Abstract
Phospholemman (PLM), when phosphorylated at serine 68, relieves its inhibition on Na+-K+-ATPase but inhibits Na+/Ca2+ exchanger 1 (NCX1) in cardiac myocytes. Under stress when catecholamine levels are high, enhanced Na+-K+-ATPase activity by phosphorylated PLM attenuates intracellular Na+ concentration ([Na+]i) overload. To evaluate the effects of PLM on NCX1 on in vivo cardiac contractility, we injected recombinant adeno-associated virus (serotype 9) expressing either the phosphomimetic PLM S68E mutant or green fluorescent protein (GFP) directly into left ventricles (LVs) of PLM-knockout (KO) mice. Five weeks after virus injection, ∼40% of isolated LV myocytes exhibited GFP fluorescence. Expression of S68E mutant was confirmed with PLM antibody. There were no differences in protein levels of α1- and α2-subunits of Na+-K+-ATPase, NCX1, and sarco(endo)plasmic reticulum Ca2+-ATPase between KO-GFP and KO-S68E LV homogenates. Compared with KO-GFP myocytes, Na+/Ca2+ exchange current was suppressed, but resting [Na+]i, Na+-K+-ATPase current, and action potential amplitudes were similar in KO-S68E myocytes. Resting membrane potential was slightly lower and action potential duration at 90% repolarization (APD90) was shortened in KO-S68E myocytes. Isoproterenol (Iso; 1 μM) increased APD90 in both groups of myocytes. After Iso, [Na+]i increased monotonically in paced (2 Hz) KO-GFP but reached a plateau in KO-S68E myocytes. Both systolic and diastolic [Ca2+]i were higher in Iso-stimulated KO-S68E myocytes paced at 2 Hz. Echocardiography demonstrated similar resting heart rate, ejection fraction, and LV mass between KO-GFP and KO-S68E mice. In vivo closed-chest catheterization demonstrated enhanced contractility in KO-S68E compared with KO-GFP hearts stimulated with Iso. We conclude that under catecholamine stress when [Na+]i is high, PLM minimizes [Na+]i overload by relieving its inhibition of Na+-K+-ATPase and preserves inotropy by simultaneously inhibiting Na+/Ca2+ exchanger.
Keywords: FXYD1, cardiac contractility, intracellular Na+ and Ca2+ regulation, sodium-binding benzofuran isophthalate, recombinant adeno-associated virus-mediated gene transfer
phospholemman (PLM), a 72-amino acid phosphoprotein with a single transmembrane domain (14), is the founding member of the FXYD family of regulators of ion transport (23). It is highly expressed in the heart (16). When phosphorylated at serine 68 by protein kinase A, PLM relieves its inhibition on Na+-K+-ATPase (8, 26) but suppresses Na+/Ca2+ exchanger (NCX1) (28). In isolated cardiac myocytes, PLM regulates contractility via its effects on either Na+-K+-ATPase (9, 26) or NCX1 (21), depending on experimental manipulations.
Since both Na+-K+-ATPase and NCX1 regulate intracellular Ca2+ concentration ([Ca2+]i) on a beat-to-beat basis (5), the effects of PLM on cardiac contractility in vivo are complex, controversial, and difficult to predict. Under basal conditions, PLM-knockout (KO) hearts contract just as well, if not better, than wild-type (WT) hearts (3, 10, 26). This is inconsistent with the hypothesis that with loss of inhibition of Na+-K+-ATPase (3, 8, 21, 26), PLM-KO hearts should exhibit lower contractility compared with WT hearts. On the other hand, when subjected to catecholamine stress resulting in high intracellular Na+ concentration ([Na+]i), WT but not PLM-KO hearts suffer a time-dependent decline in contractility (26). Despa et al. (9) proposed that in WT hearts relief of inhibition of Na+-K+-ATPase by phosphorylated PLM lowers [Na+]i. Ca2+ efflux via NCX1 is favored, resulting in reduced contractility. The role of NCX1 inhibition by phosphorylated PLM in regulation of cardiac contractility in vivo has not been elucidated.
Here, we used congenic PLM-KO hearts expressing the phosphomimetic PLM S68E mutant and green fluorescent protein (GFP) via recombinant adeno-associated virus serotype 9 (rAAV9)-mediated gene transfer. The PLM S68E mutant strongly inhibits NCX1 but has no effects on Na+-K+-ATPase (21, 28). In addition, isoproterenol (Iso) has no effects on Na+-K+-ATPase in PLM-KO myocytes (8, 26). Our experimental design allowed us to examine the effects of inhibition of NCX1 by phosphorylated PLM on cardiac contractility in vivo, without confounding influences from changes in Na+-K+-ATPase activity.
METHODS
Generation of PLM-deficient mice and animal care.
PLM-KO mice backcrossed to a pure congenic C57BL/6 background were generated as described previously (10, 24). Homozygous adult littermates ∼3 mo old were used. Mice were housed and fed on a 12:12-h light-dark cycle in the Thomas Jefferson University Animal Facility supervised by veterinary staff members. Standard care was provided to all mice used for experiments. All protocols applied to the mice in this study were approved and supervised by the Institutional Animal Care and Use Committees at Thomas Jefferson University and the University of Virginia.
Construction of rAAV9-S68E.
The coding sequence of canine cardiac PLM S68E mutant (279 bp) together with 5′-untranslated (60 bp) and 3′-untranslated (200 bp) sequences were released from pAdTrack by PCR. The 539-bp fragment was then subcloned into pTRαCARD vector with NheI and BsrGI restriction sites. Fidelity of the clone was confirmed by sequencing.
Viruses were produced by the triple transfection method with HEK293 cells (15). Cells were seeded at 1 × 107 cells per 15-cm plate the day before polyethyleneimine (PEI)-mediated transfection. Plasmids were added to serum-free Dulbecco's minimal essential medium (DMEM) in the following amounts per 15-cm plate: 7 μg of pTRαCARD S68E, 10 μg of pXR9, and 10 μg of pXX680, with a final volume of 650 μl. Then 100 μl of PEI (1 mg/ml, pH 7.4) was added and incubated for 10 min at room temperature. PEI-DNA complexes were added dropwise to cells growing in complete medium. Seventy-two hours after transfection, cells were collected by centrifugation and the cell pellet was resuspended in 10 ml of 10 mM phosphate buffer with 1 mM MgCl2 on ice. Leupeptin (10 mg/ml) was added to the resuspended cells (5 μl per 15-cm plate equivalent). Resuspended cells were sonicated (45 × 1-s pulses at 26% amplitude) on ice, and DNase I (Roche) was added to a final concentration of 25 U/plate and incubated at 37°C for 60 min.
