Abstract
Supplemental O2 is commonly employed in patients with respiratory failure; however, hyperoxia is also a potential contributor to lung injury. In animal models, hyperoxia causes oxidative stress in the lungs, resulting in increased inflammation, edema, and permeability. We hypothesized that oxidative stress from prolonged hyperoxia leads to endoplasmic reticulum (ER) stress, resulting in activation of the unfolded protein response (UPR) and induction of CCAAT enhancer-binding protein homologous protein (CHOP), a transcription factor associated with cell death in the setting of persistent ER stress. To test this hypothesis, we exposed the mouse lung epithelial cell line MLE-12 to 95% O2 for 8–24 h and evaluated for evidence of UPR induction and CHOP induction. Hyperoxia caused increased CHOP expression without other evidence of UPR activation. Because CHOP expression is preceded by phosphorylation of the α-subunit of the eukaryotic initiation factor-2 (eIF2α), we evaluated the role of double-stranded RNA-activated protein kinase (PKR), a non-UPR-associated eIF2α kinase. Hyperoxia caused PKR phosphorylation, and RNA interference knockdown of PKR attenuated hyperoxia-induced CHOP expression. In vivo, hyperoxia induced PKR phosphorylation and CHOP expression in the lungs without other biochemical evidence for ER stress. Additionally, Ddit3−/− (CHOP-null) mice had increased lung edema and permeability, indicating a previously unknown protective role for CHOP after prolonged hyperoxia. We conclude that hyperoxia increases CHOP expression via an ER stress-independent, PKR-dependent pathway and that increased CHOP expression protects against hyperoxia-induced lung injury.
Keywords: CCAAT enhancer-binding protein homologous protein, acute respiratory distress syndrome, endoplasmic reticulum stress, epithelial cell, eukaryotic initiation factor-2α, activating transcription factor-4, double-stranded RNA-activated protein kinase
oxygen supplementation is routinely used in the management of patients with acute respiratory failure. However, prolonged exposure to hyperoxia has long been recognized as a potential contributor to acute lung injury (26). In animals, prolonged exposure to hyperoxia causes lung injury, characterized by cell death, increased lung permeability, edema, and inflammation (5, 29). Although the exact mechanisms by which hyperoxia causes lung injury are incompletely understood, generation of reactive oxygen species (ROS) and cellular apoptosis appear to play important roles (1).
One potential mechanism linking ROS generation and cell death is endoplasmic reticulum (ER) stress, resulting in activation of the associated unfolded protein response (UPR) (23). The ER functions to modify proteins for subsequent exposure to the extracellular environment. ER stress occurs when proteins become misfolded, as can happen with increased oxidative stress, or when the capacity of the ER is exceeded by new protein synthesis. With ER stress, the three ER sensor proteins protein kinase RNA-like ER kinase (PERK), activating transcription factor (ATF)-6, and inositol-requiring enzyme-1α (IRE1α) are activated, leading to the UPR (4). The UPR leads to general inhibition of new protein translation via phosphorylation of eukaryotic initiation factor (eIF)-2α, while upregulating expression of specific proteins such as the chaperone protein binding protein/glucose-regulated protein-78 (BiP/GRP78) to expand ER processing capacity. In addition, with unremitting ER stress, the UPR activates mitochondrial cell death pathways partly via CCAAT enhancer-binding protein (C/EBP) homologous protein (CHOP)-dependent mechanisms (20, 28, 30).
CHOP, also known as Gadd153, is the 19.2-kDa protein product of Ddit3 and is induced during ER stress following phosphorylation of eIF2α and upregulation of ATF4 (12, 13, 31). CHOP is a transcription factor associated with apoptosis, cell cycle arrest, and inhibition of other C/EBP proteins during ER stress (28, 42). In models of cellular injury associated with ER stress, such as diabetes and ischemic brain injury, mice lacking CHOP have reduced cellular death and associated organ dysfunction (28, 31). Recently, CHOP has also been associated with nonapoptotic responses in the lung and other organs, suggesting that CHOP has more diverse functions than originally appreciated. CHOP can participate in inflammatory responses by directly regulating expression of the neutrophil chemokine IL-8/CXCL8 and of caspase-4, a component of the inflammasome (9, 25, 38). Additionally, CHOP overexpression results in increased ROS generation and podocyte adhesion to type IV collagen, suggesting roles in oxidative stress and regulation of molecules involved in cell-matrix interaction (3). Thus, in addition to its well-recognized role in apoptosis, CHOP can also contribute to inflammation, ROS generation, and altered cellular interaction with extracellular matrix.
