Abstract
The carbohydrate antigen globo H commonly found on breast cancer cells is a potential target for vaccine therapy. The objectives of this trial were to determine the toxicity and immunogenicity of three synthetic globo H-keyhole limpet hemocyanin conjugates plus the immunologic adjuvant QS-21. Twenty-seven metastatic breast cancer patients received five vaccinations each. The vaccine was well tolerated, and no definite differences were observed among the three formulations. Serologic analyses demonstrated the generation of IgM antibody titers in most patients, with minimal IgG antibody stimulation. There was significant binding of IgM antibodies to MCF-7 tumor cells in 16 patients, whereas IgG antibody reactivity was observed in a few patients. There was evidence of complement-dependent cytotoxicity in several patients. Affinity column purification supported the specificity of IgM antibodies for globo H. On the basis of these data, globo H will constitute one component of a polyvalent vaccine for evaluation in high-risk breast cancer patients.
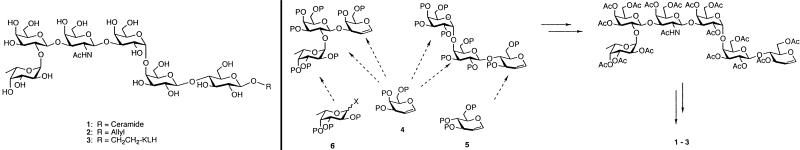
On the basis of promising clinical results, there has been renewed interest in the investigation of an immunotherapeutic approach with either monoclonal antibodies (1, 2, †) or vaccines (3–8) for the treatment of breast cancer. In particular, tumor-associated carbohydrate antigens, expressed as protein or lipid conjugates, are commonly found on breast cancer cells and may serve as potential targets for vaccine therapy. Among these, we have been particularly interested in the immunomodulatory potential of the hexasaccharide portion of the glycoceramide globo H. This antigen was identified on the human breast cancer cell line MCF-7 (9) as well as on a variety of epithelial cell tumors of ovarian, gastric, pancreatic, endometrial, prostate, and lung (small cell and nonsmall cell) origin (10). It is also expressed on the luminal surfaces of normal epithelial cells in the breast, pancreas, uterus, lung, ovary, stomach, and prostate (10). The structure of globo H was assigned by Hakomori and colleagues as Fucα1→ 2Galβ1→3GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1Cer, (1, Fig. 1) (11) and was subsequently synthesized by Bilodeau et al. (12).
Figure 1.
(Left) Globo H glycoconjugates. Globo H ceramide (1); totally synthetic globo H glycoside (2); synthetic vaccine globo H-KLH (3). (Right) Globo H total synthesis. The fully synthetic Globo H hexasaccharide was prepared by using the logic of glycal assembly in an optimally convergent stepwise manner by using galactal (4) as well as glucal (5) and fucose (6).
We have developed an approach for augmenting the immunogenicity of synthetic antigens by conjugation to the immunogenic protein carrier keyhole limpet hemocyanin (KLH) combined with the potent immunological adjuvant QS-21 (13–16). There is a variety of methods for conjugation of KLH to an antigen, including direct conjugation and conjugation with a bifunctional linker group such as 4-(4-N-maleimidomethyl) cyclohexane-1-carboxyl hydrazide (MMCCH) (17). This latter method can result in higher antigen/KLH ratios, greater yields of the antigen/KLH conjugate, and improved immunogenicity.
Mouse studies have confirmed the generation of antibodies against globo H after vaccination with KLH or CRM197 (diptheria toxoid) conjugates, prepared by a direct conjugation protocol (18, 19). There was evidence of a polyclonal antibody response, mediation of complement cytotoxicity, and binding of IgM antibodies with MCF-7 breast cancer cells (18). In patients with recurrent prostate cancer, vaccination with a globo H-KLH conjugate plus QS-21 resulted in the production of significant IgG and IgM antibody titers, predominantly IgM (20–22). Of 18 evaluable patients, 10 were found to develop IgM antibodies reactive with MCF-7 cells with no significant IgG reactivity (20). An increase in complement-dependent cytotoxicity (CDC) was observed in nine patients. Immune response did not appear dose related among the three higher doses of globo H, including 10 μg, 30 μg, and 100 μg, although the 3-μg dose yielded lower antibody titers.
On the basis of these encouraging results, we initiated a clinical trial in patients with metastatic breast cancer without evidence of disease or with stable disease on hormone therapy. The first objective of this trial was to determine the toxicity of this fully synthetic vaccine in pretreated breast cancer patients. The second objective was to determine whether immunization with a globo H-KLH conjugate plus QS-21 would generate an antibody response against globo H and breast cancer cells expressing globo H, and in particular to assess whether these antibodies could mediate CDC and antibody-dependent cell-mediated cytotoxicity (ADCC). The third objective was to evaluate whether the method of conjugation between globo H and KLH (direct or crosslinked) or the type of formulation of the vaccine (stored in saline or lyophilized) would affect the immune response. In addition to the well established “direct conjugation” strategy, we also examined the “crosslinked conjugation method” by using the bifunctional linker MMCCH (17). Finally, although clinical response was not a primary endpoint, the patient's disease status was monitored.
Patients and Methods
Patients.
