Abstract
A rise in postprandial serum triglycerides (PP-sTG) can potentiate inflammatory responses in vascular endothelial cells (ECs) and thus serves as an independent risk factor for predicting increased cardiovascular morbidity. We examined postprandial triglyceride-rich lipoproteins (PP-TGRLs) in subjects ranging from normal to hypertriglyceridemic for their capacity to alter EC acute inflammatory responses. Cultured human aortic ECs (HAECs) were conditioned with PP-TGRLs isolated from human serum at the peak after a moderately high-fat meal. VLDL particle size increased postprandially and varied directly with the subject's PP-sTG level and waist circumference. PP-TGRL particles bound to HAECs and were internalized via LDL receptor-mediated endocytosis. PP-TGRL alone did not induce an inflammatory response over the range of individuals studied. However, combined with low-dose TNF-α stimulation (0.3 ng/ml), it elicited a net 10–15% increase above cytokine alone in the membrane expression of VCAM-1, ICAM-1, and E-selectin, which was not observed with fasting TGRLs. In contrast to upregulation of ICAM-1 and E-selectin, VCAM-1 transcription and expression varied in direct proportion with individual PP-sTG and waist circumference. The extent of monocyte arrest on inflamed HAECs under shear stress also correlated closely with VCAM-1 expression induced by conditioning with PP-TGRL and TNF-α stimulation. This ex vivo approach provides a quantitative means to assess an individual's inflammatory potential, revealing a greater propensity for endothelial inflammation in hypertriglyceridemic individuals with abdominal obesity.
Keywords: atherosclerosis, dyslipidemia, triglyceride-rich lipoprotein, vascular cell adhesion molecule-1, tumor necrosis factor-α, endothelial cells
atherosclerosis has been postulated to be a postprandial phenomenon (27, 36) promoted by repetitive exposure to transiently elevated serum triglycerides (sTG) (1). Epidemiological studies have established postprandial serum triglycerides (PP-sTG) as an independent risk factor for cardiovascular disease (CVD) (26) and demonstrated the strongest correlation with adverse cardiovascular events at the postprandial peak (2). The triglyceride spike after a high-fat meal is associated with transient endothelial dysfunction, notably the impairment in flow-mediated vasodilatation (1, 32) that precedes atherosclerotic lesion formation (22). However, our understanding of the cellular- and molecular-level events in the endothelium that link epidemiological risk factors for CVD such as PP-sTG with the early mechanisms driving atherogenesis is limited.
Triglyceride-rich lipoproteins (TGRLs) constitute a heterogeneous group of particles differing in their lipid and protein components, which, in turn, determine particle size, density, receptor interactions, and metabolism (17). Biochemical studies of TGRL have revealed compositional changes concomitant with the postprandial spike in sTG, more profoundly in hypertriglyceridemic (HTG) subjects. These include enrichment of cholesterol, apolipoprotein (Apo)E, ApoC-I, and ApoC-III and depletion of ApoC-II with decreased VLDL ApoB catabolism (3, 5). However, there is a lack of understanding as to how individual heterogeneity in TGRLs can impact endothelial inflammation contributing to atherogenesis. Moreover, it is unclear the extent to which early inflammatory changes in the endothelium are reflected by current clinical measurements used to assess cardiovascular risk.
We previously established an ex vivo model that assessed the inflammatory potential of an individual's TGRLs by examining perturbations in the endothelial cell (EC) response to TNF-α, a cytokine expressed locally at sites of atherosclerotic plaque formation (28) and a biomarker of inflammation that correlates with the risk of CVD (6, 19). We studied the effects of conditioning human aortic ECs (HAECs) in a repetitive manner over several days with native PP-TGRLs isolated from normal triglyceridemic (NTG) subjects after a high-fat meal and reported an increase in VCAM-1 upregulation by TNF-α that resulted in elevated efficiency of monocyte recruitment under shear stress (31). Here, we extend the previous study to interrogate the inflammatory potential of TGRLs derived from a cohort of subjects ranging from NTG to HTG at the peak in sTG after a single high-fat meal. We hypothesized that the acute response of HAECs to simultaneous stimulation with an individual's PP-TGRLs superposed with low-dose TNF-α would provide an ex vivo measure of the influence of anthropometric characteristics and metabolic profile on systemic inflammation. We report that PP-TGRLs are not acutely inflammatory to HAECs but can augment cytokine-induced VCAM-1 expression and associated monocyte recruitment either positively or negatively in proportion to the level of the subject's PP-sTG and abdominal obesity.
MATERIALS AND METHODS
An expanded materials and methods section is available as online Supplemental Material1 and as previously described (31).
Human subjects.
This study included both NTG and HTG [fasting sTG (F-sTG) > 150 mg/dl] subjects but excluded those on lipid-lowering or anti-inflammatory medications or with a fasting blood glucose of >110 mg/dl. Volunteers were enrolled according to Institutional Review Board-approved protocols under informed consent. They fasted for 12 h and consumed a 1,230-cal test meal that was moderately high in fat (47% of calories) and saturated fat (32% of total), representative of a Western fast food diet. Standard lipid panels were determined before and 3.5 h after the meal, a time point previously demonstrated to correlate with peak levels of PP-sTG (2).
TGRL isolation and characterization.
TGRLs (<1.0063 g/ml) were isolated by ultracentrifugation (31), quantified for ApoB content (Alerchek), and tested for endotoxin using a chromogenic test kit (Associates of Cape Cod). The lipids were stored under nitrogen at 4°C and used within 3 days after isolation. TGRL particle size distribution was measured using a Nanotrac Particle Size Analyzer with FLEX software (Microtrac), which uses a controlled reference method to determine particle size based on dynamic light scattering, with a repeatability of 1% for 100-nm polystyrene. Each sample was quantified five times with a run time of 2–3 min, and the results of the five runs were averaged.
