Abstract
Early life is a dynamic period of growth for the lung and immune system. We hypothesized that ambient ozone exposure during postnatal development can affect the innate immune response to other environmental challenges in a persistent fashion. To test this hypothesis, we exposed infant rhesus macaque monkeys to a regimen of 11 ozone cycles between 30 days and 6 mo of age; each cycle consisted of ozone for 5 days (0.5 parts per million at 8 h/day) followed by 9 days of filtered air. Animals were subsequently housed in filtered air conditions and challenged with a single dose of inhaled LPS at 1 yr of age. After completion of the ozone exposure regimen at 6 mo of age, total peripheral blood leukocyte and polymorphonuclear leukocyte (PMN) numbers were reduced, whereas eosinophil counts increased. In lavage, total cell numbers at 6 mo were not affected by ozone, however, there was a significant reduction in lymphocytes and increased eosinophils. Following an additional 6 mo of filtered air housing, only monocytes were increased in blood and lavage in previously exposed animals. In response to LPS challenge, animals with a prior history of ozone showed an attenuated peripheral blood and lavage PMN response compared with controls. In vitro stimulation of peripheral blood mononuclear cells with LPS resulted in reduced secretion of IL-6 and IL-8 protein in association with prior ozone exposure. Collectively, our findings suggest that ozone exposure during infancy can result in a persistent effect on both pulmonary and systemic innate immune responses later in life.
Keywords: Toll-like receptor, lipopolysaccharide, infant
ground-level ozone is one of six criteria pollutants for which National Ambient Air Quality Standards are set by the United States Environmental Protection Agency. Of the six criteria pollutants, ozone and particulate matter pose the most significant threat to human health because of widespread exposure. Populations that are most vulnerable to the adverse effects of ozone include children and individuals with preexisting lung disease. Multiple physiological parameters enhance susceptibility in the very young, including different breathing patterns and larger lung surface area per unit of body weight compared with adults; this may affect the degree and localization of deposition for ozone (reviewed in Ref. 4). Airways injury and reparative processes are also likely to differ in children given that the human lung continues to grow through the elementary school age, primarily due to the addition of alveoli (3, 62). Epidemiologic studies in humans support a pathological association between ambient ozone levels and childhood respiratory health, including lung function deficits, respiratory allergies, increased hospital admissions, and prevalence of asthma (1, 35, 39, 45). Experimental work in animal models indicates a major role for inflammatory cells and their products in the development of airways hyperreactivity with ozone (25, 26, 52, 55, 57, 61), yet we have a very limited understanding of how ozone can mediate a direct effect on immunity, particularly in young children.
In adult human subjects and multiple animal models, inhaled ozone results in a rapid influx of leukocytes into the airways with local release of cytokines and other inflammatory mediators (reviewed in Ref. 27). The airway inflammatory cell profile of ozone is predominantly neutrophilic in adult humans and most animal models; however, there is evidence that children develop an eosinophilic response to ambient levels of exposure (14, 35, 36). Because of the nonantigenic nature of ozone, there is currently no evidence that an adaptive immune response leading to establishment of immunological memory and antibody production is induced by exposure alone. Ozone may indirectly modulate adaptive immunity by promoting the activation of antigen-presenting cells (26, 34, 38). Yet, in light of increased antigen-presenting capabilities, it is surprising that numerous rodent models show impaired pulmonary microbial clearance on exposure to ozone, a finding that is enhanced in younger animals (19, 53, 54). An important clue to the link between ozone and microbial immunity is the identification of Toll-like receptor 4 (TLR4) as an essential susceptibility gene for the inflammatory and physiological effects of ozone exposure in certain mouse strains (25, 33). TLR4 is a member of the TLR family of proteins that are expressed by cells of the innate immune system and bind to common structural motifs of pathogens (reviewed in Ref. 40). Targeted deletion mutant mice for TLR4, TLR2, and the TLR signaling adapter protein myeloid differentiation factor-88 (MyD88) exhibit an attenuated inflammatory response and no airways hyperreactivity following ozone exposure (57).
The first year of life is a period of significant maturation for both the lung and immune system, which continues into childhood (29). In the current study, we speculated that chronic inhalation of ozone during early life may have persistent effects on immunity. This hypothesis is based on known differences in lung structure and immune system development, which suggest that children and adults may have differential responses to ozone exposure. Furthermore, the long-term effects of early life air pollutant exposures on respiratory health are supported by epidemiology (16, 17, 47). To test this hypothesis, we exposed rhesus macaque monkeys to episodic ozone during the postnatal development period and subsequently evaluated the impact of prior exposure at 1 yr of age. We used a single inhaled challenge with bacterial LPS, a TLR4 ligand, to stimulate a pulmonary and systemic inflammatory response. We also determined whether prior exposure to ozone had a persistent effect on systemic immunity by ex vivo stimulation of peripheral blood mononuclear cells (PBMC) with LPS.
MATERIALS AND METHODS
Animals and ozone exposure.
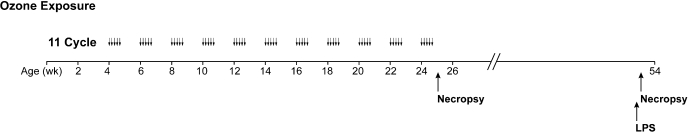
Male rhesus macaque (Macaca mulatta) infant monkeys were housed in filtered air conditions following birth. Starting at 30 days of age, animals were exposed to filtered air or 11 cycles of ozone (Fig. 1). Each cycle consisted of ozone exposure for 5 days [0.5 parts per million (ppm) at 8 h/day] followed by 9 days of filtered air. Details of ozone exposure methods for this study were previously reported (50). Animal groups not exposed to ozone remained in filtered air throughout each cycle. For animals evaluated at 6 mo of age, necropsy took place within 2.5 h following the last ozone exposure; animals ranged from 179 to 183 days of age (25–26 wk). Animals evaluated at 1 yr of age remained in filtered air housing for a period of 6 mo following 11 cycles of ozone exposure.