Patients with a history of metastatic breast cancer without evidence of disease after therapy or those with active disease who were on hormonal therapy were eligible. Four weeks had to have elapsed since prior surgery, chemotherapy, or radiation therapy, and 6 weeks since prior immunotherapy. Exclusion criteria included: a seafood allergy, a known autoimmune or immunodeficiency disorder, active steroid therapy, clinically significant New York Heart Association Class 3 or 4 disease, other active cancers (excluding basal cell or squamous carcinomas of the skin), and Karnofsky performance status ≤80. Premenopausal women were required to have a normal β human chorionic gonadotrophin level, as pregnant women were ineligible. Patients were required to have the following laboratory parameters: total lymphocyte count of ≥0.5 × 106/ml, total white blood cell count of ≥3,000 cells/mm3, serum creatinine ≤1.5 × upper limit of normal, serum aspartate aminotransferase ≤1.5 × upper limit of normal, and serum alkaline phosphatase ≤1.5 × upper limit of normal.
Pretreatment evaluation included the following within 3 weeks of protocol treatment: history and physical examination, rectal examination with hemoccult, chest x-ray, complete blood count with differential, chemistry profile, serum amylase, carcinoembryonic antigen, and CA15–3 or BR2729 level. The following studies were required within 4 weeks of treatment: computerized tomography scan of the chest, abdomen and pelvis, bone scan, and pelvic examination. All patients signed an informed consent approved by the Institutional Review Board and the Food and Drug Administration.
Treatment Plan and On-Study Evaluation.
The globo H-KLH conjugate plus QS-21 was administered s.c. during weeks 1, 2, 3, 7, and 19 for a total of 5 doses for each patient. The vaccine was usually injected into the upper arm or thigh by nurses in the Immunology Outpatient Unit. Each vaccine contained 10 μg of globo H in a globo H-KLH conjugate and 100 μg of QS-21. This dose of QS-21 had previously been confirmed as effective with minimal toxicity (23). Three versions of the globo H-KLH conjugate were evaluated: Group 1, direct conjugation of globo H and KLH plus QS-21 in saline; Group 2, crosslinked conjugation of globo H and KLH plus QS-21 in saline; Group 3, crosslinked conjugation of globo H and KLH plus QS-21 lyophilized and reconstituted with saline at the time of injection. Each version of the vaccine was evaluated in 9 patients for a total of 27 patients. Patients were sequentially assigned to a specific vaccine beginning with Group 1.
During the trial, patients were monitored for clinical toxicity and response. Laboratory studies, including complete blood count, chemistry profile, amylase level, carcinoembryonic antigen and CA15–3 or BR2729 levels, and stool hemoccult were performed intermittently as outlined in Table 1. Most patients also had repeat computerized tomography scans and bone scans during weeks 21–24.
Table 1.
Treatment schedule and on-study evaluation
| Week no.
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | 9 | 13 | 19 | 21 | q 3 mo | |
| Vaccination | x | x | x | x | x | |||||
| History/physical | x | x | x | x | ||||||
| CBC with diff | x | x | x | |||||||
| Chemistry profile | x | x | ||||||||
| Amylase level | x | x | x | |||||||
| Stool hemoccult | x | x | x | |||||||
| CEA/CA15-3 | x | x | x | |||||||
| Immune response | x | x | x | x | x | x | x | x | x | x |
CBC, complete blood count; CEA, carcinoembryonic antigen.
Vaccine Preparation.
The globo H antigen was prepared by total synthesis (see Fig. 1) (12, 17, 22, 24). The synthetic globo H construct was then conjugated to KLH (PerImmune, Rockville, MD) by two different methods. The direct method involved reductive linkage of the aldehyde group derived from 2 (Fig. 1) to the NH2 groups on KLH by using sodium cyanoborohydride. The conjugation ratio of globo H molecules conjugated to each KLH molecule was 373:1 in 7% yield, based on 2. The crosslinked method attached the aldehyde group derived from 2 (Fig. 1) to the NH2 groups on the crosslinker MMCCH (Pierce, Rockford, IL) with sodium cyanoborohydride, as previously described (17). Sulfhydryl groups on thiolated KLH were then attached to the maleimide group on the MMCCH and incubated for 2 h at room temperature. The globo H/KLH conjugation ratio was 732:1 in 29% yield, based on 2 (Fig. 1). After conjugation, the globo H-KLH conjugates were washed, filtered, and placed in individual vials containing 10 μg of conjugated globo H in saline or lyophilized and stored, to be reconstituted in saline at time of injection. In Groups 1 and 2, the globo H-KLH conjugate was mixed with 100 μg of QS-21 (Aquila Biopharmaceutical, Framingham, MA) and stored in saline. In Group 3, the lyophilized globo H-KLH conjugate was mixed with 100 μg of QS-21 immediately before vaccination. All vaccines underwent testing for sterility and immunogenicity.
Serologic Assays.
Peripheral blood was sampled at various time points (see Table 1) to determine whether IgM and IgG antibodies against synthetic globo H and tumor cells expressing globo H were generated. If feasible, patients returned every 3 months for further antibody testing.
ELISA.