After DNase I treatment, NaCl was adjusted to 0.5 M and cell debris was removed by centrifugation (2,500 g, 20 min, 4°C). Virus was precipitated from solution with PEG-8000 (final concentration 8%; Fisher) and 0.5 M NaCl on ice for 1 h, followed by centrifugation (2,500 g, 20 min, 4°C). The PEG pellet was resuspended in 0.5 M NaCl containing 10 mM sodium phosphate (pH 7.4) and briefly sonicated to disrupt the pellet. Sarkosyl (1%) was added, and the disrupted pellet was incubated with gentle shaking for 1 h. Insoluble material was removed by centrifugation (2,500 g, 20 min, 4°C). The supernatant was transferred to a 14 × 89-mm ultracentrifuge tube underlaid with 5 ml of 1.3 g/cm3 CsCl and 2 ml of 1.5 g/cm3 CsCl (both in 10 mM sodium phosphate, pH 7.4). Gradients were spun at 36,000 rpm and 18°C with a SW41 rotor (Beckman) for 20 h with no brake. The virus-containing fraction was removed by puncturing the side wall of the tube with an 18-gauge needle removing the lower most band. The extracted band was resuspended in 1.4 g/cm3 CsCl in 10 mM sodium phosphate (pH 7.4, final volume 13.2 ml) and transferred to a 16 × 76-mm Quick Seal tube (Beckman). Gradients were spun in a Ti70.1 rotor (Beckman) at 60,000 rpm and 18°C for 20 h with no brake. Gradients were fractionated from the bottom, and peak fractions were selected by refractive indexes encompassing a density of 1.42 g/cm3. Peak fractions were dialyzed against 10 mM sodium phosphate (pH 7.4), 180 mM NaCl, 0.001% Pluronic F68b, and aliquots were frozen at −80°C.
In vivo rAAV9-mediated gene transfer.
After the skin was cleaned with Betadine solution, the left chest of an anesthetized (2% inhaled isoflurane) PLM-KO mouse was opened, the heart was exteriorized, and 25 μl (total volume) of rAAV9-GFP or rAAV9-S68E (∼5 × 1011 particles) was directly injected into the anterior and posterior left ventricular (LV) wall and the apex. The heart was returned to the chest cavity and the wound sutured. The entire surgical procedure took <45 s. Typically >95% of animals survived the procedure. Survivors were allowed to recover for 5–6 wk before echocardiography, hemodynamic measurement, and heart excision. For the sake of brevity, PLM-KO mice (as well as hearts and myocytes derived from LV) injected with rAAV9-GFP or rAAV9-S68E are referred to as KO-GFP or KO-S68E mice/hearts/myocytes, respectively.
Echocardiographic and hemodynamic analyses of cardiac function.
Transthoracic two-dimensional echocardiography was performed in anesthetized (2% inhaled isoflurane) KO-GFP or KO-S68E mice with a 12-MHz probe as previously described (25, 26). For in vivo hemodynamic measurements, a 1.4-Fr micromanometer-tipped catheter (SPR-671, Millar Instruments) was inserted into the right carotid artery and advanced into the LV of lightly anesthetized [tribromoethanol-amylene hydrate (Avertin); 2.5% wt/vol, 8 μl/g ip] mice with spontaneous respirations placed on a heated (37°C) pad (25, 26). Hemodynamic parameters including heart rate (beats/min), LV end-diastolic pressure (LVEDP), and maximal first time derivative of LV pressure rise (+dP/dt) and fall (−dP/dt) were recorded in closed-chest mode, both at baseline and in response to increasing doses of Iso (0.1, 0.5, 1, 5, and 10 ng) (25, 26).
Isolation of adult murine cardiac myocytes.
Cardiac myocytes were isolated from the LV free wall and septum of KO-GFP and KO-S68E mice according to the protocol of Zhou et al. (33) and modified by us (21, 24–26). In all experiments, myocytes were used within 2–8 h of isolation, except for [Na+]i measurements, in which myocytes cultured for 18 h were used (21, 26). In all single-myocyte studies, rAAV9-infected myocytes were first identified by GFP fluorescence (excitation 475 ± 20 nm, emission 535 ± 22 nm) before measurements.
Myocyte shortening measurements.
Myocytes adherent to coverslips were bathed in 0.6 ml of air- and temperature-equilibrated (37°C), HEPES-buffered (20 mM, pH 7.4) medium 199 containing 1.8 mM extracellular Ca2+ concentration [Ca2+]o. Measurements of myocyte contraction (2 Hz) were performed as previously described (21, 24–26).
[Ca2+]i transient measurements.
Fura-2-loaded (0.67 μM fura-2 AM, 15 min, 37°C) myocytes were field stimulated to contract (2 Hz, 37°C) in medium 199 containing 1.8 mM [Ca2+]o. [Ca2+]i transient measurements, daily calibration of fura-2 fluorescent signals, and [Ca2+]i transient analyses were performed as previously described (21, 24–26).
Since both myocyte shortening and [Ca2+]i transients in myocytes paced at 2 Hz reached steady-state values in <2 min, Iso (1 μM) was routinely added 2 min after initiation of pacing.
[Na+]i measurements.
Resting [Na+]i was measured in sodium-binding benzofuran isophthalate (SBFI)-loaded [10 μM SBFI AM with 0.05% (wt/vol) Pluronic F127, 2 h, 37°C] myocytes. Myocytes were then field stimulated to contract (2 Hz, 37°C) in Tyrode solution. Measurement and calibration of SBFI signals were performed as previously described (26).
Electrophysiological measurements.
Na+-K+-ATPase current (Ipump) at pipette Na+ concentrations ([Na+]pip) of both 10 and 80 mM (21, 30), Na+/Ca2+ exchange current (INaCa) (21, 25, 28), and action potential (1 Hz) (24, 25) were measured in successfully infected myocytes (30°C) with whole cell patch clamp as previously described. In a separate series of experiments, action potential parameters (1 Hz, 30°C) were measured in successfully infected myocytes both before and 2 min after addition of Iso (1 μM).
Immunoblotting.
LV homogenates were prepared as previously described (24). For detection of PLM S68E mutant (12% SDS-PAGE, reducing conditions with 5% β-mercaptoethanol), either monoclonal B8 (1:10,000) or polyclonal C2 (1:10,000) antibody (19) was used. For detection of α1- and α2-subunits of Na+-K+-ATPase, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2), calsequestrin (7.5% SDS-PAGE, reducing conditions), and Na+/Ca2+ exchanger (7.5% SDS-PAGE, nonreducing conditions with 10 mM N-ethylmaleimide), commercially available antibodies were used as previously described (21, 24–26).
Statistics.