Cell culture and treatment protocol.
Cryopreserved HAECs derived from a 21-yr-old woman (Genlantis, passage 3) were expanded in collagen-coated (50 μg/ml, Clontech) culture flasks. Cells were maintained in EC growth medium-2 (EGM-2; Lonza) with 10% FBS (Hyclone) and 1× antibiotic-antimycotic solution (Invitrogen). They were subcultured at ∼80–90% confluence per the supplier's instructions and used for experiments at passages 4–6 (within 15 population doublings). With the exceptions noted, HAECs were conditioned with TGRLs (10 mg/dl ApoB) alone or simultaneously with the inflammatory cytokine TNF-α (0.3 ng/ml, R&D system) for 4 h. All treatments were conducted in complete media (EGM-2 supplemented with 10% FBS and 1× antibiotic-antimycotic solution). Treatments not receiving TGRLs were supplemented with an equal amount of buffer in which the isolated TGRL was suspended (196 mM NaCl and 0.3mM EDTA) to compensate for any changes in volume and media composition.
TGRL labeling and confocal imaging.
PP-TGRLs were labeled with Alexa fluor488 reactive dye (Invitrogen). Total protein content was quantified by a modified Lowry assay (Sigma). Excess dye was removed by column chromatography. Uptake of labeled PP-TGRLs was visualized by confocal microscopy. Images were analyzed in ImageJ, and integrated fluorescence intensity for each cell in the field was quantified.
Flow cytometry.
Cells were detached using an enzyme-free cell dissociation buffer (GIBCO), Fc blocked, labeled with fluorescein-conjugated antibodies against human E-selectin, ICAM-1, VCAM-1, or isotype-matched IgG control, and analyzed by FACScan flow cytometer (Becton Dickinson) with CellQuest software. Data represent the median fluorescence intensity from a single Gaussian population of 10,000 HAECs for each sample.
Cholesterol assay.
Cellular cholesterol content was determined using an Amplex Red Cholesterol Assay kit (Invitrogen), a fluorometric method that measures H2O2 produced upon hydrolysis of cholesterol esters and the subsequent oxidation of cholesterol. Fluorescence signals were measured by a FLUOstar Optima multifunctional microplate reader (BMG Labtech), and the cholesterol concentration was determined by reference to a standard curve.
RNA isolation and real-time PCR.
Total RNA was isolated using a High Pure Total RNA Isolation kit (Roche) and converted to first-strand cDNA using a Transcriptor First Strand cDNA Synthesis kit (Roche). Quantitative PCR was performed using Taqman Gene Expression Assays and Master Mix (Applied Biosystems) and a RealPlex Mastercycler (Eppendorf). Alternatively, Roche Fast Start Universal SYBR Green Master Mix reagents were used with exon-flanking primers designed using Primer3. The housekeeping genes ribosomal protein S27a (RPS27a) and acidic ribosomal protein P0 (RPLP0) were screened for their constant expression across the experimental conditions. Relative quantification was determined by the ΔΔCt method (Taqman), where Ct is threshold cycle, or by reference to standard curves (SYBR).
RNA stability assay.
To observe the impact of TGRLs on message stability, HAECs were preincubated with control media or TNF-α (1 ng/ml) for 1 h to induce a strong, consistent inflammatory gene expression response. Actinomycin D (ActD; 1 μg/ml) and/or TGRLs (10 mg/dl ApoB) were added at time 0. Transcript levels were monitored at 2, 4, and 6 h by quantitative PCR.
Monocyte adhesion assay.
Healthy normolipidemic (F-sTG < 100 mg/dl) subjects not on any medication were enrolled as monocyte donors according to Institutional Review Board-approved protocols under informed consent. Mononuclear cells were isolated from fasting blood by sedimentation over Lymphosep density separation media (MP). Monocytes were purified by repetitive centrifugation to deplete platelets and a bead-based negative isolation (Invitrogen) to remove other mononuclear cells. Each preparation was examined for purity by flow cytometry after being incubated with an Alexa fluor488-labeled antibody to the monocytic marker CD14 and used if the number of CD14+ cells exceeded 90%. They were resuspended in HEPES buffer + 0.1% human serum albumin + 1.5 mM CaCl2 at a concentration of 106 monocytes/ml and perfused over preconditioned HAEC monolayers at a shear stress of 1 dyn/cm2 for 5 min in a parallel-plate flow channel (29). The number of arrested monocytes per field was quantified by identifying their phase-bright appearance in the same focal plane as the endothelial monolayer (31). Each data point represents an average of 8 fields/channel with 2–3 channels/condition.
Data analysis.
Data were analyzed using GraphPad Prism version 5.0 software. In general, multiple groups were analyzed by repeated-measures ANOVA, and differences were assessed by the Student-Newman-Keuls posttest. Two experimental groups were compared using Student's t-test, with pairing where appropriate. Two-tailed P values of <0.05 were considered statistically significant unless otherwise indicated. Correlations between groups were assessed using Pearson's correlation coefficient (r).
RESULTS
TGRL particle composition varies with the level of donor HTG and abdominal obesity.
