOWLEDGMENTS
We thank Kathryn Mihlbachler and Demar Pitter for technical assistance and Esther Moses for manuscript preparation.
REFERENCES
- 1. Barnes PJ. Effect of beta-agonists on airway effector cells. In: Beta2-Agonists in Asthma Treatment, edited by Pauwels R, O'Byrne PM. New York: Dekker, 1997, p. 35–64 [Google Scholar]
- 2. Bouvier M, Collins S, O'Dowd BF, Campbell PT, Deblasi A, Kobilka BK, MacGregor C, Irons GP, Caron MG, Lefkowitz RJ. Two distinct pathways for cAMP-mediated down-regulation of the β2-adrenergic receptor. J Biol Chem 264: 16786–16792, 1989 [PubMed] [Google Scholar]
- 3. Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390: 88–91, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Deshpande DA, Wang WCH, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JSK, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK. Increased expression of adenylylcyclase type VI proportionately increases beta-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A 95: 1038–1043, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation 99: 1618–1622, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Green SA, Liggett SB. G-protein-coupled receptor signaling in the lung. In: The Genetics of Asthma, edited by Liggett SB, Meyers DA. New York: Dekker, 1996, p. 67–90 [Google Scholar]
- 8. Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of β2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol 13: 25–33, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ. Feedback regulation of beta-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem 274: 15971–15974, 1999 [DOI] [PubMed] [Google Scholar]
- 10. McGraw DW, Elwing JM, Fogel KM, Wang WCH, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest 117: 1391–1398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGraw DW, Liggett SB. Heterogeneity in βARK expression in the lung accounts for cell-specific desensitization of the β2-adrenergic receptor. J Biol Chem 272: 7338–7343, 1997 [DOI] [PubMed] [Google Scholar]
- 12. McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, Liggett SB. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest 116: 1400–1409, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paul RJ, de Lanerolle P. Regulation of smooth muscle contractility. In: The Genetics of Asthma, edited by Liggett SB, Meyers DA. New York: Dekker, 1996, p. 91–117 [Google Scholar]
- 14. Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene 20: 1532–1539, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem 274: 34531–34534, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Post SR, Hilal-Dandan R, Urasawa K, Brunton LL, Insel PA. Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J 11: 75–80, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tattersfield AE. Clinical studies of beta-agonists in adults. In: Beta2-Agonists in Asthma Treatment, edited by Pauwels R, O'Byrne PM. New York: Dekker, 1997, p. 283–317 [Google Scholar]
- 18. Tepe NM, Liggett SB. Functional receptor coupling to Gi is a mechanism of agonist-promoted desensitization of the β2-adrenergic receptor. J Recept Signal Transduct Res 20: 75–85, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Wang WC, Mihlbachler KA, Brunnett AC, Liggett SB. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway β2-adrenergic receptor physiologic signaling. Proc Natl Acad Sci U S A 106: 15007–15012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J Biol Chem 277: 31249–31256, 2002 [DOI] [PubMed] [Google Scholar]