Blood samples were evaluated by ELISA assays according to the following procedure (22). NUNC 96-well ELISA plates were coated with 0.1 μg of globo H-ceramide per well in ethanol and dried overnight at room temperature. Unreactive sites were blocked by incubation with 3% human serum albumin for 2 h. Serial dilutions of the patient's sera were added to the wells, left at room temperature for 1 h, and washed. Secondary antibodies, either alkaline phosphatase-labeled goat anti-human IgM or unlabeled mouse anti-human IgG, were added. For IgG detection, a tertiary antibody, alkaline phosphatase-labeled goat anti-mouse IgG (Southern Biotechnology Associates) was then added. After a 45-minute incubation, the plates were washed, developed, and read at 414 nm on the ELISA reader. The highest serum dilution with an absorbance of ≥0.100 was recorded as the antibody titer.
Fluorescence-Activated Cell Sorter (FACS).
FACS analyses were performed on pretreatment and posttreatment sera to determine whether IgM or IgG antibodies were able to bind to MCF-7 tumor cells that express globo H (22). Dilutions (1:10) of patient sera were added to the tumor cells, which were washed and mixed with 20 μl of a 1:25 dilution of goat anti-human IgM or IgG antibody (Southern Biotechnology). These antibodies were FITC-labeled. After a 30-minute incubation on ice, the cells were washed, and the percentage of positive cells and mean fluorescence was detected by a flow cytometer (FACScan, Becton Dickinson).
CDC.
Chromium release assays to assess complement-mediated cytotoxicity were performed for each patient at various time points (25). MCF-7 tumor cells were washed in FCS-free media two times, resuspended in 500 μl of media, and incubated with 100 μCi 51Cr per 10 million cells for 2 h at 37°C. The cells were then shaken every 15 min for 2 h, washed 3 times in media to achieve a concentration of approximately 20,000 cells/well, and then plated in round-bottom plates. The plates contained either 50 μl cells plus 50 μl monoclonal antibody, 50 μl cells plus serum (pre- and posttherapy), or 50 μl cells plus mouse serum as a control. The plates were incubated in a cold room on a shaker for 45 min. Human complement of a 1:5 dilution (resuspended in 1 ml of ice-cold water and diluted with 3% human serum albumin) was added to each well at a volume of 100 μl. Control wells included those for maximum release of isotope in 10% Triton X-100 (Sigma) and for spontaneous release in the absence of complement with medium alone. The plates were incubated for 2 h at 37°C, centrifuged for 3 min, and then 100 μl of supernatant was removed for radioactivity counting. The percentage of specific lysis was calculated as follows: % cytotoxicity = [(experimental release–spontaneous release)/(maximum release–spontaneous release)] × 100. A doubling of the CDC to >20% was considered significant.
ADCC.
MCF-7 tumor cells, cultured in DME plus 10% FBS, were labeled with 100 μCi 51Cr for 1 h (26). After being washed three times with culture medium, cells were resuspended at 105/ml, and 100 μl/well were plated onto 96-well round-bottom plates. Donor peripheral blood mononuclear cells were plated at a 100:1 and 50:1 ratio; 20 μl/well of pre- and postimmune patient sera or commercially available pooled human AB serum (Gemini Biological Products, Calabasas, CA) were added. After an 18-h incubation at 37°C, supernatant (30 μl/well) was harvested and transferred onto Lumaplate 96 (Packard), dried, and read in a Packard Top-Count NXT γ counter. Each measurement was carried out in triplicate. Spontaneous release was determined by cpm of tumor cells incubated with medium and maximum release by cpm of tumor cells plus 1% Triton X-100 (Sigma). Specific lysis was defined as: % specific lysis = [(experimental release–spontaneous release)/(maximum release–spontaneous release)] × 100. The percent ADCC is expressed as peak specific lysis postimmune subtracted by preimmune percent specific lysis. A doubling of the ADCC to >20% was considered significant.
Affinity Column Purification of Globo H Antibodies.
Pre- and postvaccination sera for five patients (patients nos. 11, 14, 15, 16, and 17) were selected to evaluate the specificity and to quantify the vaccine-induced antibodies. To this end, the antibodies were isolated from serum and purified through affinity chromatography. The affinity columns were designed, synthesized, and used for isolation purposes, as described (21). Briefly, the antigen was prepared by total synthesis and conjugated to the amino agarose gel (0.6 μmol globo H/ml of gel). By using 3 ml of column material, 1 ml of patient sera was added and incubated at 4°C for 1 h with gentle agitation. Column elution, collected in 1-ml fractions, was performed as described (21). Proteins were detected by using the Pharmacia LKB Ultrapsec III at 280 nm. ELISA assays by using the previously described technique were performed, with 0.2 μg globo H ceramide, and read at an absorbance of 414 nm. Purified antibodies were pooled, centrifuged in Centricon 30 tubes at 4°C for an average of 25 min, and then redissolved in 1 ml PBS. ELISA assays and protein quantification were performed on these samples, by using globo H ceramide coated plates or Ley-coated plates.
Criteria for Cessation of Treatment and Dose Reduction.
Patients with evidence of disease progression while on study requiring chemotherapy or radiation therapy were removed from the trial. Grade III or greater local or systemic toxicity according to the Common Toxicity Criteria resulted in a 50% reduction in the components of future vaccines for the individual patient. Grade III lymphopenia was excluded, because a grade 3 value was acceptable as a pretreatment value.
Biostatistical Considerations.