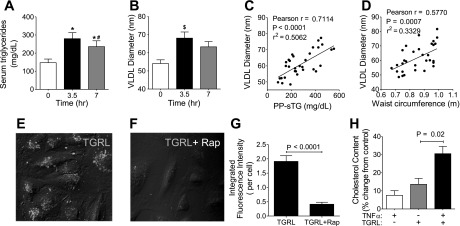
To investigate the effects of individual heterogeneity in TGRLs on EC inflammatory responses, we recruited 61 subjects with normal fasting glucose, representing a broad range in body mass index (19.8–57.9 kg/m2), waist circumference (WC; 0.64–1.32 m), and F-sTG (41–538 mg/dl) (Supplemental Tables 1 and 2). Consumption of the test meal led to a 99 ± 66% increase in sTG 3.5 h postprandially, which was significantly reduced by 7 h (Fig. 1A). Glucose, LDL, and HDL cholesterol levels were modestly decreased at 3.5 h, whereas total cholesterol and ApoB100 levels remained constant. The mean diameter of VLDL particles was increased by 26.6 ± 21.8% in response to the meal (Fig. 1B) and increased in proportion to subject's PP-sTG (r = 0.71, P < 0.0001; Fig. 1C) and WC (r =0.58, P = 0.0007; Fig. 1D). VLDL diameter correlated more weakly with a subject's F-sTG and inversely with postprandial and fasting HDL but with no other anthropometric characteristics or lipid parameters. The results demonstrate that the test meal induced a transient increase in TGRL particle size concurrent with a postprandial spike in sTG, implicating an altered TGRL composition, which reflects the level of HTG and abdominal obesity.
Fig. 1.
A–D: triglyceride-rich lipoprotein (TGRL) particle size increases postprandially and varies with subject characteristics. A: serum triglyceride levels (means ± SE) in 16 subjects at 0 (fasting), 3.5, and 7 h after the meal. Significance was determined by repeated-measures ANOVA with Student-Newman-Keuls posttest (*P < 0.001 from fasting; #P < 0.005 from 3.5 h). B: average diameter of VLDL isolated from subjects at the designated time postprandially. Significance was determined by repeated-measures ANOVA with Student-Newman-Keuls posttest ($P < 0.05 from fasting). C and D: Pearson correlations between VLDL diameter and subject postprandial serum triglycerides (PP-sTG; C) or waist circumference (WC; D). E–H: TGRL uptake by human aortic endothelial cells (HAECs) via LDL receptors (LDLRs). E–G: representative confocal images (E and F) and statistical analysis (G) of TGRL binding (means ± SE; n = 4) of HAECs incubated with Alexa fluor488-labeled TGRLs [10 mg/dl apolipoprotein (Apo)B] for 1 h alone (E) or with receptor-associated protein (RAP; 50 μg/ml; F). Significance was determined by a paired Student's t-test. H: cellular cholesterol content (means ± SE; n = 4) of HAECs incubated with TGRLs, TNF-α, or both for 4 h. Significance was determined by repeated-measures ANOVA with Student-Newman-Keuls posttest.
PP-TGRL uptake by LDLRs increases under inflammation.
Several LDL receptors (LDLRs) are constitutively expressed on HAECs, mediating EC uptake of native TGRLs and their remnant particles (RPs) by endocytosis (30). We examined PP-TGRL uptake by LDLR-related mechanisms in resting and cytokine stimulated HAECs using fluorophore-conjugated particles. Fluorescence from bound and internalized particles significantly increased as early as 15 min after the addition of TGRLs and was distributed in a punctate pattern, consistent with receptor-mediated endocytosis, which was inhibited by 78% in the presence of the LDLR antagonist receptor-associated protein and not control IgG (Fig. 1, E–G). This binding was associated with a rise in cellular cholesterol content at 4 h after exposure to PP-TGRLs, which was increased by 15% in the presence of TNF-α (Fig. 1H). These results demonstrate that PP-TGRL particles are rapidly bound by ECs via a LDLR-specific mechanism and that their internalization is increased under inflammation.
PP-TGRL enhances cytokine-induced surface expression of cellular adhesion molecules.
TNF-α is found at a picogram per milliliter level in human serum and is elevated after a high-fat meal (6). It is expressed several hundred-fold higher in atherosclerotic lesions (28) and plays an important role in atherogenesis by upregulating EC vascular cell adhesion molecules (CAMs), which support monocyte recruitment from the circulation (19, 21). To test the hypothesis that PP-TGRLs derived from our subjects would differentially impact endothelial inflammatory responses, PP-TGRLs from each subject were examined for the ability to modulate the TNF-α-induced surface expression of CAMs. PP-TGRLs alone did not elicit a significant increase in CAM expression in resting HAECs after 4 h of incubation (Supplemental Fig. I). In fact, on average, we observed a small but statistically significant reduction in VCAM-1 (−7.2%) and E-selectin (−9.8%) expression from baseline. However, acute exposure to PP-TGRLs enhanced TNF-α-induced surface expression of VCAM-1 (9.6%), ICAM-1 (13.4%), and E-selectin (13.2%). These data demonstrate that acute exposure of HAECs to PP-TGRLs isolated at their peak after a single high-fat meal can modulate the inflammatory response to cytokines.
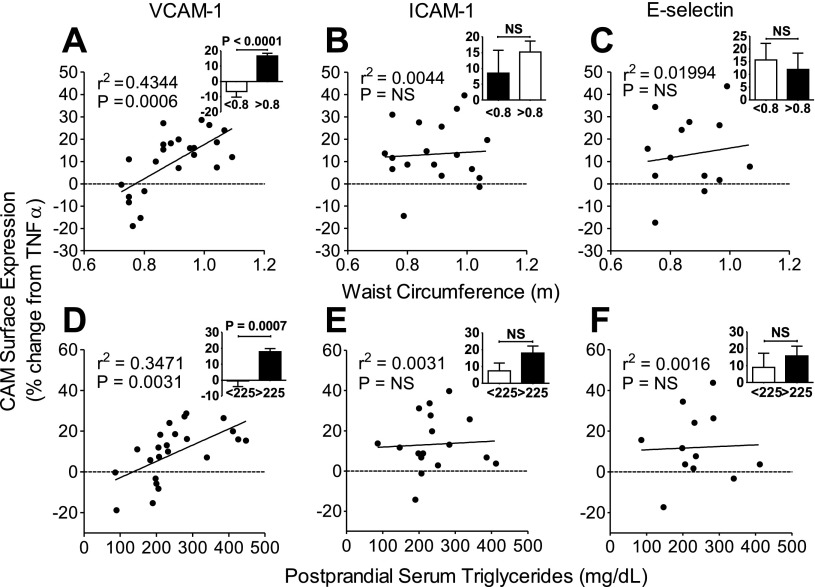
TGRL-enhanced, cytokine-induced VCAM-1 expression correlates with subject WC and PP-sTG.