Fig. 1.
Experimental timeline for postnatal episodic ozone exposure and LPS challenge. Starting at 30 days of age, infant rhesus monkeys were exposed to 11 cycles of ozone. Each cycle consisted of ozone exposure for 5 days followed by 9 days of filtered air (0.5 parts per million at 8 h/day). Animals were evaluated either at the end of 10 cycles followed by 5 days of ozone (6 mo of age) or 11 cycles followed by 6 mo of filtered air (1 yr of age). LPS challenge took place at 1 yr of age.
For LPS challenge studies, 1-yr-old animals received an aerosolized dose of 25,000 endotoxin units in PBS (E. coli O26:B6; Sigma-Aldrich, St. Louis, MO) via mask exposure ∼24 h before necropsy. The same commercial lot of LPS was used for all animals in the current study. Blood draws for complete blood count (CBC) analysis were conducted immediately before LPS challenge in addition to 6 and 22 h post-LPS challenge. CBC values were determined with a Beckman Coulter analyzer (Beckman Coulter, Miami, FL), and differential counts were obtained from blood smears. Bronchoscopy was conducted at 6 h post-LPS challenge; lavage samples for 24 h post-LPS challenge were collected at necropsy. All animal procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee. Care and housing of animals before, during, and after treatment complied with the provisions of the Institute of Laboratory Animal Resources and conforms to practices established by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International).
Bronchoalveolar lavage.
Bronchoalveolar lavage specimens were obtained by bronchoscopy of sedated monkeys or collected from the right caudal lobe at the time of necropsy. For leukocyte differentials, lavage samples were cytocentrifuged, air-dried, and stained with a modified Wright stain (Diff-Quik), and the proportion of macrophages, neutrophils, eosinophils, lymphocytes, and epithelial cells was determined by counting 300 cells per sample by light microscopy.
Cytokine ELISA.
IL-1β, IL-6, and IL-8 protein concentration in lavage and PBMC supernatants (see PBMC culture and LPS stimulation) were measured by ELISA Ready-SET-Go! kits purchased from eBioscience (San Diego, CA). TNF-α protein concentrations were measured using a DuoSet kit (R&D Systems, Minneapolis, MN). The limit of detection for ELISA assays was 2 pg/ml (IL-6), 4 pg/ml (IL-8 and IL-1β), and 5 pg/ml (TNF-α).
PBMC culture and LPS stimulation.
PBMC were prepared from blood samples collected at necropsy as previously described and cryopreserved before culture (41). The LPS strain and lot number was identical to that used for in vivo treatments in this study. PBMC were cultured in AIM V medium (Invitrogen, Carlsbad, CA) at a concentration of 2 × 105/100 μl. LPS was diluted in AIM V media and incubated with cultures for 6 or 24 h at 37°C in 5% CO2. Supernatants were collected by centrifugation of PBMC cultures.
Statistics.
All data are reported as means ± SE. Treatment and sample differences were evaluated using ANOVA (1- or 2-way) or unpaired t-test where appropriate (GraphPad Prism, La Jolla, CA).
RESULTS
Effect of postnatal episodic ozone exposure on peripheral blood and bronchoalveolar lavage leukocytes at 6 mo of age.
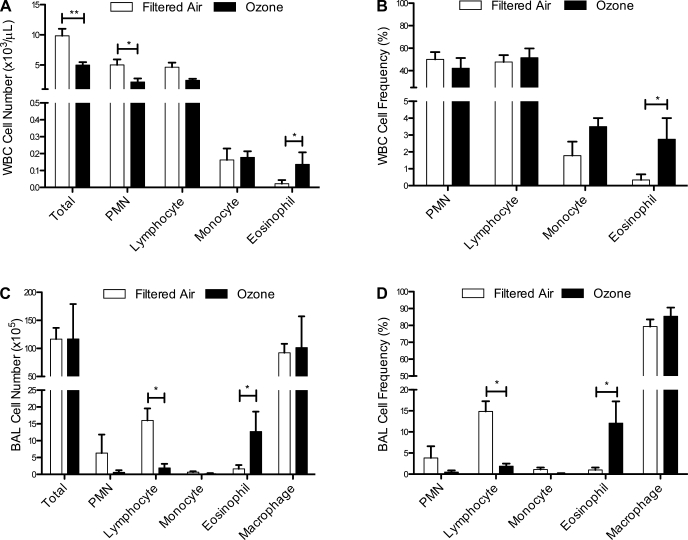
To determine whether postnatal episodic ozone exposure has an effect on circulating (blood) and airway (lavage) immune cells, we first evaluated animals at ∼6 mo of age immediately following the last 5 days of ozone. Total numbers of peripheral blood leukocytes were reduced in ozone-exposed animals compared with age-matched filtered air controls; this decrease was primarily represented in the polymorphonuclear leukocyte (PMN) population (Fig. 2A). We also observed a trend toward reduced lymphocyte numbers in response to ozone (P = 0.07). In contrast with decreased circulating PMN, ozone-exposed animals showed a significant increase in peripheral blood eosinophil number and frequency at this 6-mo time point (Fig. 2, A and B).
Fig. 2.
Effect of postnatal episodic ozone exposure on peripheral blood and lavage leukocyte populations at 6 mo of age. Total cell number (A and C) and frequency of leukocyte phenotype (B and D) were measured in filtered air- and ozone-exposed animals at 6 mo of age. Each column represents the mean ± SE values from 4 to 9 animals. *P < 0.05, **P < 0.01 compared with filtered air values. WBC, peripheral white blood cells; PMN, polymorphonuclear leukocytes; BAL, bronchoalveolar lavage.
