Abstract
The serotonin transporter (SERT) and the platelet-derived growth factor receptor (PDGFR) have been implicated in both clinical and experimental pulmonary hypertension (PH) and the facilitation of pulmonary artery smooth muscle cell (PASMC) growth. To gain a better understanding of the possible relationship of these two cell surface molecules we have explored interactions between SERT and PDGFR. We have previously demonstrated that SERT transactivates PDGFRβ in serotonin-stimulated PASMC proliferation. We now provide evidence for a role for SERT in PDGF-BB signaling and PASMC proliferation by using pharmacological inhibitors, genetic ablation, and construct overexpression of SERT. The results show that four tested SERT blockers dose dependently inhibit PDGF-stimulated human and bovine PASMC proliferation with comparable efficacy to that of PDGFR inhibitors, whereas 5-HT1B or 5-HT2A receptor inhibitors had no effect. Combinations of the SERT and PDGFR inhibitors led to synergistic/additive inhibition. Similarly, PDGF-induced PASMC proliferation was attenuated by small interfering RNA downregulation of SERT. Inhibition of SERT in PASMCs attenuated PDGF-induced phosphorylation of PDGFRβ, Akt, and p38 but not Erk. Overexpression of SERT in HEK293 cells led to enhanced Akt phosphorylation by PDGF, which was blunted by a SERT PDZ motif mutant, indicating the mechanistic need for the PDZ motif of SERT in PDGF signaling. Furthermore, coimmunoprecipitation experiments showed that SERT and PDGFRβ become physically associated upon PDGF stimulation. In total, the data show for the first time an important interactive relationship between SERT and the PDGFRβ in the production of PASMC proliferation triggered by PDGF that may be important in PH.
Keywords: cell proliferation, cell signaling interaction, pulmonary hypertension
increased proliferation of pulmonary artery smooth muscle cells (PASMCs) is a hallmark pathological feature of pulmonary arterial hypertension (PAH), a progressively lethal lung disease (21, 44). The serotonin transporter (SERT, 5-HTT), a member of the Na+/Cl−-dependent solute transporter family (SLC6A4) (3), has been shown to play a central role in the pathogenesis of PASMC proliferation in both experimental and human PAH (7). Increased SERT expression and activity associated with a greater mitogenic response to serotonin (5-hydroxytryptamine, 5-HT) are found in smooth muscle cells (SMCs) from remodeled pulmonary arteries in animal models and patients with PAH compared with those from controls (8, 10, 38). Pharmacological inhibitors of SERT abolish 5-HT-induced PASMC proliferation (8, 10, 38) and prevent and/or reverse experimental pulmonary hypertension (PH) (15, 37, 56). SERT-deficient mice develop less hypoxic PH whereas SERT-overexpressing mice show enhanced hypoxia- or monocrotaline-induced pulmonary vascular remodeling, right ventricular pressure elevation, and right ventricular hypertrophy (9, 14, 36). However, the molecular mechanisms by which SERT regulates PASMC proliferation are incompletely understood.
Our earlier work showed that SERT is responsible for 5-HT-promoted PASMC proliferation through active transport of 5-HT intracellularly accompanied by tyrosine phosphorylation of GTPase-activating protein, the formation of reactive oxygen species, and activation of Erk MAPK (25–27). Our subsequent work suggested that SERT cooperates with 5-HT receptors (5-HTRs) in the production of 5-HT-induced PASMC proliferation via the small G protein Rho, Erk, and PI3K/Akt (33, 35). A study by Guignabert et al. (14) using SM22-SERT mice (transgenic mice with SERT overexpression occurring selectively in SMCs) indicates that vascular SERT protein overexpression per se is sufficient to induce PASMC hyperplasia and subsequent development of PH despite the absence of associated changes in 5-HT bioavailability and of other stimuli like hypoxia, raising the challenging possibility that, in addition to its role in 5-HT signaling, SERT may also participate in coregulation by other mitogens of PASMC proliferation. We found earlier that SERT participates in a synergistic growth-stimulatory effect of 5-HT with other growth factors including platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF) in bovine PASMCs (BPASMCs) (28). Later, Eddahibi and colleagues (8, 10) also showed a comitogenic effect of 5-HT with PDGF requiring 5-HT internalization by the high-affinity SERT in both rat and human PASMCs (RPASMCs and HPASMCs, respectively). The underlying mechanisms that create this association between SERT and the PDGF receptor remain unexplored.
PDGFs have been studied extensively as prototypes for cellular growth factors. This family of growth factors consists of four gene products forming five homo- or heterodimeric isoforms: PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD, which bind to and activate three dimeric combinations of two structurally similar receptor tyrosine kinases (RTKs), PDGF receptors α and β (PDGFRα and PDGFRβ). Upon binding a dimeric PDGF molecule, PDGFR undergoes dimerization and autophosphorylation of tyrosine residues, which in turn triggers multiple intracellular signaling pathways that relay the receptor's signal to appropriate cellular targets. Phosphorylation of the conserved tyrosine residue in the kinase domain (Tyr-849 of PDGFRα and Tyr-857 of PDGFRβ) increases catalytic activity of the kinases, whereas autophosphorylation of tyrosine residues outside the kinase domain creates docking sites for downstream signal transduction proteins containing phosphotyrosine recognition modules such as Src homology 2 (SH2), SH3, phosphotyrosine binding, pleckstrin homology, and postsynaptic density-95/discs large/zona occludens-1 (PDZ) domains. Signaling is modulated both positively and negatively extracellularly through interaction with matrix molecules and intracellularly through cross talk with different signaling pathways (see reviews in Refs. 1, 17, 41).
PDGFB/PDGFRβ signaling plays crucial roles in the developing vasculature and vascular disease. The PDGFRβ has a unique signaling capacity for vascular smooth muscle cells (VSMCs) that the PDGFRα does not possess as indicated by multiple abnormalities occurring in VSMC development and function when the PDGFRα cytoplasmic domain is placed in the context of the PDGFRβ (24, 48). Mice deficient for PDGFB or PDGFRβ die perinatally and exhibit cardiovascular, renal, and hematological defects (30, 47), which attribute to failed recruitment of specialized VSMCs to developing capillaries in the kidney (mesangial cells) (47) and in the brain (pericytes) (32). Overactivities of PDGFB/PDGFRβ signaling are strongly implicated in the pathogenesis of PAH in patients and animal models by initiating and maintaining underlying pulmonary vascular remodeling (2, 22, 42). Inhibition of PDGFR signaling by the tyrosine kinase inhibitor imatinib mesylate has been reported to reverse PAH in animal models and to improve the clinical course of selected patients with severe PAH, and a phase III study of imatinib is in progress (6, 13, 40, 45, 49). The possible interaction of PDGFB/PDGFRβ signaling and 5-HT signaling in PAH is currently unknown. We recently demonstrated that 5-HT produces tyrosine phosphorylation of the PDGFRβ through SERT and in initial experiments showed binding of SERT to PDGFRβ upon stimulation with 5-HT (34). More recently, other growth factors like FGF have also been shown to enhance tyrosine phosphorylation of the PDGFRα (29). Recent advances indicate that PDGFRβ utilizes G protein-coupled receptor (GPCR) signaling molecules such as sphingosine-1-phosphate receptor EDG-1 (18) and G protein Gα13 (46) to transduce signals and that PDGF can transactivate GPCRs, which represent a novel mechanism for cross-communication between RTKs and GPCRs, producing an integration of stimuli that a cell receives under varying physiological conditions.