CHOP induction has been reported in the lungs of mice exposed to hyperoxia, with immunohistochemical and in situ hybridization studies localizing expression predominantly to the bronchiolar epithelium but also, to a lesser extent, throughout the lung parenchyma (27); however, the mechanism and functional consequences of hyperoxia-induced CHOP expression are unknown. We hypothesized that hyperoxia-induced lung injury results from persistent ER stress, causing increased CHOP expression and subsequent cell death. We found that hyperoxia increased CHOP expression in the lung, but contrary to our hypothesis, this increase was independent of ER stress. Furthermore, CHOP was found to confer protection, rather than increased susceptibility, to hyperoxia-induced lung injury (21). These findings suggest that CHOP has a previously unreported protective function in hyperoxia-induced lung injury that is independent of ER stress responses.
MATERIALS AND METHODS
Reagents.
The following reagents were used in these experiments: murine IgM ELISA (Bethyl Laboratories, Montgomery, TX); bicinchoninic acid protein assay (Pierce Biotechnologies, Rockville, IL); RNeasy kits for RNA isolation (Qiagen, Valencia, CA); and primer/probes for quantitative PCR assays for BiP, CHOP, and ATF4 (catalog nos. Mm00517691_m1, Mm00492097_m1, and Mm00515324_m1, respectively, Applied Biosystems, Carlsbad, CA). Antibodies to BiP (catalog no. 3177), CHOP (catalog no. 2895), β-actin (catalog no. 4970), phosphorylated tyrosine (catalog no. 9411), cleaved caspase-3 (catalog no. 9601), and total and phosphorylated eIF2α (catalog nos. 9722 and 3597) were purchased from Cell Signaling Technologies (Danvers, MA). Antibodies to total and phosphorylated double-stranded RNA-dependent protein kinase (PKR; catalog nos. sc1702 and sc101783) and PERK (catalog no. sc13073) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Primer/probe assay for hypoxanthine phosphoribosyltransferase 1 (HPRT1) was designed using RealTimeDesign software and purchased from Biosearch Technologies (Novato, CA). Primer sequences for PKR were designed using Primer3 software (34). Sequences for X-box binding protein-1 (XBP1) splice variants were previously published (11). Oligonucleotide sequences are provided in Supplemental Table S1 (see Supplemental Material for this article, available online at the Journal website).
Cell culture.
Murine alveolar epithelial (MLE-12) cells (39) were purchased from American Type Culture Collection and maintained in DMEM-F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 2% FBS, 1% insulin-transferrin-selenium, 10 nM HEPES, 10 nM β-estradiol, 2 mM l-glutathione, 1% penicillin-streptomycin, and 10 nM hydrocortisone in 5% CO2 at 37°C. Hyperoxia experiments were carried out using a sealed exposure chamber (Billups-Rothenberg, Del Mar, CA). Cells were subcultured on multiwell plates, grown to confluency, serum-deprived for 24 h, and placed inside the chamber. After the chamber was sealed, a 95% O2-5% CO2 gas mixture was flushed through the chamber at 2 lb./in.2 for ∼3–5 min. A mass spectrometer was used to verify gas composition in the inner chamber.
Primary type II alveolar epithelial cells were isolated from mice (see Supplemental Material), cultured on type IV collagen-coated 96-well plates until confluent, and exposed to hyperoxia as described above.
Mice.
The University of Washington Animal Care and Use Committee approved all experiments. Ddit3−/− mice, which do not express the protein CHOP and are on a C57BL/6 background, and wild-type (WT) C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and crossed to generate heterozygotic breeding pairs. All offspring were genotyped by PCR using protocols provided by Jackson Laboratories.
Ddit3−/− and Ddit3+/+ (CHOP-expressing, hereafter referred to as WT) female offspring were exposed to ≥95% hyperoxia for up to 72 h in an acrylic exposure chamber with a flow rate ≥5 l/min. O2 and CO2 fractions in the chamber were verified using a mass spectrometer. After hyperoxia exposure, mice were euthanized by isoflurane overdose. Left lungs were removed, weighed, and homogenized for protein or mRNA collection. Each right lung was lavaged with three 0.5-ml aliquots of PBS containing 0.6 mM EDTA for determination of cell counts, protein concentration, and IgM concentration.
In separate experiments, lungs from WT and Ddit3−/− mice exposed to 95% hyperoxia were removed and inflated to a pressure of 15 cmH2O with 4% paraformaldehyde over 24 h. Fixed lungs were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin.
In a second set of experiments, Ddit3−/− and WT mice were exposed to ∼80% O2 for up to 10 days. Mice were examined daily for predefined euthanasia criteria.
Western blot analysis.