While PP-TGRLs, on average, enhanced CAM expression in response to TNF-α in our subject pool, we observed significant interindividual variability in its priming capacity. This reflects the compositional nature of a subject's TGRLs rather than a dosage effect, since each aliquot was normalized by ApoB content to deliver the same number of particles (1 ApoB molecule/TGRL particle). We investigated whether such PP-TGRL priming capacity varied predictably with subject anthropometric characteristics or lipid profile. The increase in VCAM-1 expression (Table 1) correlated most strongly with subject WC (r = 0.66, P < 0.001; Fig. 2A) followed by PP-sTG (r = 0.59, P = 0.003; Fig. 2D) and F-sTG (r = 0.46, P = 0.026) and inversely with postprandial HDL cholesterol (r = −0.42, P = 0.046). No significant correlation was observed with other serum lipids, glucose, or body mass index. Notably, these correlations revealed that PP-TGRL could either positively or negatively modulate VCAM-1 expression over a considerable range about the mean (from a 20% drop to a 28% increase from TNF-α alone). On average, an enhancement in VCAM-1 expression was observed for PP-TGRL from subjects with PP-sTG ≥ 225 mg/dl (17.8%) or WC ≥ 0.8 m (16.7%) (Fig. 2, A and D, inset). Clinical characteristics of the groups defined by this cutoff in PP-sTG are shown in Supplemental Table 3. Overall, this cutoff was more predictive of enhanced VCAM-1 expression than one based on a clinically used criterion for HTG (F-sTG > 150 mg/dl). Although ICAM-1 and E-selectin upregulation by TNF-α were enhanced in the presence of PP-TGRLs over all subjects, these did not correlate significantly with subject WC (Fig. 2, B and C), PP-sTG (Fig. 2, E and F) or any other anthropometric or metabolic parameters (data not shown). The same priming effect on VCAM-1 expression was not observed with fasting TGRLs derived from the same donors (Supplemental Fig. 2), which overall reduced CAM expression relative to TNF-α, indicating that this is a transient response attributable to the postprandial nature of the TGRLs. Taken together, these data reveal that an acute exposure to PP-TGRLs exacerbates the inflammatory response in ECs specific to VCAM-1, which correlates directly with an individual subject's metabolic characteristics reflected by sTG and WC.
Table 1.
Correlations between subject clinical characteristics and triglyceride-rich lipoprotein-modulated VCAM-1 expression
| Pearson Correlation Coefficient | P Value (Two-Tailed) | R2 | |
|---|---|---|---|
| Waist circumference† | 0.66 | 0.0006 | 0.43 |
| Body mass index | 0.39 | 0.067 | 0.15 |
| Fasting | |||
| Triglycerides* | 0.46 | 0.026 | 0.22 |
| Total cholesterol | −0.41 | 0.055 | 0.17 |
| LDL cholesterol | −0.41 | 0.056 | 0.17 |
| HDL cholesterol | −0.39 | 0.067 | 0.15 |
| Apolipoprotein B100 | −0.24 | 0.264 | 0.06 |
| Cholesterol-to-HDL ratio | 0.24 | 0.262 | 0.06 |
| Glucose | −0.01 | 0.949 | 0.00 |
| Postprandial | |||
| Triglycerides† | 0.59 | 0.003 | 0.35 |
| HDL cholesterol* | −0.42 | 0.046 | 0.18 |
| LDL cholesterol | −0.40 | 0.083 | 0.16 |
| Total cholesterol | −0.37 | 0.079 | 0.14 |
| Glucose | −0.30 | 0.167 | 0.09 |
| Cholesterol-to-HDL ratio | 0.29 | 0.181 | 0.08 |
| Apolipoprotein B100 | −0.02 | 0.940 | 0.00 |
P < 0.05;
P < 0.01.
Fig. 2.
TGRL-enhanced VCAM-1 expression correlates with subject WC and PP-sTG. A–F: Pearson correlations between TGRL-modulated surface expression of cell adhesion molecules (CAMs) [VCAM-1 (A and D), ICAM-1 (B and E), and E-selectin (C and F)] and donor WC (A–C) or PP-sTG (D–F). HAECs were treated for 4 h with TNF-α alone or simultaneously with TGRLs. CAM expression is presented as the percent change with TGRL relative to TNF-α alone. Insets: same data for samples binned into two categories based on cutoffs in PP-sTG (225 mg/dl) or WC (0.8 m). Significance was determined by a Student's t-test. Values are means ± SE; n = 13–22. NS, not significant.
PP-TGRL modulates cytokine-induced VCAM-1 expression at the transcriptional level without affecting mRNA stability.