From the aforementioned evidence, we hypothesize that interactions may exist for SERT with PDGFR in PDGF signaling and the production of PASMC proliferation in PAH. In the present experiments we show for the first time that genetic or pharmacological targeting of SERT attenuates PDGF-BB signaling and proliferation in PASMCs and that PDGFRβ binds SERT upon its activation by PDGF-BB, suggestive of cross talk between SERT and PDGFRβ in the production of PASMC proliferation triggered by PDGF-BB that may be important in PAH.
EXPERIMENTAL PROCEDURES
Animal use.
SERT−/− rats [SERT knockout (KO), generated by Dr. Edwin Cuppen and colleagues using N-ethyl-N-nitrosourea-driven target-selected mutagenesis (19)] and the cognate wild type (WT) obtained from GenOway were used as a source of pulmonary artery cells. All animal protocols and procedures were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals.
Cell line and primary cell culture.
Primary BPASMCs and RPASMCs were isolated by a modification of the method of Ross, as previously described (28). BPASMCs were cultured in RPMI 1640 medium, containing 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 1% penicillin, and 0.5% streptomycin, and used from passages 3–5 in our study. Primary HPASMCs (Clonetics, Walkersville, MD) were cultured in the same medium and used from passages 5–9. RPASMCs (used from passages 3–5) and HEK293 cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM containing 10% FBS, 1% penicillin, and 0.5% streptomycin. Cells were maintained in a humidified 37°C incubator with 5% CO2.
[3H]thymidine incorporation.
A [3H]thymidine incorporation assay was performed to assess DNA synthesis. PASMCs seeded in 96-well plates were growth-arrested for 48 h in medium containing 0.1% FBS (for BPASMCs) or 0.2% FBS (for HPASMCs). Cells were pretreated with indicated inhibitors individually or in varying combinations for 30–60 min depending on inhibitors, followed by incubation with or without 10 ng/ml PDGF-BB (in the following text, figures, and figure legends, PDGF used in our experiments is referred to PDGF-BB) in the same medium and then labeled with 20 μCi/ml [methyl-3H]thymidine (New England Nuclear, Boston, MA) for a total of 24 h. After labeling, experiments were terminated by aspiration of medium, and the cells were harvested onto unifilter-96-well microplates (Perkin Elmer Life Sciences, Boston, MA) by use of a Tomtec harvester (Tomtec, Hamden, CT). Radioactivity was counted in a liquid microplate scintillation counter (Top CountTM from PACKARD Instrument).
siRNA transfection.
Human PASMCs were plated the day before transfection to reach 60–80% confluence the next day in medium containing 10% serum without antibiotics. The small interfering RNA (siRNA) transfections were performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in Opti-MEM according to the manufacturer's protocol. Transfections of SERT siRNA, Akt siRNA, or nontargeting siRNA were carried out in 96-well plates or 6-well plates with a final siRNA concentration of 60 nM. For cell proliferation experiments, cells were starved with 0.2% FBS medium for 24 h after overnight transfection, then treated with 20 ng/ml PDGF-BB for 24 h, and examined by the [3H]thymidine incorporation assay noted above. Gene silencing was monitored at the protein level by Western blotting of cell lysates collected 48 h following transfection.
Plasmid DNA transfection.
HEK293 cells were transiently transfected with pcDNA3.1(−) (Invitrogen) or pEYFP/SERT or pEYFP/SERT-NAE (a mutant of SERT PDZ-binding motif with COOH-terminal valine mutated into glutamate) or pLXSN/PDGFRβ constructs (kindly provided by Drs. Joël Bockaert, Philippe Marin, Michael Freissmuth, Andrius Kazlauskas, and Vijaya Ramesh; see acknowledgments) by using Lipofectamine 2000 according to the manufacturer's instructions. Experiments were carried out 48 h after transfection.
Western blotting.
The indicated treatments of cells were carried out at 37°C in serum-starved medium, as described in the figure legends. Following the treatment, the cells were rinsed with ice-cold PBS and then were lysed with solubilization buffer A (20 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100, 50 mM NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate, 1 mM EDTA, 1 mM EGTA, protease inhibitor cocktail, and phosphatase inhibitor cocktail), or buffer B (2 × Laemmli sample buffer) or buffer C (50 mmol/l Tris·HCl, pH 7.5, 150 mmol/l NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 50 mM NaF, 1 mM EDTA, protease inhibitor cocktail, and phosphatase inhibitor cocktail). Phosphorylation and total protein expression of Akt, p38, Erk, and PDGFRβ were analyzed by using phospho-site and total protein antibodies. Immunoreactive bands were bonded with horseradish peroxidase-conjugated secondary antibodies and subsequently visualized by use of an ECL Chemiluminescent Western Blotting Detection kit (Pierce, Rockford, IL). Quantification of bands was done by gel densitometry with UN-SCAN-IT gel automatic digitizing system software (Silk Scientific, Orem, UT), and protein phosphorylation was normalized for total protein band densitometry.
Coimmunoprecipitation.
Total cell lysates (700–1,000 μg protein) were prepared from HPASMCs solubilized in buffer A noted above. The extracts were clarified by centrifugation and the protein concentrations were determined by Bradford assay. Equal amounts of cell lysates were incubated with 1 μg of SERT antibody on a rotator at 4°C overnight, then 20 μl of Protein G Plus Agarose (Thermo Scientific Pierce, Rockford, IL) were added, and the incubation continued for another 4 h. The immunoprecipitates were washed three times in the solubilization buffer, heated in 40 μl of 2× Laemmli sample buffer, separated with use of 7.5% SDS-PAGE gels, transferred to polyvinylidene fluoride membrane (Millipore, Billerica, MA), and probed with PDGFRβ and SERT antibodies. The immune complexes were detected by the ECL-Plus chemiluminescent system (Amersham Pharmacia Biotech, Piscataway, NJ). In the reverse experiment, SERT was detected with SERT antibody in the precipitate when PDGFRβ was precipitated with 15 μl of PDGFRβ antibody agarose conjugate.