Cell and lung homogenates were prepared by sonication in 4°C lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100, pH 7.5) containing a combination of protease and phosphatase inhibitors (Roche, Basel, Switzerland). Lysates were cleared by centrifugation at 10,000 g for 10 min at 4°C, and supernatants were collected. For all immunoblots, 20 μg of protein per well were separated by SDS-PAGE and transferred to polyvinyl pyrrolidone membrane. Membranes were probed overnight with antibodies to the following proteins at the specified dilutions: BiP, eIF2α, PKR, and cleaved caspase-3 at 1:1,000; phosphorylated eIF2α, phosphorylated PKR, and CHOP at 1:500; and β-actin at 1:5,000. For CHOP immunoblots, immunoglobulins were precleared from lung homogenates by overnight incubation at 4°C with protein A-agarose beads (GE Healthcare, Piscataway, NJ).
For evaluation of PERK phosphorylation, cell lysates were incubated with 2 μg of anti-PERK antibody and protein A-agarose beads overnight. Immunoprecipitates were collected and analyzed as described above with antibody to phosphorylated tyrosine (1:2,000 dilution). Membranes were stripped and reprobed with antibody to PERK (1:200 dilution) as loading control and to confirm correct molecular weight of the phosphorylated tyrosine signal.
RNA isolation and RT-PCR analysis.
Total RNA was collected from cells and from lung tissue and treated with DNase. After first-strand cDNA synthesis, quantitative RT-PCR was performed using hydrolysis (Taqman) assays for ATF4, BiP, CHOP, and HPRT1. Quantitative RT-PCR for PKR was performed using SYBR Green. HPRT1 was used as internal control for all reactions.
XBP1 splicing was analyzed as previously described (20). Briefly, PCR amplification was performed on cDNA templates using the primers that span the fragment of XBP1 excised by IRE1α. PCR products were separated on a 2.5% agarose gel in 1× Tris-acetate-EDTA buffer and observed by ethidium bromide staining.
RNA interference knockdown experiments.
PKR expression was knocked down in MLE-12 cells using ON-TARGETplus SMARTpool small interfering RNA (siRNA; Dharmacon, Lafayette, CO). siRNA targeted to cyclophilin B was used as a negative control. Both sets of siRNA were used at 25 nmol with Dharmafect 1 transfection reagent (Dharmacon) for 72 h in antibiotic-free medium.
Statistical analysis.
Data were analyzed by analysis of variance and Tukey's honestly significant difference test with use of Prism 4.0 software (Graphpad). All cell culture experiments were repeated three times. All data are presented as means ± SD. Log-rank test was used to compare survival curves during prolonged 80% O2 exposure.
RESULTS
CHOP is induced by hyperoxia in vitro.
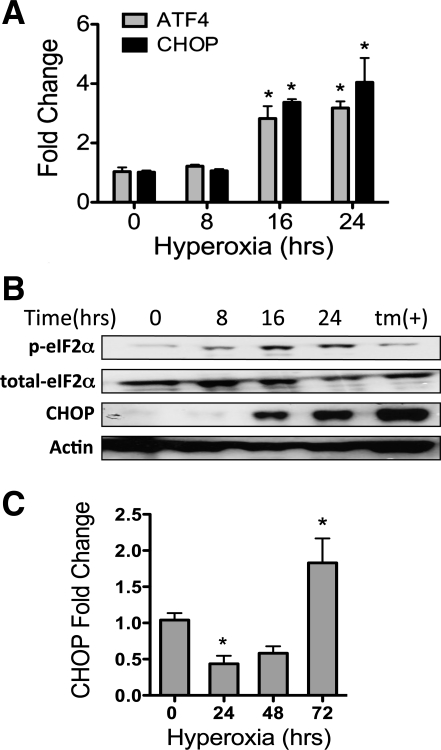
To determine whether hyperoxia induced CHOP expression, MLE-12 cells were exposed to 95% O2 for up to 24 h. CHOP mRNA was elevated nearly threefold by 16 h and fourfold by 24 h of hyperoxia compared with 0 h (Fig. 1A). ATF4, a transcription factor directly upstream of CHOP, showed a similar pattern of induction, with mRNA levels increased by 16 h and maintained through 24 h (Fig. 1A). At the protein level, CHOP was increased following 24 h of hyperoxia (Fig. 1B). Phosphorylation of eIF2α is required for upregulation of ATF4 and CHOP (14–16). Increased phosphorylation of eIF2α was observed after 8 h of hyperoxia and persisted through 24 h (Fig. 1B). To confirm these findings, primary murine type II alveolar epithelial cells were isolated and exposed to hyperoxia for up to 72 h. Isolated cells expressed the alveolar epithelial cell gene for surfactant protein C and did not express the fibroblast gene for fibroblast-specific protein-1/S100-A4 (see Fig. S1 in Supplemental Material). Interestingly, CHOP mRNA was initially lower after 24 h of hyperoxia but subsequently increased by approximately twofold after 72 h of hyperoxia in the primary alveolar epithelial cells (Fig. 1C).