All results are expressed as means ± SE. For analysis of Ipump as a function of group (KO-GFP vs. KO-S68E), [Na+]pip, and Iso, three-way ANOVA was used. For analysis of INaCa as a function of group and voltage, in vivo hemodynamic parameters as a function of group and Iso, and action potential parameters as a function of group and Iso, two-way ANOVA was used. For analysis of echocardiographic parameters, contraction and [Ca2+]i transient amplitudes, [Na+]i, [Ca2+]i, action potential parameters, and protein abundance, one-way ANOVA was used. A commercially available software package (JMP version 7, SAS Institute, Cary, NC) was used. In all analyses, P < 0.05 was taken to be statistically significant.
RESULTS
rAAV9-mediated gene transfer.
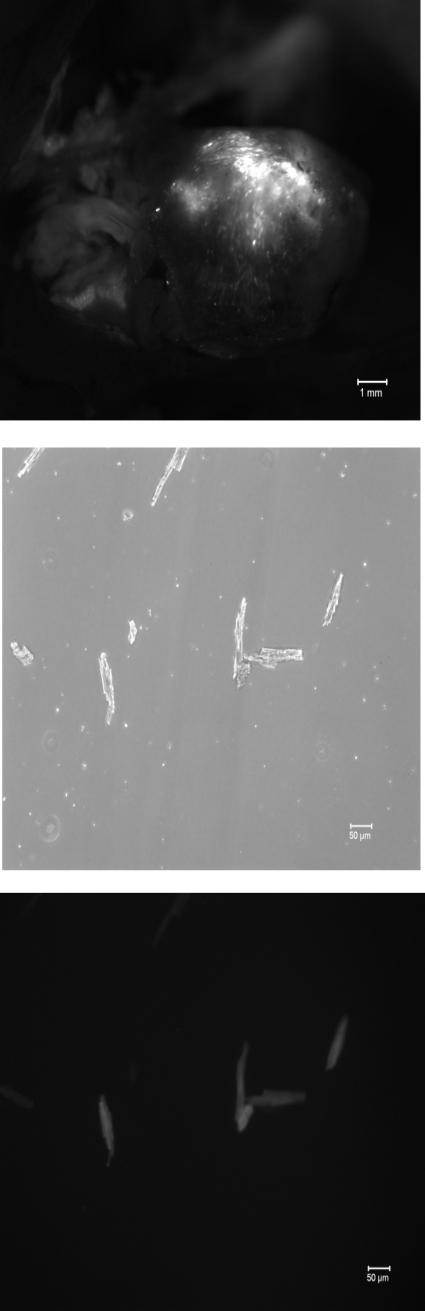
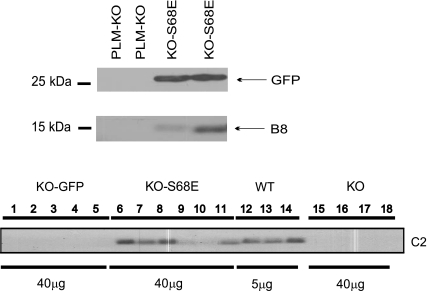
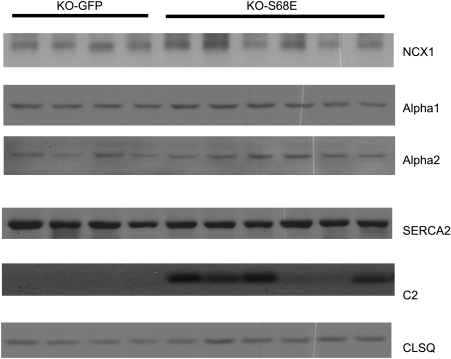
In myocytes infected with rAAV9, expression of GFP is driven by the cytomegalovirus (CMV) promoter and that of the S68E mutant is driven by the α-cardiac actin enhancer/EF1α promoter. Therefore, the S68E mutant is not “tagged” with GFP and is expected to have molecular mass similar to WT PLM. Five weeks after direct LV injection with rAAV9-GFP or rAAV9-S68E, significant areas of LV fluoresced green (Fig. 1, top), indicating successful virus-mediated gene transfer. Approximately 40% of myocytes isolated from rAAV9-injected hearts exhibited GFP fluorescence (Fig. 1, middle and bottom). This is an underestimate of the efficiency of rAAV9-mediated gene transfer by direct injection since the noninjected septum and areas of LV not injected were included in the myocyte isolation procedure. Using B8 [recognizes the NH2 terminus of dog but not rat (20) and mouse PLM (Fig. 2)] and GFP antibodies, we confirmed expression of both the dog PLM S68E mutant and GFP in PLM-KO hearts previously injected with rAAV9-S68E (Fig. 2, top). As expected, control PLM-KO hearts injected with saline demonstrated an absence of both GFP and endogenous PLM (Fig. 2). With C2 antibody, which recognizes the COOH termini of dog, rat, mouse, rabbit, pig, and human PLM (1, 6, 19, 20, 24), and assuming equal reactivity of C2 antibody against mouse PLM and dog S68E mutant, the level of expression of S68E mutant in injected PLM-KO hearts was lower than the level of endogenous PLM measured in WT hearts (Fig. 2, bottom). This observation indicates that the level of S68E expression was not supraphysiological and unlikely to distort the normal stoichiometry of interaction between PLM and NCX1 and between PLM and Na+-K+-ATPase. There were no differences in protein levels of NCX1, α1- and α2-subunits of Na+-K+-ATPase, SERCA2, and calsequestrin between KO-GFP and KO-S68E LV homogenates (Fig. 3, Table 1).
Fig. 1.
Recombinant adeno-associated virus serotype 9 (rAAV9)-mediated gene transfer in phospholemman (PLM)-knockout (KO) hearts. Top: 5 wk after injection of rAAV9-green fluorescent protein (GFP) or rAAV9-S68E into hearts of PLM-KO mice (methods), a significant area of the left ventricle (LV) exhibited green fluorescence, indicating GFP expression. Middle: transmitted light image of myocytes isolated from LV + septum of rAAV9-infected PLM-KO hearts. Bottom: fluorescent image of middle image demonstrating GFP expression in ∼50% of isolated myocytes.
Fig. 2.
rAAV9-mediated expression of GFP and PLM S68E mutant in PLM-KO hearts. Top: 5 wk after rAAV9-S68E or saline injection, LV homogenates were prepared and subjected to SDS-PAGE followed by Western blot analysis. GFP and PLM S68E mutant (detected with B8 antibody) were present in rAAV9-S68E- but not saline-injected PLM-KO hearts. Bottom: PLM or PLM S68E mutant (identified with C2 antibody) was present in wild-type (WT) and KO-S68E LV homogenates, respectively, while no C2 signal was detected in KO-GFP or PLM-KO (injected with saline) hearts. Note the differences in amount of protein loaded for WT (5 μg) and KO (40 μg) LV homogenates. Note also that WT PLM and PLM S68E mutant have similar molecular masses since expressions of GFP and PLM S68E mutant were driven by 2 separate promoters.