Antibodies and reagents.
5-HT, imipramine, paroxetine, fluoxetine, citalopram, GR55562 (Tocris), ketanserin, and smooth muscle-specific α-actin antibody were purchased from Sigma Chemical (St. Louis, MO). Imatinib was from Novartis Pharmaceutical (Basel, Switzerland). AG1296 and SB203580 were from Calbiochem (La Jolla, CA). LY294002 and phospho-specific p42/44 Erk (Thr202/Tyr204), Erk, phospho-Akt (Ser473 and Thr308), Akt, phospho-p38 (Thr180/Tyr182), p38, phospho-specific PDGFRβ (Tyr751), anti-green fluorescent protein (GFP) (rabbit) antibodies, and Akt siRNA were from Cell Signaling Technology (Beverly, MA). Antibodies of SERT (goat), PDGFRβ (P-20), PDGF-BB, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and GFP (mouse) were from Santa Cruz Biotechnology (San Diego, CA). PDGFRβ and SERT (rabbit) antibodies were from Millipore (Upstate Biotechnology, Lake Placid, NY and Chemicon International, Temecula, CA, respectively). Recombinant human PDGF-BB was from R&D Systems (Minneapolis, MN). Nontargeting siRNA and ON-TARGETplus SMARTpool siRNA for human SERT were from Thermo Scientific Dharmacon (Lafayette, CO).
Statistical analysis.
Means ± SD were calculated and statistically significant differences among groups were determined by one-way ANOVA analysis followed by post hoc comparisons, or by two-tailed unpaired Student's t-test between two groups as appropriate, with significance at P < 0.05.
RESULTS
Genetic or pharmacological targeting of SERT attenuates PDGF-induced PASMC proliferation.
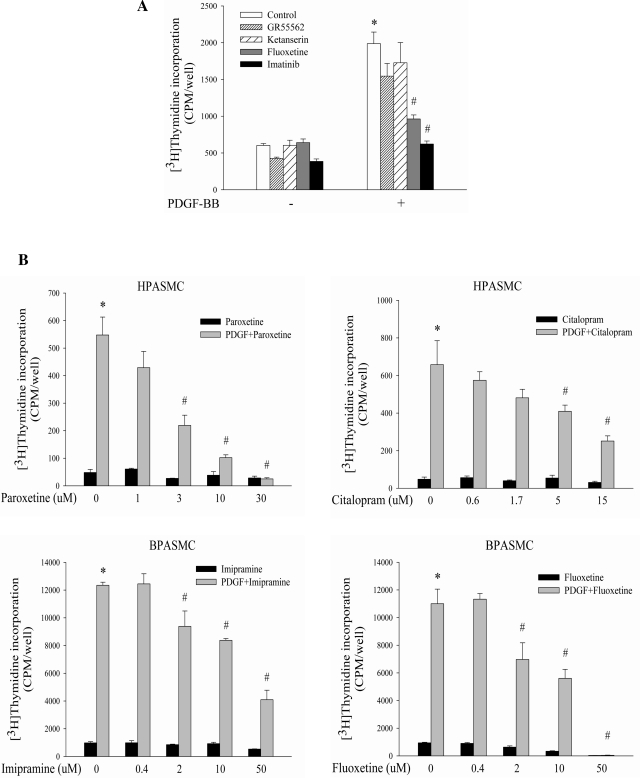
To initiate studies of possible interaction between SERT and PDGFR, we explored the role of SERT in PDGF-BB-induced DNA synthesis with pharmacological antagonists of SERT and PDGFR individually or in varying combinations at different concentrations in both primary BPASMCs and HPASMCs. We used four structurally dissimilar SERT antagonists (fluoxetine, imipramine, paroxetine, and citalopram), i.e., one tricyclic antidepressant (TCA) and three serotonin-selective reuptake inhibitors (SSRIs). We also used PDGFR inhibitors (AG1296 and imatinib) and antagonists of 5-HTR1B (GR55562) and 5-HTR2A (ketanserin). As illustrated in Fig. 1, the tested TCA and SSRIs inhibited PDGF-stimulated PASMC proliferation in a dose-dependent manner with comparable efficacy to that of AG1296 and imatinib, whereas GR55562 or ketanserin had no effect; combinations of different concentrations of SERT/PDGFR inhibitors led to significantly more pronounced inhibition of PDGF-activated cell proliferation than single drug application alone. Since higher concentrations of inhibitors like 50 μM fluoxetine and 30 μM paroxetine were found to be toxic to cells, we used lower concentrations of these reagents in subsequent experiments.
Fig. 1.
Inhibitory effects of serotonin transporter (SERT) pharmacological antagonists on PDGF-induced cell proliferation in human (H-) and bovine (B-) pulmonary artery smooth muscle cells (PASMCs). A: PDGF-induced BPASMC proliferation is inhibited by an antagonist of SERT (10 μM fluoxetine) and PDGF receptor (PDGFR) (1 μM imitinib), but not by 5-HTR2A (5 μM ketanserin) or 5-HTR1B (5 μM GR55562) antagonists. B: dose-dependent inhibitory effects of SERT antagonists (fluoxetine, imipramine, citalopram, and paroxetine) on cell proliferation by PDGF in HPASMCs and BPASMCs. C: synergistic/additive inhibition of PDGF-stimulated PASMC proliferation with combinations of SERT and PDGFR inhibitors. Representative graphs are shown as means ± SD, n = 3. *Significant difference from the untreated controls at P < 0.05. #Significant difference from cells treated with PDGF alone at P < 0.05. **Significant difference from cells treated with inhibitor alone plus PDGF at corresponding concentrations at P < 0.05.
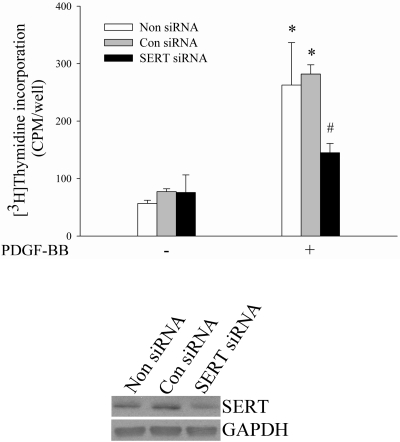
For more specific assessments of these observations than might be provided by inhibitors alone we tested genetic ablation of SERT via siRNA knockdown of SERT. We used HPASMCs and siRNA, and observed that PDGF-induced HPASMC proliferation was attenuated by siRNA downregulation of SERT (Fig. 2). From these studies we conclude that SERT participates in PDGF-stimulated PASMC proliferation.