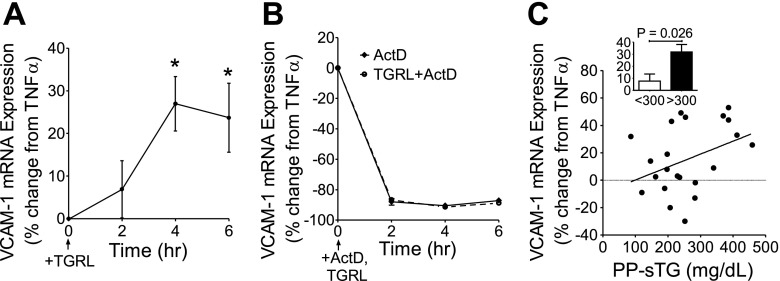
The dynamics of VCAM-1 gene expression and posttranscriptional stability in response to PP-TGRLs were assessed to determine if these could predict the upregulated protein expression. Quantitative PCR results revealed that 4 h of treatment with TGRLs alone did not induce VCAM-1 gene expression but, together with TNF-α stimulation, significantly enhanced upregulation of VCAM-1 mRNA overall by 15% (Supplemental Fig. 3). In HAECs preexposed to TNF-α for 1 h to initiate an inflammatory response, subsequent addition of PP-TGRLs overall enhanced TNF-α-stimulated VCAM-1 upregulation, which peaked at 27%, 4 h after its addition (Fig. 3A). To assess whether the increase in VCAM-1 was due to increased production or a net decrease in mRNA degradation, transcript stability was examined using ActD, an inhibitor of de novo transcription. The addition of ActD revealed a rapid turnover of VCAM-1 mRNA, as evident by a ∼90% reduction in the amount of mRNA produced by TNF-α at any given time point from 2 to 6 h after its addition (Fig. 3B). There was no difference in transcript levels in the presence or absence of PP-TGRLs, indicating that PP-TGRL priming did not change the degradation rate of VCAM-1 transcript. The priming effect of PP-TGRLs on VCAM-1 mRNA expression at 4 h was examined for variability in its response as a function of the subject's PP-sTG levels. This correlation (r = 0.3787, P = 0.0822; Fig. 3C) was not as strong as that observed for the surface expression of VCAM-1. However, TGRLs from individuals with the highest PP-sTG (>300 mg/dl) induced a significantly greater increase (average: 32%) in VCAM-1 mRNA expression (Fig. 3C, inset). In contrast, ICAM-1 and E-selectin mRNAs in response to TNF-α were modestly decreased by the presence of PP-TGRLs at 4 h and did not correlate with PP-sTG levels (Supplemental Fig. 3). Together, these results suggest that PP-TGRLs have a priming effect specific to VCAM-1 gene expression that is a function of amplified de novo transcription rather than modified RNA stability.
Fig. 3.
PP-TGRLs enhance TNF-α-induced VCAM-1 transcription without affecting mRNA stability. A and B: kinetics of VCAM-1 mRNA expression assessed by quantitative PCR at 2, 4, and 6 h after 1 h of pretreatment with TNF-α (1 ng/ml) followed by the addition of TGRL alone (A), actinomycin D (ActD, 1 μg/ml; B), or both. VCAM-1 expression is presented as the percent change with TGRLs (and/or ActD) relative to TNF-α alone at each time point (means ± SE; n = 10). Significance was determined by repeated-measures ANOVA with Student-Newman-Keuls posttest (*P < 0.05 from time = 0 h). C: Pearson correlation between TGRL-modulated VCAM-1 gene expression and donor PP-sTG. HAECs were treated for 4 h with TNF-α alone or simultaneously with TGRLs. VCAM-1 expression is presented as the percent change with TGRL relative to TNF-α alone. Inset: same data for samples binned into two categories based on the cutoff in PP-sTG (300 mg/dl). Significance was determined by Student's t-test. Values are means ± SE; n = 22.
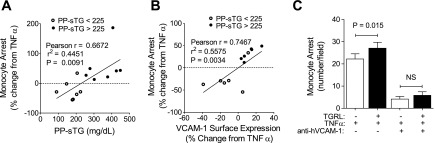
Monocyte recruitment to HAECs under shear flow increases with VCAM-1 expression under elevated PP-sTG.
Uptake of modified lipids and recruitment of monocytes to the inflamed endothelium are hallmarks of the early atherosclerotic lesion and underscore the importance of inflammation in atherogenesis (19). To provide further insights into the functional significance of VCAM-1 modulation, we assessed monocyte recruitment to HAEC monolayers primed with PP-TGRLs in a microfluidic flow channel that allowed the direct imaging of TNF-α-induced monocyte arrest under fluid shear stress (29) (Fig. 4). Monocyte arrest positively correlated both with PP-sTG (r = 0.67, P = 0.0091; Fig. 4A) and, to a greater degree, with the membrane expression of VCAM-1 (r = 0.75, P = 0.0034; Fig. 4B). A 1% change in VCAM-1 expression resulted in a corresponding 2% change in monocyte arrest. Again, we observed a critical threshold in PP-sTG of ∼225 mg/dl, above which PP-TGRLs potentiated the efficiency of monocyte arrest. Remarkable was the observation that PP-TGRLs from low PP-sTG (<225 mg/dl) subjects exerted an anti-inflammatory effect, decreasing the influence of TNF-α on VCAM-1 upregulation and monocyte arrest. Stable adhesion of monocytes was dependent on integrin binding to VCAM-1, as revealed by pretreatment with a blocking antibody that reduced adhesion by ∼80% to baseline levels (Fig. 4C). These data demonstrate that PP-TGRLs can exert both pro- and anti-inflammatory effects on TNF-α-induced VCAM-1 expression and the efficiency of monocyte recruitment.
Fig. 4.