A total of 27 patients were entered into this pilot trial to evaluate the immunogenicity of the globo H-KLH plus QS-21 vaccine. After vaccination, a serologic response included an antibody titer of ≥1:40 with a baseline titer of 0 or a ≥2-fold increase with a baseline titer of greater than 0. On the basis of prior statistical analyses (27), if more than four of nine patients achieve a serologic response, the vaccine would be considered promising.
Results
Patients.
Twenty-seven patients with metastatic breast cancer were treated during this trial between September 1997 and August 1999. Patient characteristics were comparable between the three groups, as outlined in Table 2. The median age was 46 years (range 35–63 years). Of these, 15 had a history of metastatic disease, but at the time of protocol entry had no definite evidence of disease, and 12 patients had metastatic disease that was relatively stable on hormone therapy. The number of patients with the following dominant sites of disease before protocol entry included: bone (8), lung (2), chest wall (11), liver (1), and lymph nodes (5). Metastatic disease was found in 4 patients within 6 weeks of the original diagnosis of breast cancer. For all patients, the median interval between breast cancer diagnosis and diagnosis of metastatic disease was 141 weeks (range 0–1,386 weeks); the median interval from diagnosis of metastatic disease to entry into this study was 96 weeks (range 11–446 weeks). During the trial, 22 patients were on hormone therapy, either tamoxifen, anastrazole, or megestrol acetate.
Table 2.
Patient characteristics
| Characteristics | No. of patients
|
|||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Total | |
| Number of patients | 9 | 9 | 9 | 27 |
| Stage 4 NED | 5 | 5 | 5 | 15 |
| Stage 4 stable disease | 4 | 4 | 4 | 12 |
| Patients with prior chemotherapy | ||||
| None | 2 | 1 | 1 | 4 |
| Adjuvant only | 0 | 4 | 1 | 5 |
| Metastatic only | 5 | 1 | 1 | 7 |
| Adjuvant + metastatic | 2 | 3 | 6 | 11 |
| Patients with prior radiotherapy | ||||
| None | 2 | 2 | 0 | 4 |
| Adjuvant only | 1 | 2 | 1 | 4 |
| Metastatic only | 5 | 4 | 5 | 14 |
| Adjuvant + metastatic | 1 | 1 | 3 | 5 |
| Patients on hormone therapy during the study | 6 | 9 | 7 | 22 |
NED, no evidence of disease.
Twenty-six patients received five vaccinations each, and one patient (no. 12) was removed from the trial because of disease progression in the liver after the fourth vaccine. However, all patients were considered evaluable for toxicity and serologic response. Of the 26 patients treated with 5 vaccinations each, 24 patients were treated according to schedule. Two patients experienced treatment delays. One patient (no. 17) developed an elevated amylase level, which is further described below. Another patient (no. 13) developed an infection of a breast implant requiring surgical removal and prolonged antibiotic therapy after the third vaccine. She received the fourth and fifth vaccinations during weeks 35 and 47, respectively, at the full doses.
Toxicities.
In general, the vaccine was well tolerated; local skin reactions and mild flu-like symptoms were the predominant side effects. The common toxicities observed during the trial are noted in Table 3. Local reactions at the vaccine site, including discomfort, erythema, and induration, usually ranged from 1–7 days in duration, although most resolved within 3–4 days. Flu-like symptoms, including myalgias, arthralgias, fever, and fatigue, were transient, ranging from 1–7 days duration, but often resolved within 2–3 days. Serial hemoccult stool samples revealed no evidence of gastrointestinal bleeding. Five patients noted a recall reaction at the first vaccine site after the second vaccination. Several patients complained of transient pruritis at the vaccine site, and one patient complained of grade 2 pruritis of the scalp, palms, and soles of the feet for 3 days on one occasion. Two patients experienced enlarged inguinal nodes after vaccinations on the thigh.
Table 3.
Common toxicities: The number of episodes that occurred after all vaccinations
| Toxicity | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Local skin reaction | 2 | 111 | 0 |
| Rash (not at vaccine site) | 1 | 3 | 0 |
| Arthralgias | 2 | 3 | 0 |
| Constipation | 3 | 0 | 0 |
| Cough | 4 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 |
| Fatigue | 21 | 9 | 0 |
| Fever | 14 | 10 | 0 |
| Headache | 21 | 5 | 0 |
| Myalgias | 38 | 6 | 0 |
| Nausea | 5 | 0 | 0 |
| Vomiting | 1 | 1 | 0 |
| Rigors/chills | 13 | 0 | 0 |
| Pruritis | 13 | 1 | 0 |
| Increase in amylase | 2 | 0 | 1 |
| Leukopenia | 7 | 2* | 0 |
| Neutropenia | 3 | 1† | 0 |
| Lymphopenia | 4 | 13‡ | 9§ |
| Anemia | 5 | 0 | 1¶ |
| Thrombocytopenia | 5 | 0 | 0 |
Total number of vaccinations: 134.
Both patients had pretreatment grade 1 values.
This patient had a pretreatment grade 1 value.
Twelve patients had pretreatment grade 2 values.
Six patients had pretreatment grade 3 values; 3 patients had pretreatment grade 2 values.
This patient had a pretreatment grade 3 value.
One patient developed erythematous papules on the face approximately 6 days after the second vaccine, after sun exposure. The rash resolved after 1 day of treatment with triamcinolone cream. She subsequently complained of scalp sensitivity after the fourth vaccine without further rashes. During week 19, two patients (nos. 10 and 11) complained of subjective heaviness or fatigue in the lower extremities. Neurologic and radiographic evaluations were normal; these symptoms were likely not related to the vaccine.