Fig. 2.
Downregulation of SERT using small interfering RNA (siRNA) attenuates PDGF-induced PASMC proliferation. HPASMCs were transfected with SERT siRNA and nontargeted siRNA and then evaluated for cell proliferation by a [3H]thymidine incorporation assay. The expressions of SERT and GAPDH were detected by Western blotting by using SERT and GAPDH antibody, respectively. Bar graphs shown are means ± SD, n = 4. *Significant difference from cells without PDGF treatment correspondingly (P < 0.05). #Significant difference from cells transfected with control/nontargeting (Con) siRNA plus PDGF treatment (P < 0.05). Non siRNA, no siRNA transfection.
SERT participates in PDGF-induced phosphorylation of Akt and p38 but not Erk MAPK in PASMC.
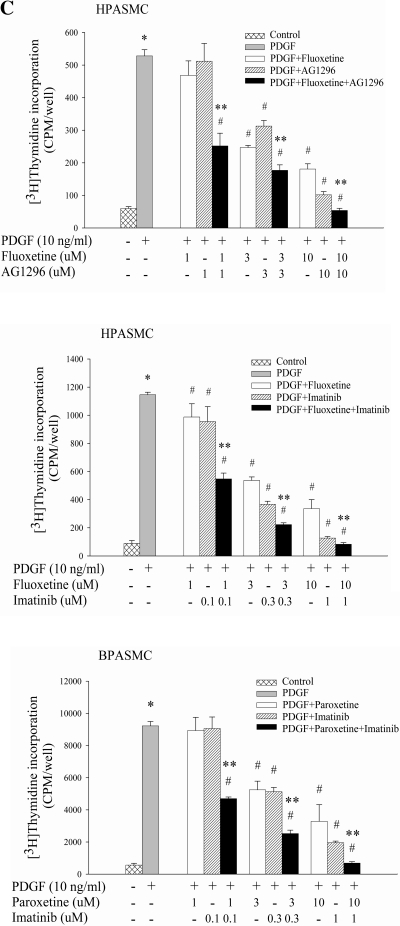
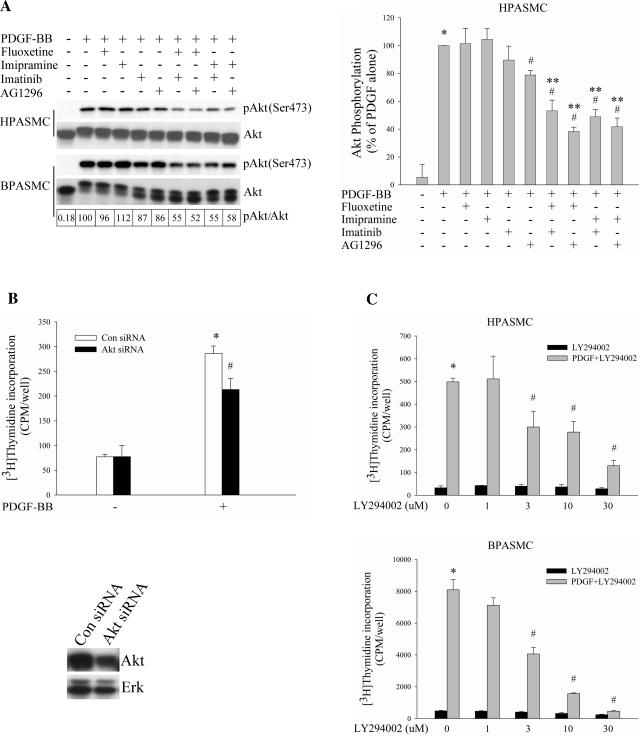
With the recognition that SERT participates in the effect of PDGF on PASMC proliferation, we began to explore possible cellular signaling pathways that might be associated with this effect. It is well documented that PI3K/Akt pathway is required for PDGF mitogenesis (11, 12, 17, 51). Thus we initially tested the effect of SERT inhibitors on activation of Akt by PDGF-BB in both HPASMCs and BPASMCs. Despite having no effect of their own on PDGF-induced activation of Akt, SERT antagonists augmented the inhibitory effect of PDGFR inhibitors on Akt phosphorylation by PDGF (Fig. 3A). This synergistic effect may also reflect cooperation of PDGFR and SERT signaling (e.g., see Fig. 1C). To determine whether in our cell system Akt pathway could be engaged in the stimulation of cell proliferation by PDGF, we treated HPASMCs with Akt siRNA and found an inhibitory effect on PDGF-induced DNA synthesis in Akt knockdown cells (Fig. 3B). We also used LY294002, a PI3K inhibitor, and observed a dose-dependent diminution in cell proliferation by PDGF in both cell types (Fig. 3C), confirming that the PDGF-induced PI3K/Akt pathway participates in cell proliferation in our cell system.
Fig. 3.
SERT participates in PI3K/Akt pathway in PDGF-stimulated PASMC proliferation. A: SERT inhibitors augment the blockade of PDGF-induced Akt phosphorylation by PDGFR inhibitors alone. Serum-starved HPASMCs and BPASMCs were preincubated with 10 μM fluoxetine, 10 μM imipramine, 1 μM imatinib, and 10 μM AG1296 individually or in combinations for 30–60 min depending on inhibitors and then were treated with 10 ng/ml PDGF for 10 min. Phosphorylation of Akt was determined by Western blot analysis of the whole cell lysate using phospho-specific antibodies, quantified as the ratios of phospho-Akt and Akt, and expressed as percentage of PDGF treatment alone (as noted for BPASMCs in boxes at bottom of figure). Bar graphs represent means ± SD for 3 independent experiments in HPASMCs. B: inhibitory effect of Akt siRNA on PDGF-induced HPASMC proliferation. Akt and Erk expressions were detected by Western blotting using Akt and Erk antibody, respectively. Bar graphs shown are means ± SD, n = 4. *Significant difference from cells without PDGF treatment (P < 0.05). #Significant difference from Con siRNA cells plus PDGF treatment (P < 0.05). C: PI3K inhibitor LY294002 dose dependently blocks HPASMC and BPASMC proliferation by PDGF. Quiescent cells were incubated with LY294002 for 60 min at indicated concentrations and then treated with 10 ng/ml PDGF for 24 h. Shown are means ± SD for n = 3. *Significant difference from untreated cells (P < 0.05). #Significant difference from cells treated with PDGF alone at P < 0.05. **Significant difference from cells treated with inhibitor alone plus PDGF (10 μM fluoxetine, 10 μM imipramine, 1 μM imatinib, and 10 μM AG1296 plus 10 ng/ml PDGF, respectively) at P < 0.05.