Monocyte recruitment to HAECs under shear flow increases with VCAM-1 expression under elevated PP-sTG. A and B: Pearson correlations between TGRL-modulated monocyte arrest and TGRL donor PP-sTG (A) or HAEC VCAM-1 surface expression (B). HAECs were treated for 4 h with TNF-α alone or simultaneously with TGRLs and exposed to monocytes isolated from healthy subjects under flow (n = 14). Monocyte arrest and VCAM-1 expression are presented as percent changes with TGRL relative to TNF-α alone. C: monocyte arrest quantified in the presence or absence of anti-VCAM-1 antibody (20 μg/ml). Values are means ± SE; n = 3–5. Significance was determined by paired Student's t-test.
DISCUSSION
This study examined the effects of HTG on the endothelial inflammatory response in a cohort of 61 subjects representing a diverse population from NTG to HTG but otherwise healthy. Cultured HAECs were treated ex vivo with PP-TGRLs isolated at their peak after a single high-fat meal. Changes in VLDL composition reflected elevated PP-sTG levels in response to the meal. PP-TGRLs bound to ECs via LDLR-mediated endocytosis and specifically modulated cytokine-induced VCAM-1 expression and monocyte arrest in a manner sensitive to individual donor metabolic characteristics. This was reflected in an exacerbated inflammatory response for subjects with PP-sTG ≥ 225mg/dl or WC ≥ 0.8 m. Our findings link common epidemiological measurements of cardiometabolic risk directly with acute markers of endothelial inflammation in a vascular mimetic model.
TGRL was isolated at a time point representative of the postprandial peak in sTG, which has been demonstrated to correlate most closely with incidence of cardiovascular morbidity (2) and to have a greater effect on endothelial dysfunction compared with the fasting state (1). The absence of a priming effect on VCAM-1 expression in response to fasting TGRL from HTG individuals in our model provided a rationale to focus on the postprandial state. Moreover, our study meal was chosen to be representative of a common fast-food meal consisting of a large percentage of the calories from fat and a high ratio of saturated fat. The composition of TGRL postprandially reflects the nature of the meal. Previous studies (1, 32) comparing the effects of an isocaloric low-fat meal to a high-fat meal on EC function demonstrated no change in sTG, oxidative stress, or flow-mediated dilatation in response to the low-fat meal. The fatty acid content of a meal has previously been shown to affect the composition and distribution of VLDL postprandially (7), to enrich ApoE and ApoC-III levels in TGRL (16), most profoundly for saturated fatty acids, and to enhance VCAM-1 and E-selectin expression by ECs (35). These observations served as a motivation to focus our investigation on a high-fat test meal.
Elevated TGRL has been associated with endothelial inflammation and dysfunction, particularly in the postprandial state (32). Previous studies have reported that TGRL increased endothelial permeability (12), inflammatory cytokine production, CAM expression (20), and oxidative stress (33), decreased nitric oxide activity, and impaired flow-mediated brachial artery vasoactivity (32). However, our experiments indicated that PP-TGRL alone is not inherently inflammatory and, based on VCAM-1 expression and monocyte recruitment under shear flow, actually exerts anti-inflammatory effects. We observed a small but statistically significant reduction in VCAM-1 and E-selectin gene and protein expression in resting HAECs in response to PP-TGRL in 60–85% of subjects. As monocyte arrest is negligible in unstimulated ECs, these observations may highlight a homeostatic feedback mechanism that maintains low basal CAM expression and function under enhanced metabolic activity, thereby countering inflammation (9).
The lack of an overt inflammatory response to PP-TGRLs implies the absence of oxidation, as oxidized lipoproteins themselves enhance inflammatory CAM expression to levels comparable to TNF-α (31). Our study used native TGRL stored such that no oxidation occurred ex vivo. In contrast to oxidized lipoproteins, which are cleared by scavenger receptors on ECs and have been directly implicated in atherogenesis (19), native TGRLs and their RPs may be bound and internalized by the LDLR family. These not only function as cargo receptors that deliver macromolecules to the cell via endocytosis but also affect cell signaling and the maintenance of cholesterol homeostasis (30). We demonstrated that LDLR-mediated endocytosis accounted for ∼80% of the cellular uptake of native TGRL in our model, which was increased under inflammation. We reason that uptake via LDLRs results in acute receptor-mediated signaling events (i.e., within 1 h of TNF-α stimulation) that can regulate a subsequent cytokine- mediated inflammatory response.
The absence of an inflammatory response to PP-TGRL treatment is consistent with our previous report (31) in which a chronic vascular injury model was applied. We previously reported that repetitive conditioning with PP-TGRLs over 3 days alone did not elicit inflammation but primed HAECs to respond to TNF-α stimulation at concentrations comparable with those observed in atherosclerotic plaques (28). Our results contrast with other recent studies (24, 25) reporting that TGRL alone was sufficient to increase the expression of a large number of inflammatory genes and to enhance VCAM-1 surface expression in HAECs. In the latter studies, NTG subjects were compared with type IV HTG subjects administered a much higher oral fat load (82% calories from fat). A higher fat load is one factor that might account for a greater inflammatory response in NTG subjects or in response to lipid alone. Our subject pool represented a continuous distribution from normal to hyperlipidemic, and our meal was typical of that associated with a Western high-fat diet. Although both studies demonstrated an increased inflammatory potential of PP-TGRLs from HTG subjects, our study demonstrated the emergence of inflammatory outcomes only upon costimulation with a low dose of TNF-α. Since TNF-α is considered a biomarker of inflammation in human serum, our results may indicate that subjects with preexisting low-grade inflammation are more susceptible to postprandial-induced endothelial dysfunction.