Ozone exposure did not have an effect on total number of bronchoalveolar lavage cells at 6 mo of age (Fig. 2C), but a significant reduction in lymphocyte number and frequency was observed (Fig. 2, C and D). Similar to findings in peripheral blood at this age, lavage from ozone-exposed animals showed a significant increase in eosinophil number and frequency compared with age-matched filtered air controls (Fig. 2D).
Effect of postnatal episodic ozone exposure on peripheral blood and bronchoalveolar lavage leukocytes at 1 yr of age.
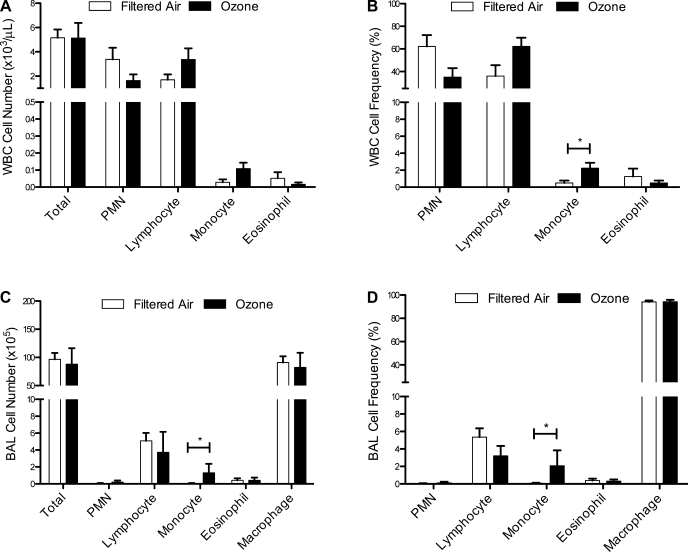
During normal immune system development in humans, peripheral blood leukocyte numbers progressively increase during the first year of life and then decline over time (8). We observed a similar trend in filtered air control monkeys, with an increase in total peripheral blood leukocytes from 3 to 6 mo of age in filtered air control monkeys (data not shown) followed by a decline at 1 yr of age, which was significant compared with values obtained at the 6-mo time point (P = 0.0267; Figs. 2A and 3A). In contrast, no significant change in number of total peripheral blood cells was observed in postnatal ozone-exposed animals evaluated at 1 yr of age compared with values obtained from ozone-exposed animals at the 6-mo time point (Figs. 2A and 3A).
Fig. 3.
Effect of postnatal episodic ozone exposure on peripheral blood and lavage leukocyte populations at 1 yr of age. Total cell number (A and C) and frequency of leukocyte phenotype (B and D) were measured in filtered air- and ozone-exposed animals at 1 yr of age. Each column represents the mean ± SE values from 4 animals. *P < 0.05 compared with filtered air values.
There was a significant interaction between exposure and cell type within peripheral blood leukocyte subsets at 1 yr of age (P < 0.05 by 2-way ANOVA); ozone-exposed animals showed a trend toward decreased PMNs and increased lymphocytes (P = 0.07 compared with filtered air controls; Fig. 3, A and B). The frequency of peripheral blood monocytes was significantly increased with prior ozone exposure (Fig. 3B). Similarly, lavage monocyte frequency and numbers were increased at 1 yr of age in ozone-exposed animals, although there was no overall effect of ozone on total lavage cell number (Fig. 3, C and D). Total numbers of lavage cells were also not affected by age, but there was a significant decline in lymphocyte counts with maturity (P < 0.05, 6-mo filtered air vs. 1-yr filtered air; Figs. 2C and 3C).
Postnatal episodic ozone exposure attenuates the systemic inflammatory response to LPS challenge at 1 yr of age.
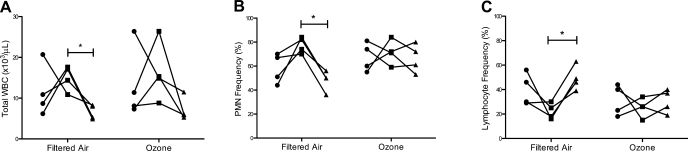
To determine whether a prior history of postnatal ozone exposure can have a persistent effect on innate immunity, we challenged 1-yr-old monkeys with a single dose of inhaled LPS and evaluated the immediate effect on peripheral blood leukocyte subsets. In age-matched filtered air control animals, LPS resulted in a time-dependent shift in peripheral blood leukocyte numbers, which peaked at 6 h postchallenge and declined at 22 h postchallenge (Fig. 4A; P < 0.05 by 1-way ANOVA). LPS also significantly affected the frequency of blood PMNs in filtered air control animals, which was maximal at 6 h post-LPS and declined at 22 h (Fig. 4B; P < 0.05 by 1-way ANOVA). Conversely, frequency of peripheral blood lymphocytes was significantly reduced at 6 h compared with 22 h in filtered air control animals (Fig. 4C; P < 0.05 by 1-way ANOVA).
Fig. 4.
Effect of postnatal episodic ozone exposure on total WBC (A), PMN frequency (B), and lymphocyte frequency (C) following LPS challenge. WBC samples were collected from filtered air- and ozone-exposed animals at 1 yr of age just before LPS instillation (●), 6 h post-LPS (■), and 22 h post-LPS (▲). Individual values from each of 4 animals are shown. *P < 0.05 for 6- vs. 22-h values.