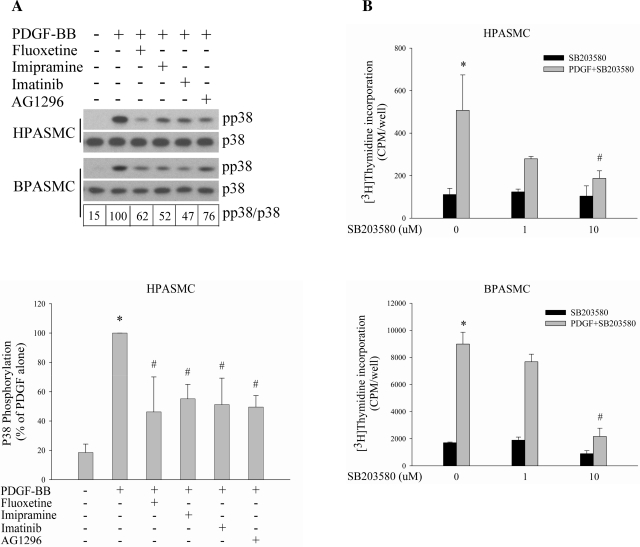
Next, we examined the effect of SERT inhibition on activation of Erk MAPK by PDGF, another principal signaling pathway responsible for PDGF-stimulated cell proliferation (17). No significant inhibitory effect was observed either by SERT inhibitors alone or in combination with PDGFR inhibitors in HPASMCs and BPASMCs (data not shown). However, we found that the PDGF-induced p38 MAPK phosphorylation was diminished by both SERT inhibitors and PDGFR inhibitors in these two types of cells (Fig. 4A), suggesting that SERT may be involved in PDGF-induced p38 MAPK activation and the consequent PASMC proliferation. To define the contribution of the p38 pathway in PASMC proliferation by PDGF, we used SB203580, a specific inhibitor of the p38 pathway, and found SB203580 dose dependently blocked PDGF-induced DNA synthesis in both PASMCs (Fig. 4B).
Fig. 4.
SERT participates in p38 MAPK pathway in PDGF-induced PASMC proliferation. A: PDGF-induced p38 phosphorylation is attenuated by SERT and PDGFR inhibitors. Quiescent HPASMCs and BPASMCs were treated with 10 ng/ml PDGF for 5 min in the absence or presence of 10 μM fluoxetine, 10 μM imipramine, 1 μM imatinib, or 10 μM AG1296. Phosphorylation of p38 was examined by immunoblotting using a phospho-specific antibody, quantified as the ratios of phospho-p38 and p38, and expressed as percentage of PDGF alone (as noted for BPASMCs in boxes below blots). Bar graphs represent means ± SD for 3 separate experiments in HPASMCs. B: P38 inhibitor SB203580 attenuates PDGF-induced DNA synthesis in a dose-dependent manner. Quiescent HPASMCs and BPASMCs were pretreated with SB203580 (1 or 10 μM) for 30 min and then stimulated with 10 ng/ml PDGF for 24 h. Shown are means ± SD for n = 3. *Significant difference from untreated cells (P < 0.05). #Significant difference from cells treated with PDGF alone at P < 0.05.
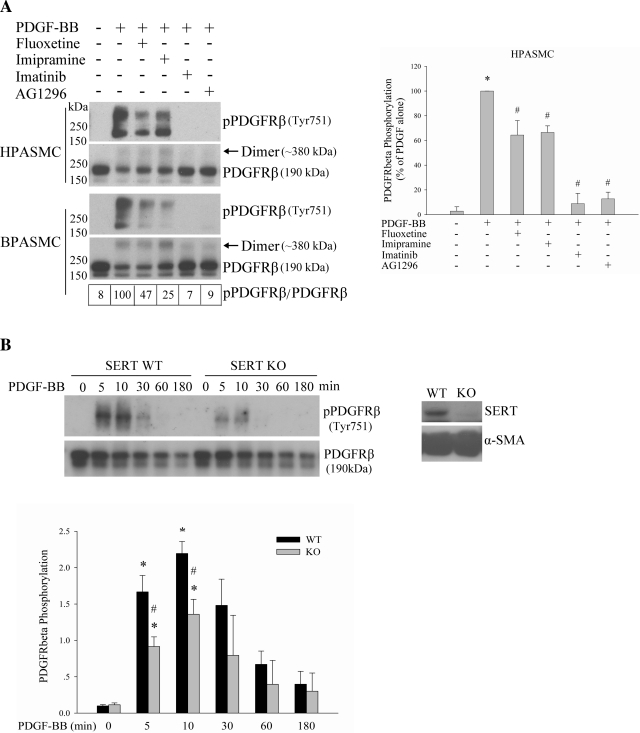
SERT ablation/inhibition partially blocks PDGFRβ phosphorylation by PDGF.
The influence of SERT on PDGF signaling prompted us to further access whether activation of the PDGFR by PDGF, an initial event for PDGF signaling, was affected by SERT. To address this question, we used SERT inhibitors and PASMCs from SERT−/− rats. Pretreatment of HPASMCs and BPASMCs with SERT inhibitors fluoxetine and imipramine followed by PDGF-BB stimulation, resulted in a partial blockade of PDGFRβ phosphorylation (at Tyr751, docking site for PI3K binding; Ref. 23), as did inhibitors of the PDGFR (imatinib and AG 1296), which almost completely blocked the effect (Fig. 5A). Comparison of PDGFRβ signaling in SERT−/− RPASMCs with that in cognate WT RPASMCs also showed that SERT ablation partially blocks PDGFRβ phosphorylation induced by PDGF (Fig. 5B). Thus our results indicate that SERT partially participates in PDGF-induced PDGFRβ phosphorylation.
Fig. 5.