Fig. 3.
rAAV9-mediated S68E expression does not change the expression of selected proteins involved in excitation-contraction coupling. LV homogenates were prepared from PLM-KO hearts injected 5 wk earlier with rAAV9-GFP or rAAV9-S68E and subjected to SDS-PAGE followed by Western blot analysis. Primary antibodies (methods) were used to detect cardiac Na+/Ca2+ exchanger (NCX1), α1- and α2-subunits of Na+-K+-ATPase, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2, phospholemman S68E mutant (C2) and calsequestrin (CLSQ). Composite results are shown in Table 1.
Table 1.
Effects of rAAV9-mediated S68E expression on levels of selected proteins
| KO-GFP | KO-S68E | |
|---|---|---|
| NCX1 | 84.4 ± 1.0 (4) | 77.1 ± 0.4 (6) |
| SERCA2 | 107.0 ± 6.4 | 96.3 ± 3.9 |
| α1, Na+-K+-ATPase | 72.4 ± 6.3 | 63.8 ± 3.1 |
| α2, Na+-K+-ATPase | 66.3 ± 5.7 | 56.2 ± 4.9 |
| PLM S68E (C2) | 0 | 14.4 ± 1.8 |
| Calsequestrin | 62.1 ± 10.2 | 61.1 ± 0.3 |
Values (in arbitrary units) are means ± SE. Numbers in parentheses are numbers of phospholemman (PLM)-knockout (KO) hearts injected with recombinant adeno-associated virus, serotype 9 (rAAV9) expressing either green fluorescent protein (GFP) or PLM S68E mutant. NCX1, cardiac Na+/Ca2+ exchanger; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase; PLM S68E (C2), PLM S68E mutant as detected by C2 antibody.
Effects of rAAV9-mediated S68E expression on in vivo cardiac performance.
By echocardiography, there were no differences in body weight, heart rate, ejection fraction, fractional shortening, LV mass, stroke volume, and cardiac output between KO-GFP and KO-S68E mice under resting conditions (Table 2). Representative raw tracings of hemodynamic response to escalating doses of Iso in catheterized, closed-chest KO-GFP and KO-S68E mice are shown in Fig. 4. Both groups demonstrated similar time courses of contractile response in response to Iso. Unlike WT hearts, in which there was a time-dependent decline in maximal +dP/dt (26), both KO-GFP and KO-S68E hearts maintained maximal +dP/dt after addition of 10 ng of Iso. Compared with KO-GFP hearts, KO-S68E hearts demonstrated significantly higher +dP/dt both at baseline and when stimulated with increasing doses of Iso (Fig. 4 and Table 2; group effect, P < 0.047, Iso effect, P < 0.0001, group × Iso interaction effect, P > 0.98). Similarly, −dP/dt was higher in KO-S68E hearts both in the presence and absence of Iso (Table 2; group effect, P < 0.0016; Iso effect, P < 0.0001; group × Iso interaction effect, P < 0.13).
Table 2.
In vivo cardiac performance of KO-GFP and KO-S68E mice
| KO-GFP | KO-S68E | |
|---|---|---|
| Echocardiography | ||
| Body weight, g | 28.0 ± 0.6 (5) | 28.0 ± 0.6 (7) |
| LVIDD, mm | 3.55 ± 0.16 | 3.40 ± 0.12 |
| LVIDS, mm | 2.09 ± 0.13 | 1.92 ± 0.09 |
| Ant, mm | 0.69 ± 0.01 | 0.71 ± 0.02 |
| PWT, mm | 0.74 ± 0.01 | 0.72 ± 0.02 |
| LV mass, mg | 67.2 ± 5.4 | 62.5 ± 5.0 |
| Heart rate, beats/min | 468 ± 19 | 464 ± 15 |
| Ejection fraction, % | 72.6 ± 2.2 | 75.6 ± 1.0 |
| Fractional shortening, % | 41.0 ± 1.8 | 43.4 ± 0.8 |
| Stroke volume, μl | 38.5 ± 4.0 | 36.1 ± 2.8 |
| Cardiac output, ml/min | 18.0 ± 2.0 | 16.6 ± 1.1 |
| In vivo catheterization | ||
| +dP/dt, mmHg/s | 6,983 ± 280 (5) | 7,584 ± 398* (6) |
| Max +dP/dt, mmHg/s | 13,795 ± 480 | 14,546 ± 600* |
| −dP/dt, mmHg/s | 7,219 ± 306 | 7,387 ± 275† |
| Max −dP/dt, mmHg/s | 10,185 ± 156 | 11,565 ± 951† |
Values are means ± SE. Numbers in parentheses are numbers of mice. KO, PLM knockout; S68E, PLM S68E mutant; LV, left ventricle; LVIDD, LV internal dimension at end-diastole; LVIDS, LV internal dimension at end-systole; Ant, anterior wall thickness; PWT, posterior wall thickness; +dP/dt, −dP/dt, 1st time derivatives of LV pressure rise and fall. LV mass is given by the formula LV mass = 1.04[(Ant + LVIDD + PWT)3 − (LVIDD)3]. Maximal +dP/dt and maximal −dP/dt are peak hemodynamic responses after 10 ng isoproterenol infusion.
P < 0.047,
P < 0.002, KO-GFP vs. KO-S68E.
Fig. 4.
rAAV9-mediated S68E expression enhances contractility response to isoproterenol (Iso) in PLM-KO hearts in vivo. In vivo catheterization was performed in anesthetized mice (methods), and maximal 1st time derivatives of LV pressure rise (+dP/dt) and fall (−dP/dt) and heart rate were continuously monitored, both at baseline and with increasing doses of Iso. A and B: representative original tracings of dP/dt in KO-GFP (A) and KO-S68E (B) mice. Arrows indicate addition of escalating doses of Iso (ng). C: averaged maximal +dP/dt achieved with each dose of Iso in 5 KO-GFP (●) and 6 KO-S68E (○) mice. Error bars are not shown if they fall within the boundaries of the symbol. Composite results are shown in Table 2.
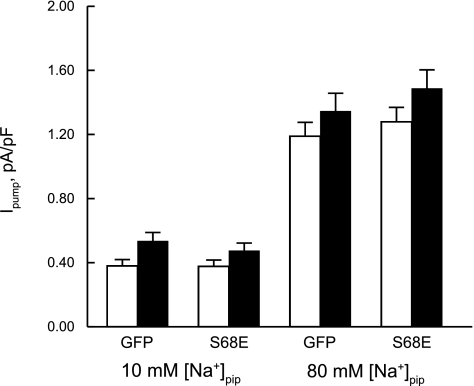
Effects of rAAV9-mediated S68E expression on INaCa and Ipump in PLM-KO myocytes.