The observation of a threshold in subject PP-sTG above which PP-TGRLs modulated an inflammatory response implies that metabolic stress can sensitize the endothelium to pathological changes (9). Changes in endothelial VCAM-1 expression proved to be the most sensitive indicator of individual variation in the inflammatory potential of PP-TGRLs. Monocyte arrest revealed a functional response that corroborated our findings at the level of VCAM-1 gene and protein expression. Notably, we observed that only a 1% change in VCAM-1 expression elicited a significant 2% increment in monocyte arrest over a dynamic range within which PP-TGRLs modulated TNF-α-induced VCAM-1 expression from a −20% drop to a 28% increase from cytokine alone. We chose to use isolated monocytes from healthy, fasting, normolipidemic subjects for this study to decrease variability and in recognition that PP-sTG can impact inflammatory responses in monocytes as well. For example, it has been recently reported that monocyte avidity for VCAM-1 is increased postprandially via a mechanism involving the upregulation of CD11c (13), where subject PP-sTG was also a reliable indicator of monocyte activation. Thus, complementary mechanisms exist in ECs and monocytes that are consistent with the enhancement of transient inflammatory responses to postprandially elevated sTG. This supports the notion that atherosclerosis may develop in metabolically disposed subjects gradually in response to repetitive postprandial inflammatory insult.
Although it is widely recognized that dyslipidemia induced by an atherogenic diet upregulates endothelial inflammatory responses, a novelty of our study was the observation of the variability in the inflammatory response and the direct correlation with donor PP-sTG and abdominal obesity. The differential capacity of PP-TGRL to alter HAEC inflammatory responses may be attributed to individual heterogeneity in particle lipid and Apo composition postprandially, reflecting both the meal and the subject's metabolic status (17). We observed that HTG subjects with more visceral fat storage produced larger triglyceride-enriched VLDL particles that were more inflammatory. Previous studies have provided evidence that ApoC-III, ApoE, and cholesterol are enriched in postprandial TGRL, particularly in HTG subjects (3, 6) or after a meal high in saturated fat (16). Variations in Apo composition could lead to differences in receptor-mediated binding and signaling events. For example, enrichment of ApoE, which serves as a potent ligand for LDLRs (4), facilitates the internalization of TGRLs and their remnant particles, whereas ApoC-III has been reported to upregulate EC VCAM-1 and ICAM-1 expression via activation of PKC-β and the NF-κB pathway (18). Heterogeneity in inflammatory responses to TGRLs can also reflect the fatty acid content of the particles released upon metabolism. An increase in circulating free fatty acids has been associated with HTG and obesity (23), and fatty acids have been demonstrated to act as pro- and anti-inflammatory modulators of the EC response to cytokine (10, 34). It has recently been demonstrated that fatty acids released upon the ex vivo lipolysis of PP-TGRLs contained neutral and oxygenated lipids that activated ROS production in ECs (33). In contrast, certain fatty acids released from TGRLs upon lipolysis may serve as agonists for peroxisome proliferator-activated receptors, which downregulate TNF-α- and VLDL-induced VCAM-1 expression (37). Thus, the EC response to lipolysis should reflect a balance between inflammatory and anti-inflammatory lipids contained in the particles (33). Notably, our study used native intact TGRLs, although it does not rule out the involvement of fatty acids released by endogenous EC metabolic activity at the membrane or after endocytosis. Additional studies are needed to demonstrate the relative role of different signaling mechanisms activated as a consequence of changes in TGRL Apo or fatty acid composition in modulating TGRL-induced pro- or anti-inflammatory signaling in our model.
The cytokine-induced surface expression of ICAM-1 and E-selectin were equally elevated by PP-TGRLs at 4 h. However, only VCAM-1 significantly correlated with the variation in a subject's lipids or anthropometric characteristics and was elevated at the transcript level. The relative membrane expression of VCAM-1 in response to TNF-α stimulation may be regulated either transcriptionally (8) or posttranscriptionally, through mechanisms that affect mRNA stability or translation (15). We propose that the relative capacity of PP-TGRLs to modulate the inflammatory response to cytokine is through altering expression and activation of transcription factors that act cooperatively with NF-κB in the CAM promoter. In this regard, TGRLs have been reported to activate the p38 pathway and the binding of NF-κB, activator protein-1, and cAMP response element-binding protein to the promoters of inflammatory genes in hyperlipidemic subjects (25). We (31) have also previously demonstrated that PP-TGRLs enhanced p38 MAPK activation that, together with cytokine stimulation, resulted in greater transcriptional activity of NF-κB. Posttranscriptional regulatory mechanisms may also contribute to differential expression of CAMs during inflammation. For example, miRNA126 has been recently shown to endogenously suppress VCAM-1 expression in ECs and inhibit translation without affecting TNF-α-induced transcription or transcript stability (15).
We demonstrated that PP-TGRLs isolated at the peak after a high-fat meal modulated a transient inflammatory response in ECs in proportion to the level of subject HTG or visceral obesity. Abdominal obesity is associated with metabolic abnormalities and an increased risk of type II diabetes and atherosclerotic CVD (11, 14). WC (>40 in. for men and >35 in. for women) is a major criterion for the diagnosis of metabolic syndrome and, together with elevated F-sTG (>150 mg/dl), may reflect a subject's propensity for more metabolically active visceral adipose tissue and elevated risk (11, 14). Our findings link simple clinical metrics with biologically relevant markers of endothelial inflammation that may provide a means for assessing an individual's response to a repeated metabolic challenge and an early measure of associated cardiovascular risk. Specifically, we demonstrated that TGRL particles from subjects with elevated PP-sTG (>225 mg/dl) and WC above a subclinical threshold of 0.8 m (∼31.5 in) for abdominal obesity correlated with enhanced VCAM-1 expression and the recruitment of monocytes, a harbinger of atherosclerosis (19).