During week 18, one patient (no. 17), who had a normal amylase level prevaccine and during week 7, developed a grade 3 elevation in the amylase level of 281 units/liter (normal 25–125 units/liter). She was without significant symptoms, and an upper abdominal ultrasound and chemistry profile were normal. A lipase level was 37 units/liter (7–60 units/liter). Fractionation of the amylase level included: total 186 units/liter (30–70 units/liter), salivary isoamylase 138 units/liter (12–137 units/liter), pancreatic isoamylase 48 units/liter (7–54 units/liter); macroamylase, none detected. By week 20, the amylase level had normalized to 102 units/liter (25–125 units/liter), and the patient was asymptomatic at week 21. She received the fifth vaccination during week 26 with a 50% reduction of all components because of the grade 3 toxicity. On that day, the amylase level was 106 units/liter and subsequently increased to 129 units/liter (25–125 units/liter) at week 28 with a normal lipase level. By week 30, the amylase level was normal and remained normal 4 and 7 months later. The etiology of the hyperamylasemia is unclear, although possible etiologies include infection, drugs, or the vaccine. Three other patients also had elevated amylase levels. These included two patients with grade 1 pretreatment amylase levels; in one patient, the value normalized by week 3, and in the other patient the grade 1 value persisted. A third patient developed a grade 1 value at week 19 that normalized by week 31.
Several patients developed grade 1 toxicity laboratory values for glucose, alkaline phosphatase, serum aspartate aminotransferase, calcium, albumin, or potassium. One patient had a pretreatment grade 3 total bilirubin level (1.6 mg/dl with a normal direct bilirubin level) that did not increase during the trial; no dose reduction was made. Another patient had a pretreatment grade 3 glucose value that was present occasionally during the trial; no dose reduction was made. One patient began the trial with a grade 1 creatinine level that transiently increased to a grade 2 value during week 2 and then returned to baseline, which was likely because of medication for a viral infection. Several patients had transient decreases in various hematologic values as outlined in Table 3. It is unclear whether these variations were directly related to the vaccine or to normal biologic fluctuations. One patient had a pretreatment grade 3 hemoglobin that continued intermittently during the trial. There was no evidence of hemolysis, and the anemia was felt to be secondary to prior high-dose chemotherapy and mild renal insufficiency. No dose reduction was made as it predated the vaccine.
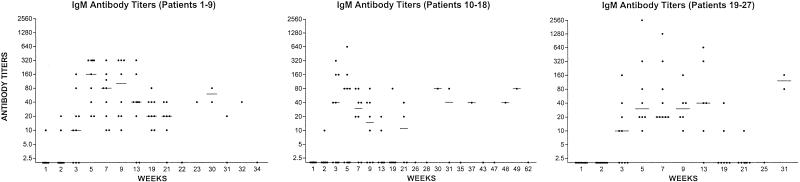
Serologic Response.
Evaluation of IgM antibody titers in patient sera was performed by ELISA (Fig. 2). Although median antibody titers were slightly higher in the first group of patients, serologic assays demonstrated no distinct differences among the three groups. Low IgG antibody titers were consistently observed (data not shown) with the following range of values: group 1 (0–40), group 2 (0–10), group 3 (0–160). The globo H vaccines generated a strong IgM response independent of formulation or linker. Flow cytometric assays to assess the percentage of MCF-7 tumor cells reactive with the patient's IgM antibodies pre- and posttherapy are presented in Table 4. Over half of the patients in this trial exhibited at least a 3-fold increase in IgM reactivity, by FACS analysis, including 4 patients in Group 1, 5 patients in Group 2, and 7 patients in Group 3. There was little reactivity of IgG antibodies with tumor cells in these assays, although 3 patients (2 in Group 1 and 1 in Group 3) demonstrated a significant increase in IgG reactivity (data not shown). CDC was analyzed by using chromium release assays. A significant increase in activity was observed in 9/27 patients (Table 4). A significant increase in ADCC was also observed in 8/27 patients including 5 patients in Group 3 (data not shown).
Figure 2.
IgM antibody reciprocal titers after vaccination with globo H-KLH conjugate + QS-21 for all patients. The line in each column indicates the median titer.
Table 4.