We previously showed that the phosphomimetic PLM S68E mutant inhibits INaCa in transfected HEK293 cells (28) and isolated adult rat (20) and mouse (21) ventricular myocytes. With rAAV9-mediated gene transfer into PLM-KO hearts, INaCa {[Ca2+]o, [Ca2+]i, extracellular Na+ concentration ([Na+]o), and [Na+]i were 5, 0.0002, 141, and 12 mM, respectively; 30°C} was significantly reduced in KO-S68E compared with KO-GFP myocytes (Fig. 5; group effect, P < 0.0001; voltage effect, P < 0.0001; group × voltage interaction effect, P < 0.0001). Our ionic solutions were biased toward measurement of outward INaCa (3 Na+ out, 1 Ca2+ in), thereby accounting for the significant group × voltage interaction effect. By contrast, Ipump (0 mV, 18 mM [K+]o, 30°C) was not different between KO-GFP and KO-S68E myocytes at [Na+]pip of both 10 and 80 mM (Fig. 6; group effect, P < 0.37; [Na+]pip effect, P < 0.0001; group × [Na+]pip interaction effect, P < 0.28) and before and after Iso (1 μM) stimulation (group × [Na+]pip × Iso interaction effect, P < 0.71). This is consistent with our previous findings that S68E mutant has no effect on Ipump in adult mouse myocytes (21).
Fig. 5.
rAAV9-mediated S68E expression inhibits Na+/Ca2+ exchange current (INaCa) in PLM-KO myocytes. Five weeks after injection with rAAV9-GFP or rAAV9-S68E into PLM-KO hearts, myocytes were isolated from LV and INaCa was measured in “green” myocytes (indicating successful infection) at 5 mM extracellular Ca2+ concentration ([Ca2+]o) and 30°C (methods). The reversal potential of INaCa in all myocytes examined was approximately −60 mV. INaCa was lower in KO-S68E (○; n = 10) than KO-GFP (●; n = 7) myocytes. Error bars are not shown if they fall within the boundaries of the symbol.
Fig. 6.
rAAV9-mediated S68E expression has no effects on Na+-K+-ATPase current (Ipump) in PLM-KO myocytes. Ipump (defined as difference in current before and after addition of 1 mM dihydroouabain) was measured at 18 mM extracellular K+ concentration ([K+]o), 0 mV, and 30°C with pipette Na+ concentration ([Na+]pip) at either 10 or 80 mM, both before (open bars) and after (filled bars) addition of 1 μM Iso. For 10 mM [Na+]pip, there are 5 KO-GFP and 7 KO-S68E myocytes. For 80 mM [Na+]pip, there are 9 KO-GFP and 9 KO-S68E myocytes.
Effects of rAAV9-mediated S68E expression on action potential in PLM-KO myocytes.
Changes in NCX1 activity are expected to alter action potential morphology, as we have shown for PLM-KO myocytes (24) and myocytes with induced overexpression of NCX1 (25). Compared with PLM-KO myocytes overexpressing GFP, KO-S68E myocytes had similar resting membrane potential, action potential amplitude, and action potential duration at 50% repolarization (APD50) (Fig. 7; Table 3, series A). Action potential duration at 90% repolarization (APD90), however, was significantly (P < 0.001) shortened in KO-S68E myocytes (Fig. 7; Table 3, series A). In a separate series of experiments examining the effects of Iso (1 μM) on action potential parameters (1 Hz), lower resting membrane potential (P < 0.05) and shortened APD90 (P < 0.0001) were observed in KO-S68E compared with KO-GFP myocytes not stimulated with Iso (Table 3, series B). The major effect of Iso was prolongation of APD90 (P < 0.025) in both KO-GFP and KO-S68E myocytes (Table 3, series B). Our observation is consistent with previous reports on prolongation of APD by Iso in adult bovine (2) and guinea pig (2, 22) ventricular myocytes and embryonic mouse ventricular myocytes (12).
Fig. 7.
Alteration of action potential morphology in PLM-KO myocytes by rAAV9-mediated S68E expression. Action potential was measured in KO-GFP (dashed line) and KO-S68E (solid line) myocytes at 30°C and 1.8 mM [Ca2+]o and paced at 1 Hz (methods). Composite data are presented in Table 3.
Table 3.
Effects of rAAV9-mediated S68E expression and isoproterenol on action potential
| Iso | KO-GFP | KO-S68E | |
|---|---|---|---|
| Series A | |||
| Resting Em, mV | −77.0 ± 1.7 (10) | −71.8 ± 2.2 (8) | |
| AP amplitude, mV | 130.2 ± 3.2 | 131.0 ± 3.5 | |
| APD50, ms | 19.6 ± 2.6 | 18.9 ± 3.7 | |
| APD90, ms | 119.0 ± 10.3 | 66.8 ± 10.7 | |
| Series B | |||
| Resting Em, mV | − | −74.8 ± 1.1 (6) | −68.4 ± 1.4 (4) |
| + | −72.3 ± 1.3 | −71.3 ± 3.1 | |
| AP amplitude, mV | − | 124.0 ± 4.2 | 121.7 ± 7.8 |
| + | 113.9 ± 4.9 | 121.1 ± 6.3 | |
| APD50, ms | − | 14.8 ± 1.8 | 11.3 ± 1.6 |
| + | 15.5 ± 1.6 | 12.6 ± 2.3 | |
| APD90, ms | − | 98.3 ± 5.4 | 67.2 ± 5.1 |
| + | 116.5 ± 4.5 | 76.6 ± 6.5 |
Values are means ± SE. Numbers in parentheses are numbers of myocytes. Em, membrane potential; AP, action potential; APD50 and APD90, action potential duration at 50 and 90% repolarization, respectively; Iso, isoproterenol (1 μM). Cells were paced at 1 Hz. Data in series A and B were obtained 10 mo apart. Comparing baseline AP parameters in KO-GFP myocytes measured in the 2 time periods showed no significant differences in resting Em (P = 0.38), AP amplitude (P = 0.25), APD50 (P = 0.22), and APD90 (P = 0.17). For AP parameters in series A, 1-way ANOVA indicates significant (P < 0.001) differences in APD90 between KO-GFP and KO-S68E myocytes. For AP parameters in series B, 2-way ANOVA indicates significant group differences in resting Em (P < 0.05) and APD90 (P < 0.0001) between KO-GFP and KO-S68E myocytes. The only effect of Iso is significant (P < 0.025) prolongation of APD90 in both groups of myocytes.
Effects of rAAV9-mediated S68E expression on [Na+]i, [Ca2+]i transients, and contraction in isoproterenol-stimulated PLM-KO myocytes.