In comparison with monkeys that were raised exclusively in filtered air for 1 yr, animals that had received postnatal episodic ozone exhibited a highly variable peripheral blood response to LPS, with no statistically significant changes in total peripheral blood cells, PMN frequency, or lymphocyte frequency relative to baseline measures (Fig. 4, A–C). However, we did observe a trend toward increased monocyte frequency at 6 h post-LPS challenge (P = 0.07 compared with filtered air, data not shown) in association with prior history of ozone exposure.
Postnatal episodic ozone exposure attenuates the pulmonary inflammatory response to LPS challenge at 1 yr of age.
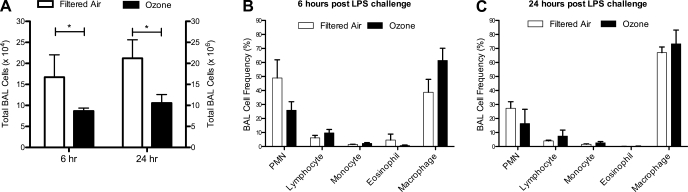
Because of concerns regarding the inflammatory effects of bronchoscopy, collection of lavage samples was limited to 6 and 24 h post-LPS challenge. Animals exposed to 11 cycles of ozone had significantly fewer total lavage cells than control animals at both 6 and 24 h post-LPS challenge (Fig. 5A). Although we obtained increased numbers of cells at the 24-h time point relative to 6 h due to collection methods at necropsy, there was no significant difference in the overall number of cells per milliliter of lavage fluid between the 6- and 24-h time points within each animal group, i.e., values for filtered air animals were similar at 6 and 24 h, and values for ozone were similar at 6 and 24 h (data not shown). A comparison between lavage cell numbers obtained at necropsy of 1-yr-old animals without LPS challenge (Fig. 3C) shows an ∼2-fold increase following LPS in filtered air animals, whereas ozone-exposed animals showed no change from baseline. One-year-old animals without LPS challenge had few lavage PMNs (Fig. 3D), but frequency increased dramatically at 6 h post-LPS (Fig. 5B) and declined by ∼2-fold at 24 h (Fig. 5C). Ozone-exposed animals showed significantly decreased lavage PMN numbers and a trend toward reduced frequency at both 6 and 24 h post-LPS compared with filtered air animals (PMN number, P < 0.05, filtered air vs. ozone; PMN frequency, P = 0.07, filtered air vs. ozone; Fig. 5, B and C).
Fig. 5.
Effect of postnatal episodic ozone exposure on lavage inflammation following LPS challenge. Total cell number (A) and frequency of leukocyte phenotype (B and C) were measured in filtered air- and ozone-exposed animals at 1 yr of age, at 6 and 24 h post-LPS. Each column represents the mean ± SE values from 3 to 4 animals. *P < 0.05 compared with filtered air values.
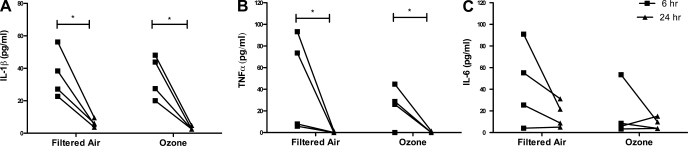
We next determined whether postnatal ozone exposure had an effect on cytokine synthesis in the context of a pulmonary inflammatory response. Lavage samples collected at 6 and 24 h post-LPS challenges were analyzed for the presence of proinflammatory cytokines IL-1β, TNF-α, and IL-6 by ELISA. Lavage obtained from 1-yr-old animals without LPS challenge show little to no detectable levels of these cytokines (data not shown). In all animals, there was an increase in cytokine concentration at 6 h post-LPS challenge, which was significantly reduced at 24 h for both IL-1β and TNF-α (Fig. 6, A–C).
Fig. 6.
Effect of postnatal episodic ozone exposure on lavage cytokine secretion following LPS challenge. IL-6 (A), TNF-α (B), and IL-1β (C) protein concentration were measured in lavage samples collected from 1-yr-old filtered air- and ozone-exposed animals at 6 h (■) and 24 h (▲) post-LPS. Individual values from each of 4 animals are shown. *P < 0.05 for 6- vs. 24-h values.
Effect of prior ozone exposure on peripheral blood response to LPS.
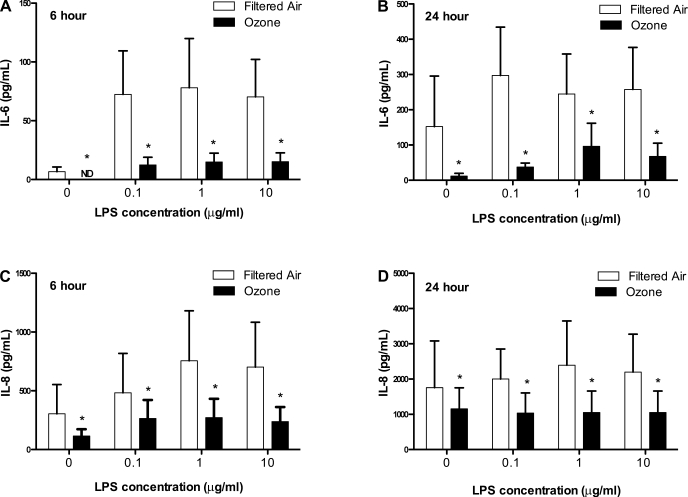
Previous studies have demonstrated that stimulation of human postnatal peripheral blood cells with TLR ligands in vitro results in a cytokine secretion profile that is distinct from that of stimulated adult cells (37, 44, 49, 60). Based on our findings of altered responses to LPS airway challenge, we investigated whether the peripheral blood response to TLR4 stimulation was persistently modulated with prior history of ozone exposure. To address this question, we cultured PBMC obtained from 1-yr-old animals that had been previously exposed to 11 cycles of ozone during postnatal development and compared the responses with age-matched filtered air control animals. PBMC cultures were treated with LPS in a dose-dependent manner, and secretion of IL-6 and IL-8 protein was measured at 6 and 24 h poststimulation. As shown in Fig. 7, we found that LPS treatment resulted in enhanced secretion of both IL-6 and IL-8 at 6 h poststimulation (Fig. 7, A and C). The overall concentration of both IL-6 and IL-8 secretion also increased over time (Fig. 7, A and B, P < 0.001, 6 vs. 24 h; Fig. 7, C and D, P < 0.001, 6 vs. 24 h). Baseline (no LPS) secretion of IL-6 and IL-8 was very low in PBMC cultures from ozone-exposed animals; IL-6 was not detectable after 6 h of culture. In conjunction with LPS stimulation, IL-6 and IL-8 secretion in PBMC cultures showed an exposure-dependent effect. PBMC from animals with prior ozone exposure showed significantly attenuated secretion of IL-6 and IL-8 (Fig. 7, A and B, P < 0.001, filtered air vs. ozone; Fig. 7, C and D, P < 0.01, filtered air vs. ozone).