Pre- and posttherapy IgM antibody titers and serologic analyses
| Patient no. | Week no. | ELISA titers | FACS % of positive cells | MFI | CDC % lysis |
|---|---|---|---|---|---|
| 1 | 2 | 0 | 10.8 | 92.6 | 3 |
| 9 | 320 | 43.6 | 197.3 | 15.7 | |
| 2 | 1 | 0 | 11 | 145.1 | 2.7 |
| 5 | 80 | 10.8 | 146.5 | 9.1 | |
| 3 | 1 | 10 | 10.4 | 65.2 | −2.8 |
| 13 | 320 | 64.7 | 154.4 | 37.3 | |
| 4 | 1 | 0 | 10.2 | 61.5 | 4.7 |
| 5 | 160 | 56.5 | 230.2 | 27.7 | |
| 5 | 1 | 0 | 11.5 | 121.4 | 6.3 |
| 5 | 320 | 30.2 | 210.0 | 30.6 | |
| 6 | 1 | 0 | 11 | 152.9 | 3.2 |
| 9 | 20 | 14.8 | 171.6 | −3.4 | |
| 7 | 1 | 0 | 10.5 | 136.4 | 8.8 |
| 5 | 320 | 53.8 | 440.5 | 30.2 | |
| 8 | 1 | 0 | 10.6 | 133.6 | 6.1 |
| 7 | 10 | 10 | 109.1 | 6.3 | |
| 9 | 1 | 0 | 11.1 | 115.1 | 2.5 |
| 9 | 40 | 31.6 | 179.9 | 22.0 | |
| 10 | 2 | 0 | 10 | 384.1 | 14.1 |
| 9 | 0 | 33.3 | 333.1 | 11.6 | |
| 11 | 1 | 0 | 10.3 | 204.2 | 13.2 |
| 5 | 640 | 20.4 | 265.2 | 25.2 | |
| 12 | 1 | 0 | 10.6 | 142.2 | 16.9 |
| 5 | 80 | 57.6 | 413.0 | 25.7 | |
| 13 | 1 | 0 | 9.4 | 52.1 | 0.4 |
| 5 | 0 | 24 | 50 | 10.4 | |
| 14 | 1 | 0 | 10.2 | 141 | 31.8 |
| 9 | 40 | 29.8 | 270.7 | 48.3 | |
| 15 | 1 | 0 | 9.7 | 27.4 | 0.4 |
| 5 | 0 | 57.6 | 94.7 | 21.4 | |
| 16 | 1 | 0 | 9.3 | 90.5 | 11.5 |
| 5 | 80 | 36.5 | 170.8 | 9.7 | |
| 17 | 1 | 0 | 9.9 | 166.9 | 56.3 |
| 5 | 160 | 69.9 | 434.6 | 55.8 | |
| 18 | 1 | 0 | 10.4 | 52.3 | 2.9 |
| 5 | 0 | 71.1 | 168.2 | 0.7 | |
| 19 | 1 | 0 | 9.9 | 70 | 6.1 |
| 13 | 40 | 47.9 | 167.4 | 12.8 | |
| 20 | 1 | 0 | 10.2 | 108.1 | 1.4 |
| 5 | 40 | 58.2 | 476.5 | 35.7 | |
| 21 | 1 | 0 | 9.8 | 83.1 | 13.9 |
| 5 | 20 | 59 | 222.8 | 42.6 | |
| 22 | 1 | 0 | 10 | 111.1 | 2.9 |
| 9 | 40 | 56.8 | 309.4 | 11.5 | |
| 23 | 1 | 0 | 9.8 | 71.8 | 4.9 |
| 9 | 160 | 59.3 | 330.2 | 13.0 | |
| 24 | 1 | 0 | 10.2 | 83.5 | 2.9 |
| 5 | 80 | 43.7 | 218.1 | 17.9 | |
| 25 | 1 | 0 | 9.8 | 99.0 | 24.8 |
| 5 | 2560 | 67.6 | 512.1 | 58.0 | |
| 26 | 1 | 0 | 10.2 | 76.9 | 5.3 |
| 5 | 20 | 21.2 | 90.9 | 11.1 | |
| 27 | 1 | 0 | 10.3 | 48.7 | 8.5 |
| 9 | 10 | 10.3 | 26.9 | 16.2 |
MFI, mean fluorescence intensity. Bold numbers indicate a significant change.
To further evaluate response to vaccination, posttherapy antibodies detected in sera from five patients were isolated and purified by using affinity columns bearing the synthetic globo H antigen. All anti-globo H activity was removed from the sera, and the presence of IgM antibodies was confirmed. The IgM antibodies induced by vaccination demonstrated reactivity with globo H, whereas they proved unreactive with the tumor-associated carbohydrate antigen Ley, as assessed by ELISA (data not shown). To further demonstrate this specificity, an affinity column bearing the Ley antigen was incubated with sera from patient no. 11. No significant antibody binding was observed.
Clinical Response.
Although clinical response was not a primary endpoint of this trial, the following observations were made. Of the 15 patients without evidence of disease (NED) at the beginning of this trial, all remained free of disease at the conclusion of the protocol (weeks 21–24) except for patient no. 8, who developed progression of disease in the chest wall. Of the remaining 14 patients, 10 remain NED, and 4 had progression of disease, with a median followup (interval from date on study to date of most recent evaluation) of 107.5 weeks (range 85–153 weeks).
Of the 12 patients who began this trial with stable disease, 9 remained with stable disease, and 3 had disease progression at the conclusion of the protocol (weeks 21–24). One patient (no. 12) progressed in the liver and received only 4 vaccinations. The other two patients (nos. 21 and 22) had progression of disease in lymph nodes and increasing CA15–3 levels at the conclusion of the protocol. Of the remaining 9 patients, 5 remain with stable disease and 4 had progression, with a median followup (interval from date on study to date of most recent evaluation) of 111 weeks (range 61–152 weeks).
Of the 27 patients, 3 have subsequently died of disease (nos. 5, 8, and 21).