Fig. 1.
Hyperoxia induces CCAAT enhancer-binding protein homologous protein (CHOP) in vitro. A: fold change in CHOP and activating transcription factor (ATF)-4 mRNA normalized to hypoxanthine ribosyltransferase (HPRT1) from murine alveolar epithelial (MLE-12) cells exposed to 95% O2-5% CO2 for up to 24 h. B: representative immunoblots of whole cell lysates for phosphorylated eukaryotic initiation factor (eIF)-2α (p-eIF2α), total eIF2α, CHOP, and actin from MLE-12 cells exposed to 95% O2-5% CO2 for up to 24 h. tm, Lysate from cells exposed to 5 μM tunicamycin for 24 h to induce ER stress. C: fold change in CHOP mRNA normalized to HPRT1 from primary mouse lung type II epithelial cells exposed to 95% O2-5% CO2 for up to 72 h. Quantitative PCR data summarize 3 separate experiments for each time. *P < 0.05 vs. 0 h.
The UPR is not activated by hyperoxia.
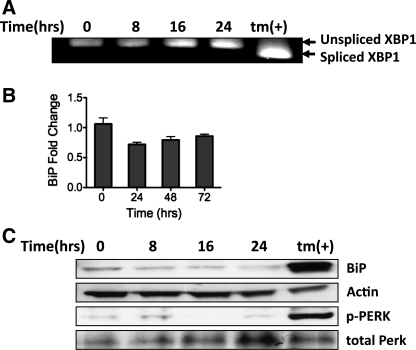
We hypothesized that CHOP induction during hyperoxic exposure was associated with ER stress. To identify the presence of ER stress, we evaluated several components of the UPR in MLE-12 cells exposed to hyperoxia. As part of the UPR, IRE1α alternatively splices XBP1 mRNA (4, 41). Whereas treatment with tunicamycin, an inducer of ER stress, caused alternative splicing of XBP1, XBP1 splicing was conspicuously absent after hyperoxic exposure (Fig. 2A). During ER stress, BiP, an ER chaperone protein, is upregulated via ATF6- and XBP1-dependent mechanisms. However, BiP expression was unchanged at mRNA and protein levels in response to hyperoxia (Fig. 2, B and C). The third branch of UPR activation involves the phosphorylation of PERK, which is directly upstream of eIF2α, ATF4, and CHOP. In correlation with the other data, no increase in phosphorylated PERK was observed with hyperoxia (Fig. 2C). Thus, contrary to our hypothesis, we found no evidence of ER stress or the UPR in MLE-12 cells exposed to hyperoxia, suggesting that eIF2α phosphorylation and downstream CHOP induction occurred via an alternative mechanism.
Fig. 2.
Hyperoxia does not cause general activation of the unfolded protein response (UPR). A: representative agarose gel electrophoresis of X-box binding protein (XBP1) PCR product demonstrating different-sized cDNA corresponding to spliced and unspliced XBP1 mRNA. mRNA collected from MLE-12 cells was exposed to 95% O2-5% CO2 for up to 24 h or 5 μM tunicamycin (tm) for 6 h. B: fold change in binding protein (BiP) mRNA normalized to HPRT1 from MLE-12 cells exposed to 95% O2-5% CO2 for up to 24 h. Data summarize 3 separate experiments for each time. C: representative immunoblots of whole cell lysates for BiP, phosphorylated protein kinase RNA-like endoplasmic reticulum kinase (p-PERK), total PERK, and actin from MLE-12 cells exposed to 95% O2-5% CO2 for up to 24 h or 5 μM tunicamycin for 24 h.
Hyperoxia induces CHOP expression via PKR activation.
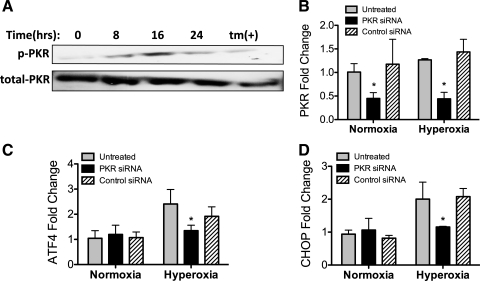
Because our data suggested that CHOP was not induced via the UPR, we considered other pathways that can lead to CHOP induction. PKR is an eIF2α kinase that is activated following binding to double-stranded RNA, an intermediate product of viral replication, but can also be activated by binding to the PKR-activating protein (PACT)/retinal homeobox protein Rx (RAX) (8, 32). Because H2O2 induces PACT phosphorylation and PKR activation (15), we speculated that oxidative stress during hyperoxia may also activate PKR.