SERT partially participates in PDGFRβ phosphorylation by PDGF. A: inhibition of SERT by SERT inhibitors partially blocks PDGF-induced PDGFRβ phosphorylation. Quiescent HPASMCs and BPASMCs were treated with 10 ng/ml PDGF for 10 min with and without pretreatment with 10 μM fluoxetine, 10 μM imipramine, 1 μM imatinib, or 10 μM AG1296 for 30–60 min depending on inhibitors. The phosphorylation of PDGFRβ was analyzed by immunoblotting using a phospho-PDGFRβ (Tyr751) antibody. The relative PDGFRβ activation levels were defined as the ratios of receptor phosphorylation and the receptor levels and expressed as percentage of PDGF alone. Values for BPASMCs are shown in boxes at bottom of top left. Bar graphs (right) represent means ± SD for 3 independent experiments in HPASMCs. *Significant difference from the untreated controls at P < 0.05. #Significant difference from PDGF-treated cells at P < 0.05. B: SERT ablation diminishes PDGFRβ phosphorylation by PDGF. Quiescent SERT−/− and cognate wild-type (WT) rat PASMCs (RPASMCs) were treated with 10 ng/ml PDGF for the indicated time, and phosphorylation of PDGFRβ was determined. The expressions of SERT and α-smooth muscle actin (α-SMA) were detected by Western blotting using SERT and α-SMA antibody, respectively. Quantification of PDGFRβ phosphorylation is shown as means ± SD for n = 3 in bar graph. *Significant difference from the correspondingly untreated SERT−/− and WT cells at P < 0.05. #Significant difference from WT cells treated with PDGF at corresponding time at P < 0.05. “Dimer” noted in Figs. 5 and 6 refers to PDGFRβ, of which the molecular mass is ∼380 kDa. KO, knockout.
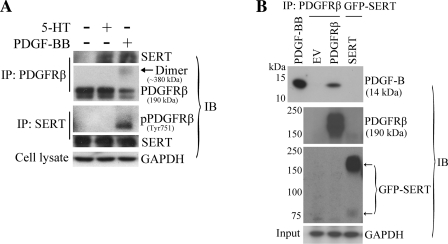
SERT and PDGFRβ are physically associated by PDGF.
Our evidence of the functional relationship between PDGFR and SERT led us to question whether a physical interaction occurs between these two cell surface molecules when PDGFR is activated by PDGF. Therefore, we conducted experiments employing a reciprocal coimmunoprecipitation method from extracts of HPASMCs treated with or without PDGF-BB. Using a polyclonal PDGFRβ antibody to immunoprecipitate PDGFRβ, we detected immunoreactive bands of SERT in cells with PDGF treatment. Here, we used 5-HT treatment as a positive control that was demonstrated in our previous study (34). Similar results were obtained in reciprocal experiments with pulldown of SERT and detection of the associated PDGFRβ (Fig. 6A). Our data show that SERT binds with phosphorylated PDGFRβ in a complex upon stimulation with PDGF, suggesting that SERT and PDGFRβ form a signaling platform that might function as a unit to promote PDGF signaling and cell proliferation. We did not observe a direct interaction of PDGF-BB with SERT in our separate immunoprecipitation experiments (Fig. 6B), also suggesting that the interaction occurs between the receptors. Since these observations occurred under serum-starvation conditions without added exogenous 5-HT, we suspect that SERT as a protein independent of 5-HT is important for its functional and physical interaction with PDGFRβ triggered by PDGF. Also, since 5-HT stimulation can induce SERT-PDGFRβ binding (34) (Fig. 6A) and 5-HT internalization is necessary to the comitogenic effect of 5-HT with PDGF (8, 10, 28), studies of 5-HT fluxes across the cell membrane as a regulator of the extent of binding may merit further investigation.
Fig. 6.
Physical interaction between SERT and PDGFRβ. A: PDGF-dependent association of SERT with PDGFRβ in PASMC. Quiescent HPASMCs were stimulated with and without 10 ng/ml PDGF or 1 μM 5-HT for 10 min. A reciprocal coimmunoprecipitation method was employed followed by immunoblotting to detect the physical association of SERT and PDGFRβ with PDGF and 5-HT treatment using SERT and PDGFRβ antibodies. Total GAPDH from whole cell lysates as loading control was detected using the anti-GAPDH antibody. Representative blots are shown. B: PDGF-BB does not bind with SERT directly but rather with PDGFRβ. HEK293 cells were transiently transfected with pcDNA3.1(−) [empty vector (EV)], pEYFP/SERT, pEYFP/SERT-NAE, or pLXSN/PDGFRβ constructs. After 48 h of transfection, cells were lysed and precipitated with green fluorescent protein (GFP) antibody (anti-mouse) and PDGFRβ antibody, respectively. The immunoprecipitates were then incubated with 50 ng of PDGF-BB, and PDGF-BB binding was determined by immunoblotting with a PDGF-B antibody. Pulldown of SERT and PDGFRβ were also determined by GFP antibody (anti-rabbit) and PDGFRβ antibody, respectively. Total GAPDH from whole cell lysates (input) was detected using the anti-GAPDH antibody as loading control. Representative blots are shown. IP, immunoprecipitation; IB, immunoblotting.
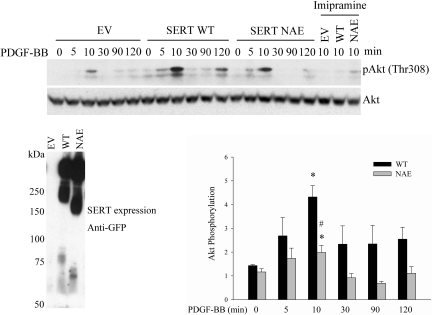
COOH-terminal PDZ-binding motif of SERT is necessary for PDGF signaling in HEK293 cells.
It was shown by Chanrion et al. (5) that the COOH-terminal PDZ binding motif of SERT is critical for protein functional modulation. We wished to determine whether the SERT PDZ motif participates in the functional interactions between SERT and PDGFRβ. Therefore, using a construct overexpression approach, we extended our experiments to HEK293 cells, which have very low endogenous SERT expression but express the PDGFR endogenously to permit studies of PDGF signaling (52). For these experiments constructs of empty vector (EV), SERT wild type (SERT WT), and SERT PDZ mutant (SERT NAE) were transfected into the HEK293 cells and phosphorylation of Akt by PDGF was determined. As shown in Fig. 7, PDGF time dependently stimulated Akt phosphorylation in the HEK293-transfected cells. The phosphorylation of Akt was weak in EV cells, enhanced in WT cells, and blunted in NAE cells. Consistently, the SERT inhibitor imipramine blocked the Akt phosphorylation in all the three transfectants. Thus our results indicate that exogenous SERT is required for the PDGF-induced Akt phosphorylation, and the SERT NAE attenuates this Akt phosphorylation. The expression of the transiently transfected SERT (WT and NAE) constructs was confirmed by probing a Western blot of the anti-GFP (rabbit) immunoprecipitated with anti-GFP (mouse) antibody.