In conclusion, we applied a reductionist approach to evaluate the inflammatory potential of an individual's PP-TGRLs and demonstrated a direct correlation between the spike in circulating triglycerides after a meal and its capacity to specifically alter the expression of VCAM-1 and monocyte recruitment on the aortic endothelium using a rapid and reliable lab-on-a-chip assay.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-082689 (to S. I. Simon and A. G. Passerini) and a Howard Hughes Medical Institute Med Into Grad Fellowship (University of California-Davis; to Y. I. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Soichiro Yamada for confocal microscopy and Dr. R. Michael Gower and Dr. Anne A. Knowlton for thoughtful discussion on the manuscript.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Bae JH, Bassenge E, Kim KB, Kim YN, Kim KS, Lee HJ, Moon KC, Lee MS, Park KY, Schwemmer M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 155: 517–523, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298: 309–316, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Batal R, Tremblay M, Barrett PHR, Jacques H, Fredenrich A, Mamer O, Davignon J, Cohn JS. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J Lipid Res 41: 706–718, 2000. [PubMed] [Google Scholar]
- 4. Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature 341: 162–164, 1989. [DOI] [PubMed] [Google Scholar]
- 5. Bjorkegren J, Karpe F, Milne RW, Hamsten A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low density lipoproteins isolated from fasting and postprandial plasma. J Lipid Res 39: 1412–1420, 1998. [PubMed] [Google Scholar]
- 6. Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Brit J Nutr 93: 3–9, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Chan JW, Motton D, Rutledge JC, Keim NL, Huser T. Raman spectroscopic analysis of biochemical changes in individual triglyceride-rich lipoproteins in the pre- and postprandial state. Anal Chem 77: 5870–5876, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J 9: 899–909, 1995. [PubMed] [Google Scholar]
- 9. Davies PF, Civelek M, Fang Y, Guerraty MA, Passerini AG. Endothelial heterogeneity associated with regional athero-susceptibility and adaptation to disturbed blood flow in vivo. Semin Thromb Hemost 36: 265–275, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr 71: 213s-–223s., 2000. [DOI] [PubMed] [Google Scholar]
- 11. Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28: 1039–1049, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol 292: H2745–H2753, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 31: 160–166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grundy SM. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol 47: 1093–1100, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 105: 1516–1521, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jackson KG, Wolstencroft EJ, Bateman PA, Yaqoob P, Williams CM. Greater enrichment of triacylglycerol-rich lipoproteins with apolipoproteins E and C-III after meals rich in saturated fatty acids than after meals rich in unsaturated fatty acids. Am J Clin Nutr 81: 25–34, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Kannel WB, Vasan RS. Triglycerides as vascular risk factors: new epidemiologic insights. Curr Opin Cardiol 24: 345–350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 114: 681–687, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Lundman P, Eriksson MJ, Silveira A, Hansson LO, Pernow J, Ericsson CG, Hamsten A, Tornvall P. Relation of hypertriglyceridemia to plasma concentrations of biochemical markers of inflammation and endothelial activation (C-reactive protein, interleukin-6, soluble adhesion molecules, von Willebrand factor, and endothelin-1). Am J Cardiol 91: 1128–1131, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mano T, Masuyama T, Yamamoto K, Naito J, Kondo H, Nagano R, Tanouchi J, Hori M, Inoue M, Kamada T. Endothelial dysfunction in the early stage of atherosclerosis precedes appearance of intimal lesions assessable with intravascular ultrasound. Am Heart J 131: 231–238, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Mostaza JM, Vega GL, Snell P, Grundy SM. Abnormal metabolism of free fatty acids in hypertriglyceridaemic men: apparent insulin resistance of adipose tissue. J Intern Med 243: 265–274, 1998. [DOI] [PubMed] [Google Scholar]
- 24. Norata GD, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, Eriksson P, Catapano AL. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis 193: 321–327, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Norata GD, Grigore L, Raselli S, Seccomandi PM, Hamsten A, Maggi FM, Eriksson P, Catapano AL. Triglyceride-rich lipoproteins from hypertriglyceridemic subjects induce a pro-inflammatory response in the endothelium: molecular mechanisms and gene expression studies. J Mol Cell Cardiol 40: 484–494, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298: 299–308, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM, Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb 12: 1336–1345, 1992. [DOI] [PubMed] [Google Scholar]
- 28. Rus HG, Niculescu F, Vlaicu R. Tumor necrosis factor-α in human arterial wall with atherosclerosis. Atherosclerosis 89: 247–254, 1991. [DOI] [PubMed] [Google Scholar]
- 29. Schaff UY, Xing MM, Lin KK, Pan N, Jeon NL, Simon SI. Vascular mimetics based on microfluidics for imaging the leukocyte–endothelial inflammatory response. Lab Chip 7: 448–456, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrin Met 13: 66–74, 2002. [DOI] [PubMed] [Google Scholar]
- 31. Ting HJ, Stice JP, Schaff UY, Hui DY, Rutledge JC, Knowlton AA, Passerini AG, Simon SI. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-α. Circ Res 100: 381–390, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 79: 350–354, 1997. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res 50: 204–213, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L, Lim EJ, Toborek M, Hennig B. The role of fatty acids and caveolin-1 in tumor necrosis factor α-induced endothelial cell activation. Metabolism 57: 1328–1339, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams CM, Maitin V, Jackson KG. Triacylglycerol-rich lipoprotein-gene interactions in endothelial cells. Biochem Soc Trans 32: 994–998, 2004. [DOI] [PubMed] [Google Scholar]
- 36. Zilversmit DB. Atherogenesis–postprandial phenomenon. Circulation 60: 473–485, 1979. [DOI] [PubMed] [Google Scholar]
- 37. Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA 100: 2730–2735, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.