We found that PKR was phosphorylated after 16 h of hyperoxia (Fig. 3A). To determine whether CHOP induction resulted from PKR activation, PKR expression was knocked down in MLE-12 cells. MLE-12 cells were treated with siRNA targeting PKR or cyclophilin B (negative control) for 72 h prior to collection (48 h prior to hyperoxia). PKR was significantly reduced by specific siRNA treatment (Fig. 3B; see Supplemental Fig. S2). PKR knockdown attenuated hyperoxia-induced ATF4 expression (Fig. 3C) and CHOP expression (Fig. 3D). Thus hyperoxia induced CHOP via a PKR-dependent, UPR-independent pathway in MLE-12 cells.
Fig. 3.
Double-stranded RNA-dependent protein kinase (PKR) is activated by hyperoxia and induces downstream CHOP expression. A: representative immunoblots of whole cell lysates for phosphorylated PKR (p-PKR) and total PKR from MLE-12 cells exposed to 95% O2-5% CO2 for up to 24 h or 5 μM tunicamycin for 24 h. B–D: PKR, ATF4, and CHOP mRNA expression normalized to HPRT1 in MLE-12 cells exposed to transfection reagent alone (untreated), PKR small interfering RNA (siRNA), or control (cyclophilin B) siRNA (25 nM for 72 h). Cells were exposed to normoxia (21% O2) or hyperoxia (95% O2) for the final 24 h. Data summarize 3 independent experiments. *P < 0.05.
Hyperoxia induces CHOP independent of ER stress in vivo.
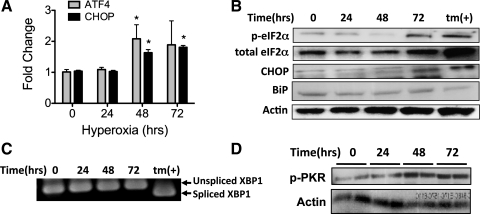
To confirm that hyperoxia induces CHOP expression in vivo, mice were exposed to 95% O2 for up to 72 h. CHOP and ATF4 mRNA levels significantly increased by 1.5- to 2-fold after 48 h of hyperoxia (Fig. 4A). Increased levels of phosphorylated eIF2α and CHOP protein were observed at 72 h of hyperoxia (Fig. 4B).
Fig. 4.
Hyperoxia increases CHOP expression and PKR phosphorylation but does not cause general activation of endoplasmic reticulum (ER) stress responses. A: ATF4 and CHOP mRNA normalized to HPRT1 from lungs of mice exposed to 95% O2 for up to 72 h (n = 5/group). *P < 0.05. B: representative immunoblots of phosphorylated and total eIF2α, CHOP, BiP, and actin from whole lung lysates of mice exposed to 95% O2 for up to 72 h. tm, MLE-12 cells treated with 5 μM tunicamycin for 24 h. C: XBP1 PCR product demonstrating different-sized cDNA corresponding to spliced and unspliced XBP1 mRNA. mRNA collected from lungs of mice was exposed to 95% O2 for up to 72 h. tm, MLE-12 cells treated with 5 μM tunicamycin for 6 h. D: immunoblots of phosphorylated PKR and actin from lung lysates of mice exposed to 95% O2 for up to 72 h.
To determine whether the UPR is activated with hyperoxia in vivo, BiP expression and XBP1 alternative slicing were measured in the lungs of mice exposed to 95% O2 for up to 72 h. Consistent with the cell culture results, BiP induction (Fig. 4B) and XBP1 alternative splicing (Fig. 4C) were absent in the lungs of mice exposed to hyperoxia. However, 48 h of hyperoxia did cause PKR phosphorylation in the lung (Fig. 4D). Together, these data suggest that hyperoxia increases CHOP expression via a UPR-independent pathway, likely involving PKR phosphorylation of eIF2α.
CHOP-deficient mice have increased lung injury with hyperoxia.