Baseline [Na+]i was not different among WT (7.3 ± 1.1 mM; n = 18), KO-GFP (5.8 ± 0.8 mM; n = 21), and KO-S68E (6.8 ± 0.8 mM; n = 15) myocytes (P = 0.47). Pacing myocytes at 2 Hz for 2 min increased [Na+]i by 4–5 mM in both KO-GFP and KO-S68E myocytes (Fig. 8A). Iso (1 μM) addition resulted in monotonic increase in [Na+]i in KO-GFP myocytes, similar to PLM-KO myocytes studied under identical conditions (26). After Iso addition, increases in [Na+]i in KO-S68E myocytes paralleled those in KO-GFP myocytes for the first 3 min but then reached a plateau (Fig. 8A). The time course of [Na+]i is different from that of WT myocytes, in which after reaching a peak following Iso addition, [Na+]i continues to decline with time (9, 26).
Fig. 8.
Changes in intracellular Na+ concentration ([Na+]i), intracellular Ca2+ concentration ([Ca2+]i) transient, and contraction amplitudes with pacing and Iso treatment in KO-GFP and KO-S68E myocytes. A: increases in [Na+]i (Δ[Na+]i) were measured at 37°C in sodium-binding benzofuran isophthalate (SBFI)-loaded myocytes (methods). After resting [Na+]i was obtained (at −1 min), pacing (2 Hz) was started at “time 0.” Iso (1 μM) was added at ∼2 min after initiation of pacing when both [Ca2+]i transient (9) and contraction amplitudes (unpublished observations) have reached steady state. There are 15 KO-GFP (●) and 11 KO-S68E (○) myocytes. For comparison, Δ[Na+]i from 5 WT (□) myocytes (26) are also shown. B: [Ca2+]i transient amplitudes were measured at 37°C in fura-2-loaded myocytes paced at 2 Hz (methods). After ≥2 min of pacing, Iso (1 μM) was added. To facilitate comparison between myocytes, [Ca2+]i transient amplitudes are normalized to the maximum values observed for each myocyte. There are 17 KO-GFP (●) and 20 KO-S68E (○) myocytes. For comparison, [Ca2+]i transients from 8 WT (□) myocytes (26) are also shown. C: contraction amplitudes were measured at 37°C in myocytes paced at 2 Hz and normalized to the maximum values observed for each myocyte. After ≥2 min of pacing, Iso (1 μM) was added. There are 9 KO-GFP (●) and 14 KO-S68E (○) myocytes. For comparison, normalized contraction amplitudes from 6 WT (□) myocytes (26) are also shown. Error bars are not shown if they fall within the boundaries of the symbol.
At baseline, both systolic and diastolic [Ca2+]i values were similar between KO-GFP and KO-S68E myocytes paced at 2 Hz (Table 4). Iso addition resulted in significantly higher peak systolic and diastolic [Ca2+]i in KO-S68E compared with KO-GFP myocytes (Table 4). [Ca2+]i transient amplitudes in both KO-GFP and KO-S68E myocytes reached maximum at 2–3 min after Iso addition and remained stable thereafter (Fig. 8B). The time course is different than that observed in WT myocytes, which suffer a time-dependent decline in [Ca2+]i transient amplitude after reaching peak values (9, 26).
Table 4.
Effects of rAAV9-mediated S68E expression on [Ca2+]i transients
| Iso | KO-GFP | KO-S68E |
|---|---|---|
| Diastolic [Ca2+]i, nM | ||
| − | 126 ± 7 (17) | 118 ± 7 (20) |
| + | 109 ± 8 (12) | 143 ± 10* (16) |
| Systolic [Ca2+]i, nM | ||
| − | 236 ± 12 | 219 ± 11 |
| + | 345 ± 21 | 426 ± 24* |
| t1/2 of [Ca2+]i transient decline, ms | ||
| − | 106 ± 3 | 121 ± 6 |
| + | 68 ± 3 | 71 ± 3 |
Values are means ± SE. Numbers in parentheses are numbers of myocytes isolated from 4 KO-S68E and 2 KO-GFP hearts. Myocytes were paced at 2 Hz. Intracellular Ca2+ concentration ([Ca2+]i) values were measured before and 2 min after addition of 1 μM Iso. t1/2, half-time.
P < 0.025, KO-GFP vs. KO-S68E.
The half-time (t1/2) of [Ca2+]i transient decline is an estimate of in situ SR Ca2+ uptake activity (31). There were no differences in t1/2 of [Ca2+]i transient decline between KO-GFP and KO-S68E myocytes paced at 2 Hz, both in the absence and presence of Iso (Table 4). This observation is in agreement with similar SERCA2 protein levels between KO-GFP and KO-S68E hearts (Table 1).
When myocytes were paced at 2 Hz, contraction amplitudes reached maximum at ∼2 min after Iso addition and showed little decline in both KO-GFP (from 98.5 ± 1.2% to 85.4 ± 3.6%) and KO-S68E (from 97.0 ± 0.9% to 80.5 ± 3.3%) myocytes (Fig. 8C). By contrast, after reaching maximal values following Iso addition, contraction amplitudes in WT myocytes decline significantly with time (from 95.1 ± 1.7% to 68.5 ± 6.2%) (26).
DISCUSSION
Adeno-associated viral vectors possess several advantages over other vectors in mediating exogenous gene delivery to the heart. They are not pathogenic in humans (4), and they provide sustained myocardial transduction (7, 34). Of the nine AAV serotypes, AAV serotypes 6 and 9 have the earliest (<7 days after intracoronary injection) and highest expression of the transgene in the heart (34). In the present study, we injected rAAV9 directly into the LV of mice, which resulted in successful expression of the transgene as detected by immunoblotting, fluorescence imaging, and functional measurements. On the basis of detection of GFP fluorescence in myocytes isolated from LV and septum, ∼40% of myocytes were successfully transduced by direct LV injection with rAAV9. This is a lower bound of the estimate of rAAV9-mediated gene transfer efficiency since the septum was not accessible to injection and only part of the LV was injected.
In WT hearts stimulated with Iso, PLM phosphorylated at serine 68 simultaneously inhibits NCX1 (28) but relieves its inhibition on Na+-K+-ATPase (8, 26). Inhibition of NCX1 is expected to enhance contractility, while disinhibiting Na+-K+-ATPase would result in decreased inotropy (9, 26). This complexity makes it very difficult to critically evaluate the two opposing effects of PLM on cardiac contractility. We reasoned that by expressing PLM S68E mutant (which inhibits NCX1 but not Na+-K+-ATPase) (21) in the PLM-KO background, a model with “pure” NCX1 inhibition but without concomitant enhancement of Na+-K+-ATPase activity would result. This novel model would allow us to isolate the effects of NCX1 inhibition by phosphorylated PLM on cardiac contractility.