Discussion
Overall, this study demonstrates that vaccination of metastatic breast cancer patients with a globo H-KLH plus QS-21 vaccine can generate an immunogenic response with minimal toxicity. Several conclusions can be drawn. First, the most common toxicities were transient local skin reactions at the vaccine site and mild flu-like symptoms. The amylase level was monitored closely, because globo H is expressed on normal pancreatic cells. One patient exhibited a significant grade 3 amylase level that resolved within 2 weeks and did not require medical therapy. Whether this episode was directly related to the vaccine, other medications, or an infection cannot be definitively determined, but it did not recur with an additional immunization. Another patient developed a transient grade 1 amylase level and was also asymptomatic. The two patients with pretreatment grade 1 levels did not exhibit any significant increase in these values. Pancreatitis was not observed in a trial after vaccination of 18 prostate cancer patients with a globo H vaccine (20). Therefore, it is unlikely that this vaccine would stimulate episodes of clinically significant pancreatitis. Two patients exhibited vague neurologic symptoms without clinically significant findings. Neither patient has exhibited evidence of a connective tissue disease to suggest an autoimmune reaction related to the vaccine. We observed no significant changes in hematologic, renal, metabolic, or hepatic function. Transient alterations in these values are unlikely related to the vaccine and may be because of normal biologic fluctuations. The toxicities we observed in this trial are similar to those observed in a previous breast cancer vaccine trial and are likely due to the immunological adjuvant QS-21 (28).
Second, vaccination of breast cancer patients with this vaccine was able to stimulate moderate IgM antibody titers in the majority of participants. IgG reactivity was stimulated to a lesser extent with lower peak titers. In most patients, the peak antibody titers seemed to occur between weeks 5 and 13, with a subsequent decrease despite a fifth vaccination at week 19. This pattern was also observed in the prostate cancer globo H vaccine trial (20). The peak antibody titers and duration of antibody detection were greater in a previous breast cancer vaccine trial with a MUC1-KLH conjugate plus QS-21 (28). In that trial, although the same vaccination schedule was used, the antibody titers in the 9 patients were higher and remained elevated for a minimum of 106 weeks following the last vaccination. This difference probably reflects the relative immunogenicity of the synthetic MUC1 peptide and globo H glycolipid antigens. It is unclear at this time whether the level of the antibody titers has a significant impact on clinical outcome. Intuitively, it would seem that a prolonged duration of detectable antibody levels would be optimal in terms of clinical outcome.
Significant binding of the patients' IgM antibodies to MCF-7 tumor cells (a tripling of the values between pre- and posttherapy samples) was observed in 16 of the 27 patients by FACS analysis. IgG antibody reactivity was observed by FACS analysis in only three patients. Nine patients also demonstrated increased CDC, which is encouraging because potential antitumor mechanisms of antibodies involve binding to the tumor cell surface and induction of complement-mediated lysis. In comparison to a previous MUC1 vaccine trial, the frequency of induction of significant antibody reactivity against MCF-7 cells was similar (28). Evidence of ADCC was observed in 8/27 patients including 5 patients in Group 3.
Third, no significant differences were observed in antibody titers by ELISA assays, flow cytometric analyses, CDC, or ADCC between the three formulations of globo H vaccine in this trial. Further analysis by using affinity column purification supports the observation that the IgM antibodies induced by vaccination in this trial seemed to be specific for globo H, in that reactivity against a second fucose containing pentasaccharide, Ley, was not detected. The use of the MMCCH bifunctional linker group, which greatly improved the conjugation yield, did not appear to decrease the globo H specificity or tumor cell reactivity of the antibodies induced.
Fourth, although clinical response was not a primary endpoint of this trial, several observations can be made. During or at the conclusion of the trial, 4 of the 27 patients developed progression of disease. With a median followup of approximately 2 years, of the other 23 patients, 10 remain without evidence of disease, and 5 have stable disease. It is not possible to correlate immunologic response with clinical response from this trial because of patient heterogeneity, small numbers of patients, and use of hormone therapy during this study.
In summary, vaccination with the globo H-KLH plus QS-21 vaccine can induce antibodies reactive with globo H-positive tumor cells that mediate CDC and ADCC. As we observed no significant differences in immunologic response among the three different versions of the vaccine, we plan to use the crosslinked method of conjugation because of the higher antigen/KLH yields. We are currently exploring additional approaches to augmenting the titer and duration of the antibody response, including various vaccination schedules, use of a second carrier protein, and additional immune adjuvants. It is important to consider that not all breast tumor cells may display the globo H antigen. Thus, targeting globo H antigen alone may result in limited clinical impact. Therefore, we plan to incorporate the globo H antigen into a polyvalent vaccine containing several other antigens (Ley, TF, sTn, etc.). With a full complement of antigen-based vaccines and a set of affinity columns to complement serological analyses, the simultaneous targeting of multiple antigens may result in the most significant clinical impact.
Acknowledgments
We are grateful for the motivation and compliance of the participants in this trial. We thank Dr. George Sukenick of the Sloan Kettering Institute NMR Core Facility (CA-08748) for mass spectral and NMR analyses. We also thank the members of the Breast Cancer Service, Department of Medicine, at Memorial Sloan-Kettering Cancer Center (MSKCC) and other physicians for patient referrals, and the nurses on the Outpatient Immunology Unit at MSKCC. This work was supported by the National Institutes of Health [Grant CA28824 (S.J.D.)], Spore in Breast Cancer (2P50CA68425–06), and the Breast Cancer Research Foundation. Postdoctoral fellowship support from the Selma and Lawrence Ruben Foundation is gratefully acknowledged by L.J.W.