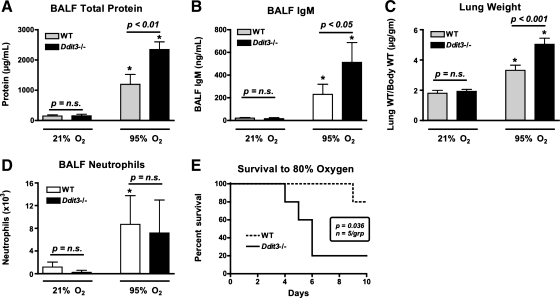
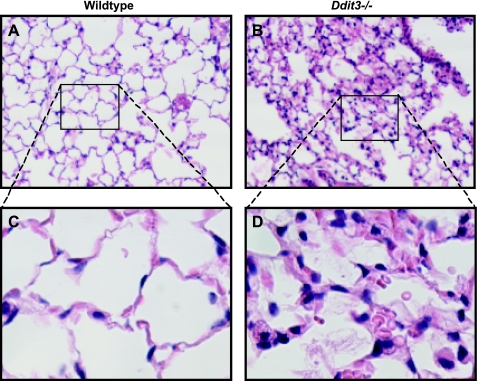
To determine if CHOP is involved in the pathogenesis of hyperoxia-induced lung injury, Ddit3−/− mice were compared with WT mice after 72 h of hyperoxia (≥95% O2). Exposure to hyperoxia resulted in elevated bronchoalveolar lavage fluid (BALF) total protein concentrations and increased lung weights (Fig. 5, A and C). Hyperoxia also caused a significant increase in BALF IgM concentration (Fig. 5B). Elevated BALF IgM, which normally exists as a pentameric multimer with a molecular weight of ∼900,000, is a marker of lung permeability to very large proteins, indicating disruption of the alveolar-capillary barrier. Unexpectedly, total protein and IgM concentrations in BALF were higher and normalized lung weights were greater in Ddit3−/− than WT mice. Hyperoxia also resulted in a modest increase in BALF polymorphonuclear cell count; however, there was no difference between WT and Ddit3−/− mice (Fig. 5D). Consistent with the quantitative findings of increased permeability and lung weights, histological sections from the lungs of Ddit3−/− mice demonstrated thickened alveolar septae and increased intra-alveolar exudate compared with WT mice following exposure to hyperoxia (Fig. 6). Because apoptosis may play a role in acute lung injury-associated barrier dysfunction and because CHOP is associated with initiation of apoptosis in the setting of ER stress, we evaluated for activation of caspase-3. Immunoblot revealed no differences in levels of activated caspase-3 between WT and Ddit3−/− mice exposed to hyperoxia for 72 h (see Supplemental Fig. S3).
Fig. 5.
Mice lacking CHOP have increased lung permeability in response to hyperoxia. A–D: bronchoalveolar lavage fluid (BALF) total protein concentration, BALF IgM concentration, lung weight normalized to starting body weight, and BALF polymorphonuclear (neutrophil) cell count in Ddit3−/− and wild-type (WT) mice exposed to 72 h of normoxia (21% O2) or hyperoxia (95% O2). E: survival of Ddit3−/− and WT mice exposed to 80% O2 for up to 10 days. *P < 0.05 vs. 21% O2.
Fig. 6.
Mice lacking CHOP have greater alveolar septal thickening and intra-alveolar exudate than WT mice following hyperoxia exposure. A–D: hematoxylin-eosin-stained sections of lungs from WT and Ddit3−/− mice following 76 h of exposure to 95% O2. Original magnification: ×100 (A and B) and ×400 (C and D).
We attempted to evaluate the role of CHOP over longer periods of hyperoxia exposure. However, four of five Ddit3−/− mice did not survive exposure to 95% O2 for 90 h. In contrast, all but one of the WT mice survived. To determine whether CHOP is important for survival at lower levels of hyperoxia, mice were exposed to 80% O2 for up to 10 days. By day 10, only 20% of Ddit3−/− mice survived, in contrast to 80% of WT mice (P = 0.036; Fig. 5E).
DISCUSSION
In this study, we examined the role of ER stress and CHOP induction in hyperoxia-induced lung injury. The important findings of this study are as follows: 1) hyperoxia induced CHOP expression in cultured lung epithelial cells and intact mouse lungs in vivo; 2) CHOP induction in response to hyperoxia was independent of other biochemical evidence of ER stress; 3) PKR was required for maximal CHOP expression with hyperoxia; and 4) mice lacking CHOP had increased lung permeability and decreased survival in response to hyperoxia.
CHOP is a transcription factor previously implicated in a variety of cellular stress responses. CHOP is induced by ATF4, which in turn is expressed following phosphorylation of eIF2α. eIF2 is essential for initiation of protein translation, and phosphorylation of its α-subunit inhibits new protein synthesis (33). CHOP expression is classically described as part of the ER stress response, where it results from eIF2α phosphorylation by PERK (28). However, our data show that, in the setting of hyperoxia, CHOP expression is primarily regulated by PKR-mediated eIF2α phosphorylation in the absence of other evidence of ER stress as measured by activation of the UPR.