Compared with PLM-KO hearts expressing GFP, PLM-KO hearts expressing the phosphomimetic PLM S68E mutant had no changes in protein levels of NCX1, Na+-K+-ATPase, and SERCA2. In KO-S68E myocytes, NCX1 inhibition was confirmed by reduction in INaCa. Another observation that supported NCX1 inhibition in KO-S68E myocytes was when myocytes were subjected to rapid pacing and Iso stimulation, conditions that favored [Ca2+]i efflux via forward Na+/Ca2+ exchange, increases in [Na+]i reached a plateau rather than the monotonic [Na+]i increase exhibited by KO-GFP myocytes. Decreased [Na+]i correlated with significantly elevated diastolic [Ca2+]i in Iso-stimulated KO-S68E myocytes. A third piece of evidence supporting NCX1 inhibition in KO-S68E myocytes was shortening of APD90. In rodent myocytes with short APD, NCX1 mediates net Ca2+ efflux at the shoulder of the action potential. This is because the action potential is already repolarizing before the [Ca2+]i transient reaches its peak, and INaCa is predominantly inward (3 Na+ in, 1 Ca2+ out) during this phase of the action potential (18). Inhibition of NCX1 in KO-S68E myocytes would be expected to shorten APD90. It is important to emphasize that INaCa but not Ipump was suppressed in KO-S68E myocytes, consistent with our previous results in isolated rat myocytes (20), cultured PLM-KO myocytes (21), and transfected HEK293 cells (28).
The major finding is that despite only ∼40% of LV myocytes expressing the transgene, KO-S68E hearts had significantly better +dP/dt when stimulated with increasing doses of Iso. In PLM-KO hearts, in which Iso does not have any effects on Na+-K+-ATPase (8, 26), expression of S68E mutant, which inhibits Na+/Ca2+ exchanger but not Na+-K+-ATPase (21, 26), should result in enhanced cardiac contractility. Indeed, increased inotropy was associated with lower [Na+]i and higher diastolic [Ca2+]i in Iso-stimulated KO-S68E myocytes, consistent with inhibition of forward Na+/Ca2+ exchange. Unlike WT myocytes (9, 26), when subjected to rapid pacing and Iso stimulation neither KO-GFP nor KO-S68E myocytes suffered a time-dependent decline in [Ca2+]i transient and contraction amplitudes. This observation supports the lack of effect of Iso on Na+-K+-ATPase in KO-GFP and KO-S68E myocytes.
In light of the results of previous (3, 8–10, 19–21, 24, 26, 28, 32) and present studies, the functional significance of PLM in the heart is beginning to emerge. In isolated rat myocytes in which PLM is overexpressed (19) or in mouse myocytes in which PLM is genetically absent (24), the effects of PLM on contractility and [Ca2+]i transients are not obvious under physiological conditions (1.8 mM [Ca2+]o, 1 Hz, 37°C). Only when the thermodynamic driving force for NCX1 is altered by varying [Ca2+]o (0.6 or 5.0 mM) are the effects of PLM on [Ca2+]i transients and myocyte contractility evident (13, 19, 20, 24, 32). Likewise, modulation of Na+-K+-ATPase activity by PLM has little effect in resting myocytes since basal [Na+]i is similar between WT and PLM-KO myocytes (8, 26). Therefore, under resting conditions, PLM is functionally quiescent.
Under stressful conditions when catecholamine levels are high, both [Na+]i and [Ca2+]i are elevated because of increased depolarizations and Ca2+ entry via L-type Ca2+ channels. Increased Ca2+ entry must be balanced by greater Ca2+ efflux via NCX1, thereby bringing more Na+ into the myocyte. Under these conditions, stimulation of Na+-K+-ATPase by phosphorylated PLM serves to limit myocyte Na+ overload and reduce the risk for arrhythmogenesis, but at the apparent expense of decreased inotropy (9). Indeed, recent in vivo studies support this concept in that with Iso +dP/dt rapidly rises to a peak followed by decline in WT but not PLM-KO hearts (26). However, reduced inotropy under conditions of fight or flight is clearly not in the best interests of the animal. Our present observation that “pure” NCX1 inhibition by the phosphomimetic S68E mutant resulted in enhanced inotropy in Iso-stimulated KO-S68E hearts suggests that phosphorylated PLM serves two important functions under stressful conditions: minimizing the risks of arrhythmogenesis (by enhancing Na+-K+-ATPase activity) and preserving inotropy (by inhibiting Na+/Ca2+ exchanger).
There are limitations to the present study. The first is that our method of gene delivery by direct rAAV9 injection only affected ∼40% of LV myocytes. This limitation should not affect the results of single-cell studies since only successfully infected (“green”) cells were examined. In addition, despite <50% infection efficacy, enhanced +dP/dt was already apparent in KO-S68E hearts compared with KO-GFP hearts. More efficient gene delivery methods such as tail vein (34) or retroorbital sinus injection of virus and novel mouse models with inducible expression of S68E mutant in the PLM-KO background would improve the signal-to-noise ratio in whole animal studies. The second limitation is the assumption that the S68E mutant faithfully mimicked the phosphorylated form of PLM. This appears reasonable since in HEK293 cells coexpressing NCX1 and WT PLM the S68E mutant reproduces the inhibitory effects of forskolin on INaCa (28). The third limitation is that in our attempt to compare expression level of S68E mutant in rAAV9-transduced PLM-KO hearts to that of PLM present in WT hearts, we assumed equal reactivity of our C2 antibody against dog S68E mutant and mouse WT PLM. Our C2 antibody was raised against a 16-amino acid peptide fragment of the COOH terminus of rat PLM (19), which differs from dog S68E mutant (glutamate 68) and mouse PLM (threonine 69) by 1 amino acid. In addition, C2 antibody preferentially recognizes unphosphorylated PLM (30). These two considerations suggest that our assumption of equal reactivity may not be entirely correct. The fourth concern is that baseline +dP/dt in KO-S68E hearts appeared to be higher than that in KO-GFP hearts, despite similar baseline systolic and diastolic [Ca2+]i measured in isolated myocytes. Noninvasive measurements of cardiac performance, however, demonstrated no differences in resting cardiac output and ejection fraction between KO-GFP and KO-S68E mice under inhaled anesthesia. We speculate that injected anesthesia and the surgical manipulation involved in exposing the internal carotid artery and insertion of the catheter might have resulted in higher circulating catecholamine levels, thereby enhancing +dP/dt in KO-S68E hearts at “baseline.” Finally, we have ignored the recent report that in transfected HEK293 cells PLM regulates the gating kinetics of L-type Ca2+ channels (27). This novel observation would render the interpretation of the effects of PLM expression and phosphorylation on cardiac contractility extremely complex. However, heterologous expression model systems often do not reproduce a protein's native milieu. A good example is that the stimulation of L-type Ca2+ current by adrenergic agonists, so readily observed in cardiac myocytes (17, 29), has yet to be reproduced in heterologous expression systems (11). In addition, overexpression of proteins resulting in supraphysiological levels may distort the normal stoichiometry of interaction between proteins. At the present time, it may be premature to consider the effects of PLM on Ca2+ channel gating on cardiac contractility until the effects are authenticated in cardiac myocytes.