Abbreviations
- KLH
keyhole limpet hemocyanin
- CDC
complement-dependent cytotoxicity
- ADCC
antibody-dependent cell mediated cytotoxicity
- MMCCH
4-(4-N-maleimidomethyl) cyclohexane-1-carboxyl hydrazide
- FACS
fluorescence-activated cell sorter
Footnotes
Norton, L., Slamon, D., Leyland-Jones, B., Wolter, J., Fleming, T., Eirmann, W., Baselga, J., Mendelsohn, J., Bajamonde, A., Ash, M. & Shak, S. (1999) Proc. Am. Soc. Clin. Oncol. 18, 127a (abstr.).
References
- 1.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz C C, Dantis L, Sklarin N T, Seidman A D, Hudis C A, Moore J, et al. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 2.Cobleigh M A, Vogel C L, Tripathy D, Robert N J, Scholl S, Fehrenbacher L, Wolter J M, Paton V, Shak S, Lieberman G, Slamon D. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 3.Winter S F, Sekido Y, Minna J D, McIntire D, Johnson B E, Gazdar A F, Carbone D P. J Natl Cancer Inst. 1993;85:2012–2018. doi: 10.1093/jnci/85.24.2012. [DOI] [PubMed] [Google Scholar]
- 4.Darnell R B. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones P C, Sze L L, Liu P Y, Morton D L, Irie R F. J Natl Cancer Inst. 1981;66:249–254. [PubMed] [Google Scholar]
- 6.Livingston P O, Zhang S, Lloyd K O. Cancer Immunol Immunother. 1997;45:1–9. doi: 10.1007/s002620050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston P O, Wong G Y C, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves M J, Helling F, Ritter G, Oettgen H F, Old L J. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 8.Reddish M A, MacLean G D, Poppema S, Berg A, Longenecker B M. Cancer Immunol Immunother. 1996;42:303–309. doi: 10.1007/s002620050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard S, Tagliabue E, Canevari S, Fossati G, Colnaghi M I. Cancer Res. 1983;43:1295–1300. [PubMed] [Google Scholar]
- 10.Zhang S, Cordon-Cardo C, Zhang H S, Reuter V E, Adluri S, Hamilton W B, Lloyd K O, Livingston P O. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Bremer E G, Levery S B, Sonnino S, Ghidoni R, Canevari S, Kannagi R, Hakomori S. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 12.Bilodeau M T, Park T K, Hu S, Randolph J T, Danishefsky S J, Livingston P O, Zhang S. J Am Chem Soc. 1995;117:7840–7841. [Google Scholar]
- 13.Kensil C R, Patel U, Lennick M, Marciani D. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 14.Zhang S, Graeber L A, Helling F, Ragupathi G, Adluri S, Lloyd K O, Livingston K O. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 15.Helling F, Shang A, Calves M, Zhang S, Ren S, Yu R K, Oettgen H F, Livingston PO. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 16.Livingston P O, Ragupathi G. Cancer Immunol Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragupathi G, Koganty RR, Qui D, Lloyd K O, Livingston P O. Glycoconjug J. 1998;15:217–221. doi: 10.1023/a:1006936826730. [DOI] [PubMed] [Google Scholar]
- 18.Ragupathi G, Park T K, Zhang S, Kim I J, Graber L, Adluri S, Lloyd K O, Danishefsky S J, Livingston P O. Angew Chem Int Ed Engl. 1997;36:125–128. [Google Scholar]
- 19.Perico M E, Mezzanzanica D, Luison E, Alberti P, Panza L, Russo G, Canevari S. Cancer Immunol Immunother. 2000;49:296–304. doi: 10.1007/s002620000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slovin S F, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann W G, Fazzari M, Dantis L, et al. Proc Natl Acad Sci, USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z-G, Williams L J, Zhang X-F, Zatorski A, Kudryashov V, Ragupathi G, Spassova M, Bornmann W, Slovin S F, Scher H I, et al. Proc Natl Acad Sci USA. 2000;97:2719–2724. doi: 10.1073/pnas.97.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ragupathi G, Slovin S F, Adluri S, Sames D, Kim I J, Kim H M, Spassova M, Bornmann W G, Lloyd K O, Scher H I, et al. Angew Chem Int Ed Engl. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Livingston P O, Adluri S, Helling F, Yao T J, Kensil C R, Newman M J, Marciani D. Vaccine. 1994;12:1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 24.Danishefsky S J, Bilodeau M T. Angew Chem Int Ed Engl. 1996;35:1381–1419. [Google Scholar]
- 25.Dickler M N, Ragupathi G, Liu N X, Musselli C, Martino D J, Miller V A, Kris M G, Brezicka F T, Livingston P O, Grant S C. Clin Cancer Res. 1999;5:2773–2779. [PubMed] [Google Scholar]
- 26.Livingston P O, Zhang S, Adluri S, Yao T-J, Graeber L, Ragupathi G, Helling F, Fleischer M. Cancer Immunol Immunother. 1997;43:324–330. doi: 10.1007/s002620050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao T J, Begg C B, Livingston P O. Biometrics. 1996;52:992–1001. [PubMed] [Google Scholar]
- 28.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao T J, Panageas K, Moynahan M, Houghton A, Norton L, Livingston P O. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]