We also found that CHOP induction during hyperoxia was protective against disruption of the alveolar-capillary barrier in the lung and that, consequently, the primary abnormality in Ddit3−/− mice during hyperoxia was increased permeability independent of significant differences in polymorphonuclear cell recruitment or caspase-3 activation. In addition to increased lung permeability, Ddit3−/− mice had increased mortality compared with WT mice at two different levels of hyperoxia. This protective effect of CHOP contrasts with CHOP induction during ER stress, which is typically associated with cellular death and associated organ dysfunction (40). Recently, multiple studies have shown that CHOP has diverse functional effects beyond apoptosis (3, 9, 25, 38), suggesting that the specific function of CHOP is contextually related. For example, ER stress-induced apoptosis requires not only CHOP (28) but also the endoribonuclease activity of IRE1α, an independent component of the UPR (11). In contrast to its function in the setting of ER stress, CHOP can function in proinflammatory responses through direct regulation of IL-8/CXCL8 and caspase-4, a component of the inflammasome (2, 3, 9, 38), and through indirect regulation of monocyte chemoattractant protein 1 (MCP-1/CCL2) (24). Additionally, PKR phosphorylation is required for maximal cytokine response to Toll-like receptor (TLR)2/4 activation in macrophages (6), raising the following question: Does CHOP play an unrecognized proinflammatory role during microbial infection via a PKR-dependent mechanism? Thus modulation of Toll-like receptor signaling pathways represents another way in which CHOP potentially modulates the lung response to bacterial infection and tissue injury, resulting in the release of endogenous ligands. The specific mechanism(s) by which PKR-regulated CHOP induction preserves alveolar-capillary permeability are unknown but may represent a potential novel pathway that can be manipulated to attenuate lung edema severity during acute respiratory distress syndrome.
Our results complement a recently published study by Gewandter et al. (10), who report minimal activation of a BiP reporter construct and no evidence of activation of the three ER sensor proteins, PERK, ATF6, and IRE1α, in two different human lung epithelial cell lines exposed to hyperoxia. Importantly, they also report that hyperoxia increases cell death in response to concurrent exposure to tunicamycin. A mechanism for this augmented cell death was not identified; however, the authors showed that overexpression of the ER chaperone protein BiP did not prevent the augmented cell death associated with hyperoxia. Our data suggest that hyperoxia-induced, PKR-mediated CHOP induction may be the mechanism by which hyperoxia augments cell death during ER stress. This is supported by the recent report that PKR activation by its endogenous activator PACT is required for maximal CHOP induction and cell death following tunicamycin-induced ER stress (36). We speculate that PKR activation and CHOP induction during O2 therapy or in response to viral activation may promote further lung injury in diseases associated with epithelial ER stress, such as idiopathic pulmonary fibrosis (18, 19) and smoking-related lung disease (14, 16, 17, 22). Further studies evaluating the role of PKR-induced CHOP expression in ER stress-associated cellular injury are needed to evaluate this possibility.
Whereas our data identify a PKR-dependent pathway, other eIF2α kinases could also potentially contribute to hyperoxia-induced CHOP induction. Besides PERK and PKR, heme-regulated inhibitor kinase (HRI) and general control nondepressible protein 2 (GCN2) are eIF2α kinases expressed in mammals (35). HRI is primarily expressed in reticulocytes and is activated upon heme or iron deficiency (7), diminishing the likelihood that it plays an important role in our model. GCN2, which is classically activated upon amino acid deprivation, also cannot be excluded as a possible contributor in hyperoxia-induced eIF2α phosphorylation and CHOP induction (12).
Our data show that hyperoxia increases CHOP expression in cultured alveolar epithelial cells and in the lungs of mice. However, it is unknown whether CHOP expression in alveolar epithelial cells in vivo is the only or even primary mechanism by which CHOP limits lung permeability changes in the lungs of mice. In the setting of lung injury, increased permeability typically occurs in response to disruption of the alveolar-capillary barrier, and it is possible that CHOP induction in pulmonary endothelial cells is important. Data of oxidative stress-induced CHOP expression in pulmonary endothelial cells are not available; however, oxidative stress associated with exposure to oxidized low-density lipoproteins does induce CHOP in cultured human coronary artery endothelial cells (37). It is also possible that induction of CHOP in a cell type other than alveolar epithelium or endothelium may play an important role in attenuation of lung permeability with hyperoxia. CHOP mRNA is expressed in bronchiolar epithelium and, less prominently, throughout the lung parenchyma in response to hyperoxia (27).