Fig. 7.
Effect of postnatal episodic ozone exposure on LPS stimulation of peripheral blood mononuclear cells (PBMC) from 1-yr-old monkeys. PBMC from 1-yr-old filtered air- and ozone-exposed animals were treated with LPS in vitro. Cultures were evaluated at either 6 (A and C) or 24 (B and D) h for cytokine secretion. Concentration of IL-6 (A and B) and IL-8 (C and D) protein were measured in culture supernatant collected at listed time points. Each bar represents the mean ± SE of 2 data points obtained from each of 4 animals per group. *P < 0.05 compared with filtered air values. ND, not detected.
DISCUSSION
In this study, we investigated the immunomodulatory impact of early life ozone exposure. A cyclic (episodic) regimen of ozone was used to mimic the repetitive nature of ambient air pollution exposures in humans. We first evaluated the immediate effects of exposure on the postnatal immune system and found that ozone reduced circulating leukocyte numbers after 11 cycles, particularly in the PMN subset. Yet, eosinophil counts were increased in peripheral blood, a finding that was paralleled in lung lavage. These ozone-mediated shifts in leukocyte subpopulations were not maintained in animals that were allowed to mature to 1 yr of age under filtered air conditions; only blood and lavage monocytes increased in association with prior ozone exposure. Based on findings of enhanced monocyte frequency at 1 yr of age, we expected that stimulation of the innate immune system with a TLR ligand would elicit a differential effect in animals with prior ozone exposure history. Whereas the systemic and pulmonary immune system of animals with a history of postnatal ozone exposure contained more monocytes and therefore more cells expressing the receptor for LPS (TLR4), we instead found a diminution of both blood and lavage cell responses following inhaled LPS challenge compared with controls. Surprisingly, we also observed that the long-term effects of ozone were retained within the peripheral blood compartment, such that ex vivo LPS stimulation of PBMC from 1-yr-old animals with a prior history of exposure showed a significant reduction in IL-6 and IL-8 secretion compared with age-matched animals raised exclusively in filtered air.
In adult rhesus monkeys, acute ozone exposure (0.96 ppm/8 h) results in neutrophilic airways inflammation (30). Consistent with the reported inflammatory effects of ozone in rhesus monkeys, a recent investigation in adult cynomolgus monkeys also shows that PMNs are the major leukocyte population recruited into the lung following acute ozone exposure (1 ppm/6 h) (23). In the current study, we found that airways inflammation in 6-mo-old monkeys immediately following 5 mo of episodic exposure at 0.5 ppm/8 h is eosinophilic in phenotype. Furthermore, we also observed a significant increase in the eosinophil population within peripheral blood, indicating recent stimulation of bone marrow release. PMN were not only suppressed in the circulation of ozone-exposed infant monkeys, but also were found in minimal numbers in lavage. It is possible that the inflammatory response to chronic or episodic ozone exposure in nonhuman primates is generally eosinophilic in nature. However, 5-day exposure of 6-mo-old rhesus monkeys at 0.5 ppm/8 h shows a similar profile of airways eosinophilia without a PMN component (data not shown), therefore suggesting that this response is unique to young animals. Eosinophils may be the initial granulocyte response to ozone in young monkeys but could ultimately transition to a PMN granulocyte response as monkeys mature. Indeed, prolonged ozone exposure of the adult bonnet macaque monkey (0.5 ppm/8 h) for up to 90 days results in an airway inflammatory profile consisting of both PMNs and eosinophils (11). Furthermore, Hyde et al. (30) reports significant eosinophil influx within intrapulmonary mucosa of adult rhesus monkeys following acute exposure. Although adult human subjects develop a neutrophilic airways inflammatory response to experimental ozone exposure (reviewed in Ref. 27), the observation of eosinophil activation in young children living in regions of high ambient ozone levels suggests that our findings in infant monkeys may be comparable with that observed in the human population (14).
The effects of episodic ozone on peripheral blood and lung inflammation at 6 mo of age, as well as increased monocytes at 6 mo postexposure, demonstrate that ozone has both immediate and long-term effects on immunity. We do not know whether alterations of peripheral blood and lavage leukocyte populations in association with early life ozone persist beyond the developmental time points evaluated in this study; there are no comparable human studies reported in the literature. It can be speculated that elevated numbers of blood and airway monocytes are due to increased bone marrow recruitment, and there is evidence of enhanced pulmonary expression of hematopoietic cytokines and monocyte chemokines following acute ozone exposure (48, 63). Our finding of increased blood and airway monocytes in association with postnatal episodic ozone followed a long period of filtered air housing after the last exposure, which suggests the possibility that cellular sources for monocyte recruitment were modulated in an epigenetic fashion as one possible explanation.