In summary, we successfully expressed the phosphomimetic PLM S68E mutant in PLM-KO hearts by injecting recombinant adeno-associated virus directly into the LV. PLM-KO myocytes expressing S68E mutant demonstrated inhibition of Na+/Ca2+ exchanger but not Na+-K+-ATPase and abbreviated APD. In vivo, PLM-KO hearts expressing the S68E mutant had enhanced inotropy compared with PLM-KO hearts expressing GFP, especially when stimulated with Iso. We conclude that under stressful conditions inhibition of Na+/Ca2+ exchanger by phosphorylated PLM preserved inotropic response, while relief of inhibition of Na+-K+-ATPase minimized [Na+]i and [Ca2+]i overload and the risk of arrhythmogenesis.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO1-HL-58672 and RO1-HL-74854 (J. Y. Cheung); RO1-HL-91096 (J. E. Rabinowitz); RO1-HL-56205, RO1-HL-61690, RO1-HL-85503, PO1-HL-75443, and PO1-HL-91799 (W. J. Koch); and PO1-HL-91799 (Project 2; to A. M. Feldman); by the Pennsylvania Research Formulary Fund (A. M. Feldman), and by American Heart Association Scientist Development Grant F64702 (T. O. Chan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem 280: 19875–19882, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Belardinelli L, Isenberg G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res 53: 287–297, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Bell JR, Kennington E, Fuller W, Dighe K, Donoghue P, Clark JE, Jia LG, Tucker AL, Moorman JR, Marber MS, Eaton P, Dunn MJ, Shattock MJ. Characterization of the phospholemman knockout mouse heart: depressed left ventricular function with increased Na-K-ATPase activity. Am J Physiol Heart Circ Physiol 294: H613–H621, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Berns KI, Linden RM. The cryptic life style of adeno-associated virus. Bioessays 17: 237–245, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bossuyt J, Ai X, Moorman JR, Pogwizd SM, Bers DM. Expression and phosphorylation of the Na-pump regulatory subunit phospholemman in heart failure. Circ Res 97: 558–565, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chu D, Sullivan CC, Weitzman MD, Du L, Wolf PL, Jamieson SW, Thistlethwaite PA. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J Thorac Cardiovasc Surg 126: 671–679, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97: 252–259, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Despa S, Tucker AL, Bers DM. PLM-mediated activation of Na/K-ATPase limits [Na]i and inotropic state during β-adrenergic stimulation in mouse ventricular myocytes. Circulation 117: 1849–1855, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia LG, Donnet C, Bogaev RC, Blatt RJ, McKinney CE, Day KH, Berr SS, Jones LR, Moorman JR, Sweadner KJ, Tucker AL. Hypertrophy, increased ejection fraction, and reduced Na-K-ATPase activity in phospholemman-deficient mice. Am J Physiol Heart Circ Physiol 288: H1982–H1988, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J Biol Chem 283: 34738–34744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu W, Yasui K, Arai A, Kamiya K, Cheng J, Kodama I, Toyama J. Beta-adrenergic modulation of L-type Ca2+-channel currents in early-stage embryonic mouse heart. Am J Physiol Heart Circ Physiol 276: H608–H613, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Mirza MA, Zhang XQ, Ahlers BA, Qureshi A, Carl LL, Song J, Tucker AL, Mounsey JP, Moorman JR, Rothblum LI, Zhang TS, Cheung JY. Effects of phospholemman downregulation on contractility and [Ca2+]i transients in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 286: H1322–H1330, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem 266: 11126–11130, 1991 [PubMed] [Google Scholar]
- 15. Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 115: 2506–2515, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Presti CF, Jones LR, Lindemann JP. Isoproterenol-induced phosphorylation of a 15-kilodalton sarcolemmal protein in intact myocardium. J Biol Chem 260: 3860–3867, 1985 [PubMed] [Google Scholar]
- 17. Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium concentration. J Physiol 192: 479–492, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shattock M, Bers DM. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol Cell Physiol 256: C813–C822, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Song J, Zhang XQ, Carl LL, Qureshi A, Rothblum LI, Cheung JY. Overexpression of phospholemman alters contractility and [Ca2+]i transients in adult rat myocytes. Am J Physiol Heart Circ Physiol 283: H576–H583, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, Cheung JY. Serine 68 of phospholemman is critical in modulation of contractility, [Ca2+]i transients, and Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 288: H2342–H2354, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Song J, Zhang XQ, Wang J, Cheskis E, Chan TO, Feldman AM, Tucker AL, Cheung JY. Regulation of cardiac myocyte contractility by phospholemman: Na+/Ca2+ exchange vs. Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 295: H1615–H1625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song Y, Shryock JC, Knot HJ, Belardinelli L. Selective attenuation by adenosine of arrhythmogenic action of isoproterenol on ventricular myocytes. Am J Physiol Heart Circ Physiol 280: H2789–H2795, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68: 41–56, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Tucker AL, Song J, Zhang XQ, Wang J, Ahlers BA, Carl LL, Mounsey JP, Moorman JR, Rothblum LI, Cheung JY. Altered contractility and [Ca2+]i homeostasis in phospholemman-deficient murine myocytes: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 291: H2199–H2209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Chan TO, Zhang XQ, Gao E, Song J, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger transgene: altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents, and action potential duration. Am J Physiol Heart Circ Physiol 297: H590–H601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Gao E, Song J, Zhang XQ, Li J, Koch WJ, Tucker AL, Philipson KD, Chan TO, Feldman AM, Cheung JY. Phospholemman and β-adrenergic stimulation in the heart. Am J Physiol Heart Circ Physiol 298: H807–H815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Gao G, Guo K, Yarotskyy V, Huang C, Elmslie KS, Peterson BZ. Phospholemman modulates the gating of cardiac L-type calcium channels. Biophys J 98: 1149–1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang XQ, Moore RL, Tillotson DL, Cheung JY. Calcium currents in postinfarction rat cardiac myocytes. Am J Physiol Cell Physiol 269: C1464–C1473, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Zhang XQ, Moorman JR, Ahlers BA, Carl LL, Lake DE, Song J, Mounsey JP, Tucker AL, Chan YM, Rothblum LI, Stahl RC, Carey DJ, Cheung JY. Phospholemman overexpression inhibits Na+-K+-ATPase in adult rat cardiac myocytes: relevance to decreased Na+ pump activity in postinfarction myocytes. J Appl Physiol 100: 212–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang XQ, Ng YC, Moore RL, Musch TI, Cheung JY. In situ SR function in postinfarction myocytes. J Appl Physiol 87: 2143–2150, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Zhang XQ, Qureshi A, Song J, Carl LL, Tian Q, Stahl RC, Carey DJ, Rothblum LI, Cheung JY. Phospholemman modulates Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 284: H225–H233, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci 3: 81–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]