Fig. 7.
SERT PDZ mutant blunts PDGF-induced Akt phosphorylation in HEK293 cells. HEK293 cells were transiently transfected with EV, SERT WT, or SERT NAE constructs. After 48 h of transfection, cells were serum starved and then stimulated with 10 ng/ml PDGF for the indicated time. All 3 transfectants (EV, WT, NAE) were also pretreated with 10 μM imipramine for 30 min and then stimulated with 10 ng/ml PDGF for 10 min. Phosphorylation of Akt was determined by immunoblotting using a phospho-Akt antibody at Thr308. The expression levels of SERT and its NAE mutant were examined by immunoblotting with antibody to GFP. Quantification of Akt phosphorylation from 3 repeat experiments is shown as means ± SD at bottom right. *Significant difference from the correspondingly untreated SERT WT and SERT NAE cells at P < 0.05. #Significant difference from SERT WT cells treated with PDGF for 10 min at P < 0.05.
DISCUSSION
Although both SERT and PDGFRβ are implicated in PAH, prior to our demonstration that SERT transactivates PDGFRβ in 5-HT-triggered PASMC proliferation (34) no previous studies have addressed a possible relationship between these two cell surface molecules. Here, we show a new role for SERT in cooperating with PDGFRβ in PDGF-BB signaling and proliferation in PASMCs.
With the use of four structurally dissimilar SERT-specific antagonists and SERT-targeted siRNA, we initially demonstrated the inhibition of PDGF-BB-stimulated proliferation in HPASMCs and BPASMCs and the synergistic/additive inhibition of PASMC proliferation with combinations of SERT and PDGFR inhibitors. These findings strongly indicate that SERT participates in PDGF-BB-triggered PASMC proliferation and that a functional collaboration between SERT and PDGFR is required for effective cellular response to PDGF.
We next explored the impact of SERT on PDGF-BB signaling responsible for PASMC growth. Our results showed that although SERT inhibitors fluoxetine and imipramine did not influence Akt phosphorylation by PDGF they markedly augmented the inhibitory effect of PDGFR inhibitors, AG1296 and imatinib, on the Akt phosphorylation. Conversely, HEK293 cells overexpressing SERT exhibited enhanced Akt phosphorylation by PDGF. These data suggest that SERT may participate in PDGF-induced Akt activation, subsequently regulating proproliferative and/or prosurvival gene expression and ultimately driving cell growth in PASMCs. Interestingly, even with coapplications SERT and PDGFR inhibitors failed to completely block Akt phosphorylation by PDGF in our PASMCs; however, SERT blockers (10 μM fluoxetine and 10 μM imipramine) partially and PDGFR inhibitors (1 μM imatinib and 10 μM AG1296) almost completely blocked the phosphorylation of the PDGFRβ at Tyr751 at their tested concentrations. Consistent with our results, Li et al. (31) also observed that 1 μM imatinib slightly inhibited Akt phosphorylation whereas it completely blocked PDGFRβ phosphorylation by PDGF in human aortic SMCs. As possible explanations for these observations in SMCs, we hypothesize the following: 1) Both the α and β subunits of PDGFR transduce Akt activation signals with similar binding affinity with PDGF (12, 16) although with a higher expression of PDGFRβ in SMCs (1), but with no or less influence of SERT on the PDGFRα. Further studies will be needed to determine whether there is any relationship between SERT and PDGFRα as in the present study we focused on PDGFRβ. Less likely, the portion of activated PDGFRβ not affected by SERT inhibitors is sufficient to activate the PI3K/Akt pathway. 2) In addition to SERT, Akt full activation by PDGF may require the contribution of other modulators or signaling pathways. 3) PDGF may stimulate synergistic cross talk between the PI3K/Akt pathway and other proproliferative pathways, such as Ras/MAPK cascade (20, 43) and Rho family GTPase (53), which in turn amplify the PI3K/Akt activation downstream of PDGFR. In any case, our results suggest that SERT and PDGFRβ cooperatively influence Akt activation, consequently coregulating PDGF-triggered proliferation in the PASMCs. Since the data suggest that the Akt pathway may not be solely responsible for PDGF-induced PASMC proliferation, we thus determined whether SERT could impact the PDGF-BB-stimulated MAPK pathway. Although inhibition of SERT failed to block Erk activation, fluoxetine and imipramine significantly diminished p38 phosphorylation, suggesting a role for SERT in the activation of p38 but not Erk MAPK by PDGF. Since the p38 pathway is required for PDGF-induced DNA synthesis [by this study in both HPASMCs and BPASMCs and by Nègre-Aminou et al. (39) in human SMCs], the impact of SERT on PDGF-mediated p38 phosphorylation may be responsible for the role of SERT in PDGF-induced cell proliferation. Together, our results indicate that the inhibition of PDGF-induced proliferation by SERT inhibition may be caused by attenuation of PDGFRβ phosphorylation, sequentially and/or in parallel, resulting in downregulation of the PDGFRβ-mediated PI3K/Akt and p38 signaling pathways.
In support of the cell proliferative and cellular signaling relationships between SERT and PDGFRβ, we also have found a physical association of SERT and PDGFRβ following PDGF stimulation. The nature of this association is unknown at the present time. The binding could be assisted by another protein such as a scaffold, anchoring, or adaptor protein. One good candidate for this action could be the PDZ domain containing proteins that link SERT to PDGFRβ in a complex via their multiple PDZ domains binding to the PDZ recognition motifs of PDGFRβ and SERT, respectively, as exemplified by Na+/H+ exchanger regulatory factor linking the N-cadherin/catenin complex to the PDGFRβ via its two PDZ domains (50). Our data show that a mutant of the SERT PDZ-binding motif blunted PDGF-BB signaling in HEK293 cells. Another candidate could be Src, which has well-documented binding with phosphorylated PDGFR via its SH2 domain (17) and has been shown to be associated with SERT in a complex by coimmunoprecipitation in human platelets; this action upregulates 5-HT transport by mediating SERT Tyr-phosphorylation (54). The SERT/PDGFRβ association could also be mediated by reported integrins (4, 55).