The suppressive effect of ozone on the airway inflammatory response to LPS in this study is consistent with previous data obtained in mouse, rat, and rabbit models of microbial infection. Indeed, the detrimental impact of ozone exposure on host defense mechanisms in the lung was originally described over 50 years ago in an animal model of Klebsiella pneumoniae infection (42). Since this time, the role of ozone in susceptibility to infection by multiple microbial organisms as well as variable responsiveness by age, strain, and species has been documented by a number of investigators (19, 20, 54). Additionally, the sequence of exposures has a significant impact on the type of inflammatory response generated, such that preexposure to LPS results in an enhanced ozone-mediated cytokine response, whereas preexposure to ozone results in a suppressed LPS-mediated cytokine response (32). The primary mechanism for reduced bacterial clearance following acute ozone is via impairment of alveolar macrophage phagocytosis (19). In vitro exposure of human alveolar macrophages to ozone results in a similar reduction of phagocytosis, accompanied by reduced cytokine secretion when subsequently challenged with LPS (5). It is not known whether suppression of alveolar macrophage function by ozone translates to increased susceptibility to respiratory infections in the human population, but epidemiology does support increased hospital admissions for pneumonia and COPD in areas of high ambient ozone (51, 58). In this study, the observed attenuation of inflammatory responses to LPS challenge in young rhesus monkeys is similar to previously published effects of ozone on attenuated host defense mechanisms during microbial infection, but our data are the first to show an effect with episodic exposures. Comparatively, chronic ozone exposure negates the initial immunosuppressive effect of acute exposure on alveolar macrophages in a murine model (18). We were also able to discern a significant effect of ozone in circulating leukocyte populations, both before and after LPS challenge. Our data clearly show that the immunosuppressive effects of ozone are not limited to cells within the lung and that the ability of cells within the peripheral blood compartment to respond following LPS challenge is also negatively affected by prior exposure.
Recently, there has been growing support that one of the mechanisms by which ozone exposure may induce airway injury and hyperresponsiveness in rodent models is dependent on TLRs (25, 57). There are limited data in the human population regarding environmental effects on TLRs, however, a recent study by Gold et al. (21) provides strong evidence that birth seasonality has a significant effect on cord blood responses to innate immune ligands; children born in summer months showed reduced cytokine synthesis following stimulation of cord blood with various TLR ligands. Our data showing reduced responsiveness to LPS challenge in ozone-exposed rhesus monkeys supports a direct link between ozone and TLR4, although we do not yet know whether protein or gene expression of TLR4 has been affected. Acute ozone exposure can induce altered TLR4 distribution on murine alveolar macrophages, but cytokine responsiveness to LPS is enhanced in this model, suggesting an alternative response mechanism in primate species (28). Alterations of TLR4 responsiveness are evident in other respiratory diseases, such as cystic fibrosis, suggesting that this pathway is a particularly vulnerable target for modulation (31). How ozone can directly affect the function and/or expression of TLR4 and other surface receptors remains poorly understood. It is possible that ozone can affect cellular signaling via modification of lipid products derived from epithelial membranes (reviewed in Ref. 27). An important question remains as to how oxidative stress events on the surface of airways can transmit a TLR4-modulating response within peripheral blood, as observed in our study. Alveolar macrophages are a likely immune cell target for ozone inhalation, but there is currently no evidence to support migratory efflux of this leukocyte population from the lung back into the systemic circulation.
Maternal exposures to air pollutants (diesel exhaust particles, residual fly ash, and particulate matter) can prime the postnatal airway response to a secondary antigenic or oxidative stress challenge (2, 12, 22). Our study is the first to experimentally demonstrate a persistent suppressive effect of early life exposure to air pollutants on innate immunity. As with the cellular mechanisms of ozone-mediated receptor modification, the molecular pathways that maintain persistence of an aberrant response, independent of genetic polymorphism, are unclear. In a similar system, challenge of postnatal rats with LPS can subsequently attenuate the systemic cytokine LPS response in adult rats (10). It has been proposed that the early life effects on LPS responsiveness are dependent on reprogramming of the neuroimmune axis, such that a reduction of plasma corticosterone levels via specific inhibition of COX-2 activity prevented alteration of adult immune responses (43). However, we were able to elicit differential responsiveness to LPS stimulation in peripheral blood cell in vitro, independent of neural stimulation, suggesting that the persistent effects of postnatal ozone in monkeys are inherent to TLR4 and/or associated signaling pathways.
Another unexpected finding in this study is that the effect of postnatal ozone on the LPS response is maintained within the peripheral blood compartment for at least 6 mo after the exposure period. We do not know what immune cell type is responsible for the deficient response to LPS in monkeys, but it is unlikely to be a granulocyte as we used cryopreserved cells for our in vitro stimulation assays. The monocyte may be a good candidate, as it can serve as the precursor for the alveolar macrophage response; future studies will investigate the phagocytic properties of the macrophage population in 1-yr-old monkeys from this study. The rate of turnover for rhesus monkey monocytes in the circulation is unknown, but human monocytes have a half-life of 3 days (56); as such, it is likely that a bone marrow-derived population has been persistently affected. A recent investigation using a human monocyte leukemic cell line to investigate mechanisms of LPS tolerance clearly shows that epigenetic regulation of an environmental challenge relies on a complex strategy of both transcriptional and translational silencing of cytokine mRNA (9). Epigenetic regulation of ozone has yet to be defined, although studies with inbred strains of mice suggest that parental imprinting can transmit enhanced susceptibility to ozone injury (46).