In summary, our data show that hyperoxia induces expression of CHOP in the lungs, in vivo, and in lung epithelial cells, in vitro, by a mechanism independent of the UPR. Furthermore, we identify PKR phosphorylation as a key step required for expression of CHOP during hyperoxia. Surprisingly, we found that CHOP is protective in a murine model of hyperoxic lung injury. This protective effect of CHOP is in contrast to its reported role in models of ER stress-associated and inflammatory cellular injury. Specifically, deletion of CHOP resulted in increased disruption of the alveolar epithelial permeability barrier and increased mortality. This difference in permeability was not associated with differences in neutrophil recruitment. We conclude that PKR-dependent induction of CHOP is protective in lungs exposed to hyperoxic conditions by a novel mechanism independent of modulation of neutrophilic inflammation. Whether the protection associated with CHOP induction is secondary to modulation of cell death pathways, alteration in inflammatory responses, or some other mechanism remains to be determined.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-086883.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dowon An for assistance with the in vivo mouse studies and Gil Sucheol for assistance with isolation of mouse primary alveolar epithelial cells.
REFERENCES
- 1. Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73–78, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Beck KC, Vettermann J, Rehder K. Gas exchange in dogs in the prone and supine positions. J Appl Physiol 72: 2292–2297, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Bek MF, Bayer M, Muller B, Greiber S, Lang D, Schwab A, August C, Springer E, Rohrbach R, Huber TB, Benzing T, Pavenstadt H. Expression and function of C/EBP homology protein (GADD153) in podocytes. Am J Pathol 168: 20–32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22: 487–508, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bhandari V, Elias JA. The role of angiopoietin 2 in hyperoxia-induced acute lung injury. Cell Cycle 6: 1049–1052, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cabanski M, Steinmuller M, Marsh LM, Surdziel E, Seeger W, Lohmeyer J. PKR regulates TLR2/TLR4-dependent signaling in murine alveolar macrophages. Am J Respir Cell Mol Biol 38: 26–31, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2α kinase: relevance to anemias. Blood 109: 2693–2699, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Acquisto F, Ghosh S. PACT and PKR: turning on NF-κB in the absence of virus. Sci STKE 2001: RE1, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol 176: 6245–6253, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Gewandter JS, Staversky RJ, O'Reilly MA. Hyperoxia augments ER-stress-induced cell death independent of BiP loss. Free Radic Biol Med 47: 1742–1752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138: 562–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hengstermann A, Muller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic Biol Med 44: 1097–1107, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem 274: 15427–15432, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 8: 229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol 38: 541–550, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294: L1119–L1126, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science 318: 944–949, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lozon TI, Altemeier WA. Chop deficient mice have augmented lung injury with hyperoxia (Abstract). Am J Respir Crit Care Med 179: A4168, 2009 [Google Scholar]
- 22. Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med 180: 1196–1207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Morse E, Schroth J, You YH, Pizzo DP, Okada S, Ramachandrarao S, Vallon V, Sharma K, Cunard R. TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP-1. Am J Physiol Renal Physiol 299: F965–F972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Namba T, Tanaka K, Ito Y, Ishihara T, Hoshino T, Gotoh T, Endo M, Sato K, Mizushima T. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response, in the development of colitis. Am J Pathol 174: 1786–1798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nash G, Blennerhassett J, Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artificial ventilation. Laval Med 39: 59–64, 1968 [PubMed] [Google Scholar]
- 27. O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC. p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 278: L552–L559, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann NY Acad Sci 1010: 405–416, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14: 996–1007, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Raven JF, Baltzis D, Wang S, Mounir Z, Papadakis AI, Gao HQ, Koromilas AE. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2α phosphorylation. J Biol Chem 283: 3097–3108, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Raven JF, Koromilas AE. PERK and PKR: old kinases learn new tricks. Cell Cycle 7: 1146–1150, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Sadler AJ. Orchestration of the activation of protein kinase R by the RNA-binding motif. J Interferon Cytokine Res 30: 195–204 [DOI] [PubMed] [Google Scholar]
- 36. Singh M, Fowlkes V, Handy I, Patel CV, Patel RC. Essential role of PACT-mediated PKR activation in tunicamycin-induced apoptosis. J Mol Biol 385: 457–468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thum T, Borlak J. LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium. J Biol Chem 283: 19456–19464, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Vij N, Amoako MO, Mazur S, Zeitlin PL. CHOP transcription factor mediates IL-8 signaling in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 38: 176–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 90: 11029–11033, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.