In summary, we know from the present studies that previously unidentified relationships exist between SERT and the PDGFRβ in PASMCs and that these relationships participate in PDGF-BB- and 5-HT-induced PASMC proliferation, which are outlined in Fig. 8. There also is a physical interaction between these two cell surface molecules. The physical association of SERT and PDGFRβ by PDGF stimulation suggests that SERT may serve as a modulator facilitating transmission of signaling from PDGFR to its associated signaling molecules, and that the activated PDGFRβ in turn enhances SERT activity of 5-HT internalization, and thereby subsequent PASMC processes. The cell signaling interaction is not a simple linear and sequential one. These findings are of potential clinical interest and may provide new insight into the pathogenesis of PAH. The relationship of the PDGF/PDGFR and 5-HT/SERT signaling may have further significance as both may need to be inhibited in the regulation of PAH. Also, these findings have prompted us to further pursue studies of any relationship between SERT and other growth factors such as EGF and FGF that may be associated with smooth muscle proliferation and PAH.
Fig. 8.
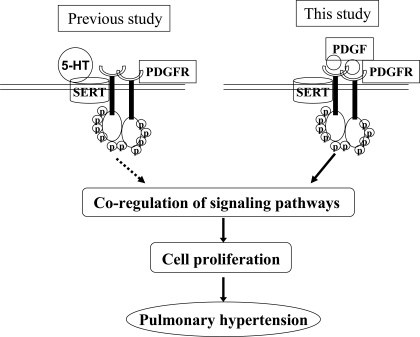
Proposed model of interactions between SERT and PDGFRβ in the regulation of PASMC proliferation in pulmonary arterial hypertension (PAH). SERT transactivates PDGFRβ in the production of PASMC proliferation by 5-HT (our previous study) (34), and SERT participates in PDGF-BB signaling and mitogenesis (this study). The 2 smooth muscle cell surface molecules are physically associated by 5-HT and PDGF, and their association facilitates signal transduction to promote cell proliferation, consequently triggering PAH. Circled p denotes phosphorylated PDGFR.
GRANTS
This work was supported by National Institutes of Health Grant HL085260 (to B. L. Fanburg).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful for the generous gifts of pEYFP/SERT and pEYFP/SERT-NAE constructs from Drs. Joël Bockaert, Philippe Marin (Institut de Génomique Fonctionnelle, Universités Montpellier I & II, Montpellier, France), and Michael Freissmuth (Institut für Pharmakologie, Universität Wien, Wien, Austria), and of the pLXSN/PDGFRβ construct from Drs. Andrius Kazlauskas and Vijaya Ramesh (Schepens Eye Research Institute, Harvard Medical School, and Massachusetts General Hospital, Boston, MA). We are also grateful to Dr. Edwin Cuppen and colleagues for generating the SERT−/− rat model for us to use.
REFERENCES
- 1. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balasubramaniam V, Le Cras TD, Ivy DD, Grover TR, Kinsella JP, Abman SH. Role of platelet-derived growth factor in vascular remodeling during pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 284: L826–L833, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66–70, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest 118: 1544–1552, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, Bockaert J, Marin P. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci USA 104: 8119–8124, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chhina MK, Nargues W, Grant GM, Nathan SD. Evaluation of imatinib mesylate in the treatment of pulmonary arterial hypertension. Future Cardiol 6: 19–35, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol 155: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res 84: 329–336, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 105: 1555–1562, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 108: 1141–1150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fantl WJ, Escobedo JA, Martin GA, Turck CW, del Rosario M, McCormick F, Williams LT. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell 69: 413–423, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 353: 1412–1413, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation 111: 2812–2819, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell 80: 213–223, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1378: F79–F113, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science 291: 1800–1803, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, Nieuwenhuizen OF, Cools AR, Ronken E, Cremers T, Schoffelmeer AN, Ellenbroek BA, Cuppen E. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience 146: 1662–1676, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 268: 100–102, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jankov RP, Kantores C, Belcastro R, Yi S, Ridsdale RA, Post M, Tanswell AK. A role for platelet-derived growth factor beta-receptor in a newborn rat model of endothelin-mediated pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 288: L1162–L1170, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kazlauskas A, Cooper JA. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell 58: 1121–1133, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell 7: 343–354, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Lee SL, Wang WW, Fanburg BL. Association of Tyr phosphorylation of GTPase-activating protein with mitogenic action of serotonin. Am J Physiol Cell Physiol 272: C223–C230, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Lee SL, Wang WW, Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radic Biol Med 24: 855–858, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol Lung Cell Mol Physiol 277: L282–L291, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res 68: 1362–1368, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem 284: 6329–6336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Li L, Blumenthal DK, Masaki T, Terry CM, Cheung AK. Differential effects of imatinib on PDGF-induced proliferation and PDGF receptor signaling in human arterial and venous smooth muscle cells. J Cell Biochem 99: 1553–1563, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGFβ receptor in pulmonary artery smooth muscle cells. FASEB J 21: 2725–2734, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 36. MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation 109: 2150–2155, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, Eddahibi S. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med 168: 487–493, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res 94: 1263–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Nègre-Aminou P, van Erck M, van Leeuwen RE, Collard JG, Cohen LH. Differential effect of simvastatin on various signal transduction intermediates in cultured human smooth muscle cells. Biochem Pharmacol 61: 991–998, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med 145: 152–153, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science 278: 2075–2080, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Herve P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 81–88, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370: 527–532, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Rubin LJ. Primary pulmonary hypertension. N Engl J Med 336: 111–117, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY. The G protein G alpha(13) is required for growth factor-induced cell migration. Dev Cell 10: 707–718, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8: 1888–1896, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15: 205–213, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Ten Freyhaus H, Dumitrescu D, Bovenschulte H, Erdmann E, Rosenkranz S. Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol 98: 265–267, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Theisen CS, Wahl JK, 3rd, Johnson KR, Wheelock MJ. NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell 18: 1220–1232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valius M, Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell 73: 321–334, 1993 [DOI] [PubMed] [Google Scholar]
- 52. Wang C, Buck DC, Yang R, Macey TA, Neve KA. Dopamine D2 receptor stimulation of mitogen-activated protein kinases mediated by cell type-dependent transactivation of receptor tyrosine kinases. J Neurochem 93: 899–909, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol 4: 509–513, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zarpellon A, Donella-Deana A, Folda A, Turetta L, Pavanetto M, Deana R. Serotonin (5-HT) transport in human platelets is modulated by Src-catalysed Tyr-phosphorylation of the plasma membrane transporter SERT. Cell Physiol Biochem 21: 87–94, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem 284: 16693–16703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu SP, Mao ZF, Huang J, Wang JY. Continuous fluoxetine administration prevents recurrence of pulmonary arterial hypertension and prolongs survival in rats. Clin Exp Pharmacol Physiol 36: e1–e5, 2009 [DOI] [PubMed] [Google Scholar]