It should be noted that the infant monkey population evaluated in our study was limited to males, which may have an impact on experimental outcomes. The rationale for focusing exclusively on males in this investigation is based on epidemiology, which suggests that male children are more susceptible to the development of lung disease, particularly respiratory distress syndrome and asthma (reviewed in Ref. 6). During early childhood lung development, surfactant production is delayed in male neonates (13), and the normal growth pattern of the male lung results in narrowing of the airways compared with the female lung (24); these physiological factors may contribute to deposition of inhaled pollutants, which can affect the severity of the inflammatory response. In addition, studies in rhesus macaques show that females have significantly higher numbers of circulating lymphocytes and monocytes, which could also impact on the immune response to environment (59). We expect that future studies will expand our analysis to females to experimentally address the influence of sex on immune response to air pollutant exposure.
In summary, using an infant rhesus monkey model, our findings indicate that chronic inhalation of ozone during the postnatal growth period results in a persistent attenuation of both pulmonary and systemic innate immune responses to LPS later in life. To the best of our knowledge, this study is the first to experimentally demonstrate that the molecular pathways that respond to oxidative stress retain memory, such that cells within the lung and peripheral blood compartments no longer yield a robust inflammatory response to a stimulus of innate immunity. We do not yet know whether these early life environmental perturbations are prolonged beyond the time points evaluated in this study. It will also be important to establish whether airway challenge by other TLR ligands is sensitive to postnatal ozone exposure; epidemiology and animal models suggest a link between ozone and virus infections (reviewed in Ref. 7). These studies, along with future experiments evaluating the impact of early life exposures on the adaptive immune response to microbial challenges, will provide important mechanistic support for the long-term detrimental effects of air pollution on respiratory disease.
GRANTS
This study was supported by National Institutes of Health Grants P01-ES-011617, P01-ES-000628, R01-HL-081286, R01-HL-097087, P51-RR-000169, and T32-HL-007013.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the contributions of Sarah Davis, Paul-Michael Sosa, Sona Santos, Joan Gerriets, Rebbeca Fishman, Justin Fontaine, Lei Putney, Louise Olsen, and Brian Tarkington for technical support. We thank Dr. Candice Clay for expert editorial assistance during the preparation of this manuscript.
REFERENCES
- 1. Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001–2004. Environ Res 110: 294–301, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Auten RL, Potts EN, Mason SN, Fischer B, Huang Y, Foster WM. Maternal exposure to particulate matter increases postnatal ozone-induced airway hyperreactivity in juvenile mice. Am J Respir Crit Care Med 180: 1218–1226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Balinotti JE, Tiller CJ, Llapur CJ, Jones MH, Kimmel RN, Coates CE, Katz BP, Nguyen JT, Tepper RS. Growth of the lung parenchyma early in life. Am J Respir Crit Care Med 179: 134–137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bateson TF, Schwartz J. Children's response to air pollutants. J Toxicol Environ Health A 71: 238–243, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Becker S, Madden MC, Newman SL, Devlin RB, Koren HS. Modulation of human alveolar macrophage properties by ozone exposure in vitro. Toxicol Appl Pharmacol 110: 403–415, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, Zeldin DC. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18: 308–313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol 19: 1135–1146, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, Hooijkaas H, van Dongen JJ. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 130: 388–393, 1997 [DOI] [PubMed] [Google Scholar]
- 9. El Gazzar M, McCall CE. MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J Biol Chem 285: 20940–20951, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J 19: 1519–1521, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Eustis SL, Schwartz LW, Kosch PC, Dungworth DL. Chronic bronchiolitis in nonhuman primates after prolonged ozone exposure. Am J Pathol 105: 121–137, 1981 [PMC free article] [PubMed] [Google Scholar]
- 12. Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol 38: 57–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleisher B, Kulovich MV, Hallman M, Gluck L. Lung profile: sex differences in normal pregnancy. Obstet Gynecol 66: 327–330, 1985 [PubMed] [Google Scholar]
- 14. Frischer T, Studnicka M, Halmerbauer G, Horak F, Jr, Gartner C, Tauber E, Koller DY. Ambient ozone exposure is associated with eosinophil activation in healthy children. Clin Exp Allergy 31: 1213–1219, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 351: 1057–1067, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, Lurmann F, Avol E, Kunzli N, Jerrett M, Peters J. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 369: 571–577, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gilmour MI, Hmieleski RR, Stafford EA, Jakab GJ. Suppression and recovery of the alveolar macrophage phagocytic system during continuous exposure to 0.5 ppm ozone. Exp Lung Res 17: 547–558, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Gilmour MI, Park P, Doerfler D, Selgrade MK. Factors that influence the suppression of pulmonary antibacterial defenses in mice exposed to ozone. Exp Lung Res 19: 299–314, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Gilmour MI, Park P, Selgrade MK. Ozone-enhanced pulmonary infection with Streptococcus zooepidemicus in mice. The role of alveolar macrophage function and capsular virulence factors. Am Rev Respir Dis 147: 753–760, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Gold DR, Bloomberg GR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, O'Connor GT, Wood RA, Burger MS, Wright RJ, Witter F, Lee-Parritz A, Sperling R, Sadovsky Y, Togias A, Gern JE. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol 124: 1078–1087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, Kimura H. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A 70: 688–695, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Hicks A, Kourteva G, Hilton H, Li H, Lin TA, Liao W, Li Y, Wei X, March T, Benson J, Renzetti LM. Cellular and molecular characterization of ozone-induced pulmonary inflammation in the cynomolgus monkey. Inflammation 33: 144–156, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Hoffstein V. Relationship between lung volume, maximal expiratory flow, forced expiratory volume in one second, and tracheal area in normal men and women. Am Rev Respir Dis 134: 956–961, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Hollingsworth JW, 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med 170: 126–132, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hollingsworth JW, Free ME, Li Z, Andrews LN, Nakano H, Cook D, N Ozone activates pulmonary dendritic cells and promotes allergic sensitization through a Toll-like receptor 4-dependent mechanism. J Allergy Clin Immunol 125: 1167–1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc 4: 240–246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, Fong A, Foster WM, Schwartz DA. Ambient ozone primes pulmonary innate immunity in mice. J Immunol 179: 4367–4375, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy 55: 688–697, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Hyde DM, Hubbard WC, Wong V, Wu R, Pinkerton K, Plopper CG. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol 6: 481–497, 1992 [DOI] [PubMed] [Google Scholar]
- 31. John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol 42: 424–431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnston CJ, Holm BA, Finkelstein JN. Sequential exposures to ozone and lipopolysaccharide in postnatal lung enhance or inhibit cytokine responses. Exp Lung Res 31: 431–447, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, Eleff SM, DiSilvestre D, Holroyd KJ. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet 17: 475–478, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Koike E, Kobayashi T. Ozone exposure enhances antigen-presenting activity of interstitial lung cells in rats. Toxicology 196: 217–227, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Kopp MV, Bohnet W, Frischer T, Ulmer C, Studnicka M, Ihorst G, Gardner C, Forster J, Urbanek R, Kuehr J. Effects of ambient ozone on lung function in children over a two-summer period. Eur Respir J 16: 893–900, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Kopp MV, Ulmer C, Ihorst G, Seydewitz HH, Frischer T, Forster J, Kuehr J. Upper airway inflammation in children exposed to ambient ozone and potential signs of adaptation. Eur Respir J 14: 854–861, 1999 [DOI] [PubMed] [Google Scholar]
- 37. La Pine TR, Joyner JL, Augustine NH, Kwak SD, Hill HR. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B Streptococci. Pediatr Res 54: 276–281, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, Hazucha MJ, Devlin RB, Peden DB. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol 120: 719–722, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Lin S, Liu X, Le LH, Hwang SA. Chronic exposure to ambient ozone and asthma hospital admissions among children. Environ Health Perspect 116: 1725–1730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Miller LA, Gerriets JE, Tyler NK, Abel K, Schelegle ES, Plopper CG, Hyde DM. Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys. Toxicol Appl Pharmacol 236: 39–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller S, Ehrlich R. Susceptibility to respiratory infections of animals exposed to ozone. I. Susceptibility to Klebsiella pneumoniae. J Infect Dis 103: 145–149, 1958 [DOI] [PubMed] [Google Scholar]
- 43. Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J Neurosci 30: 7975–7983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLos One 5: e10407, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parker JD, Akinbami LJ, Woodruff TJ. Air pollution and childhood respiratory allergies in the United States. Environ Health Perspect 117: 140–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prows DR, Shertzer HG, Daly MJ, Sidman CL, Leikauf GD. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat Genet 17: 471–474, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, McDonnell W, Loomis D, Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med 176: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Rusznak C, Devalia J, Sapsford R, Davies R. Ozone-induced mediator release from human bronchial epithelial cells in vitro and the influence of nedocromil sodium. Eur Respir J 9: 2298–2305, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Schaub B, Bellou A, Gibbons FK, Velasco G, Campo M, He H, Liang Y, Gillman MW, Gold D, Weiss ST, Perkins DL, Finn PW. TLR2 and TLR4 stimulation differentially induce cytokine secretion in human neonatal, adult, and murine mononuclear cells. J Interferon Cytokine Res 24: 543–552, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, Baker GL, Pantle LM, Joad JP, Pinkerton KE, Wu R, Evans MJ, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol 191: 74–85, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Schwartz J. PM10, ozone, and hospital admissions for the elderly in Minneapolis-St. Paul, Minnesota. Arch Environ Health 49: 366–374, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 164: 602–607, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Thomas GB, Fenters JD, Ehrlich R, Gardner DE. Effects of exposure to ozone on susceptibility to experimental tuberculosis. Toxicol Lett 9: 11–17, 1981 [DOI] [PubMed] [Google Scholar]
- 54. Van Loveren H, Rombout PJ, Wagenaar SS, Walvoort HC, Vos JG. Effects of ozone on the defense to a respiratory Listeria monocytogenes infection in the rat. Suppression of macrophage function and cellular immunity and aggravation of histopathology in lung and liver during infection. Toxicol Appl Pharmacol 94: 374–393, 1988 [DOI] [PubMed] [Google Scholar]
- 55. Verhein KC, Jacoby DB, Fryer AD. IL-1 receptors mediate persistent, but not acute, airway hyperreactivity to ozone in guinea pigs. Am J Respir Cell Mol Biol 39: 730–738, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Whitelaw DM. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet 5: 311–317, 1972 [DOI] [PubMed] [Google Scholar]
- 57. Williams AS, Leung SY, Nath P, Khorasani NM, Bhavsar P, Issa R, Mitchell JA, Adcock IM, Chung KF. Role of TLR2, TLR4, and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. J Appl Physiol 103: 1189–1195, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Wong TW, Lau TS, Yu TS, Neller A, Wong SL, Tam W, Pang SW. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup Environ Med 56: 679–683, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xia HJ, Zhang GH, Wang RR, Zheng YT. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cell Mol Immunol 6: 433–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yerkovich ST, Wikstrom ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr Res 62: 547–552, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol 289: L627–L635, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respir Physiol 67: 269–282, 1987 [DOI] [PubMed] [Google Scholar]
- 63. Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol Lung Cell Mol Physiol 274: L39–L46, 1998 [DOI] [PubMed] [Google Scholar]