Abstract
Advancing age is a major risk factor for coronary artery disease. Endothelial dysfunction accompanied by increased oxidative stress and inflammation with aging may predispose older arteries to greater ischemia-reperfusion (I/R) injury. Because coronary artery ischemia cannot be induced safely, the effects of age and habitual endurance exercise on endothelial I/R injury have not been determined in humans. Using the brachial artery as a surrogate model of the coronary arteries, endothelial function, assessed by brachial artery flow-mediated dilation (FMD), was measured before and after 20 min of continuous forearm occlusion in young sedentary (n = 10, 24 ± 2 yr) and middle-aged (n = 9, 48 ± 2 yr) sedentary adults to gain insight into the effects of primary aging on endothelial I/R injury. Young (n = 9, 25 ± 1 yr) and middle-aged endurance-trained (n = 9, 50 ± 2 yr) adults were also studied to determine whether habitual exercise provides protection from I/R injury. Fifteen minutes after ischemic injury, FMD decreased significantly by 37% in young sedentary, 35% in young endurance-trained, 68% in middle-aged sedentary, and 50% in middle-aged endurance-trained subjects. FMD returned to baseline levels within 30 min in young sedentary and endurance-trained subjects but remained depressed in middle-aged sedentary and endurance-trained subjects. Circulating markers of antioxidant capacity and inflammation were not related to FMD. In conclusion, advancing age is associated with a greater magnitude and delayed recovery from endothelial I/R injury in humans. Habitual endurance exercise may provide partial protection to the endothelium against this form of I/R injury with advancing age.
Keywords: flow-mediated dilation, endothelium, brachial artery
among the major causes of mortality in the United States, cardiovascular disease ranks first, and advancing age is a major risk factor for cardiovascular disease (30) with vascular endothelial dysfunction contributing to this age-related increase in cardiovascular risk (31). The mechanisms underlying these vascular changes with age are incompletely understood, but reductions in the bioavailability of nitric oxide (NO) and increases in oxidative stress and inflammation play important roles (12, 48). With increased levels of oxidative stress and inflammation, aging arteries may be more vulnerable to ischemia-reperfusion (I/R) injury, and habitual exercise training may attenuate this by improving NO bioavailability and lowering oxidative stress and inflammation (19, 32, 49, 51), but studies in humans are lacking because such methodologies require ischemia of the coronary arteries.
Accordingly, the primary aim of this study was to determine the magnitude of I/R injury and rate of recovery from 20 min of forearm occlusion on conduit artery endothelial function, as assessed by brachial artery flow-mediated dilation (FMD), in young and middle-aged men and women who were sedentary or performed habitual endurance exercise. Middle-aged men and women who were free of overt cardiovascular disease were specifically recruited because the incidence of myocardial infarction doubles between the ages of 45 to 64 yr (33) and to isolate the effects of primary aging on the recovery from I/R injury in the endothelium. Brachial artery FMD was chosen as a surrogate model of the coronary arteries because this measure is strongly associated with gold-standard measures of coronary artery function (2). We hypothesized that after I/R injury, the fall in endothelial function would be greater and the rate of recovery would be slower in sedentary middle-aged than in young adults and that these changes would be associated with circulating markers of antioxidant capacity, inflammation, and metabolites of NO bioavailability. In addition, we hypothesized that endurance-trained middle-aged adults would recover more quickly from endothelial I/R injury than their age-matched sedentary counterparts.
METHODS
Subjects.
A total of 37 apparently healthy sedentary or endurance-trained adults (32 men and 5 women) ages 18–40 and 41–65 yr, respectively, were recruited from The University of Texas at Austin and surrounding community (Table 1). All subjects were normotensive (<140/90 mmHg), nonobese [body mass index (BMI) < 30 kg/m2], nonsmoking, free of overt cardiovascular or other chronic diseases, were not taking any cardiovascular-acting medications as assessed by a medical history questionnaire, and were not currently taking any herbal supplements or refrained from taking herbal supplements for the 2 wk before testing. Self-reported exercise training status was verified by maximal oxygen consumption (Table 1). Endurance-trained subjects reported cycling and/or running at a moderate to strenuous exercise intensity for 8.6 ± 0.7 h/wk. Sedentary subjects reported engaging in no exercise or <2 h of exercise per wk for the past year. The Human Research Committee reviewed and approved all procedures, and written informed consent was obtained from all subjects.
Table 1.
Selected subject characteristics
| Young Sedentary | Young Endurance Trained | Middle-Aged Sedentary | Middle-Aged Endurance Trained | |
|---|---|---|---|---|
| Men/Women | 8/2 | 7/2 | 8/1 | 8/1 |
| Age, yr | 24 ± 2 | 25 ± 1 | 48 ± 2*† | 50 ± 2*† |
| Body mass, kg | 63 ± 3 | 66 ± 3 | 85 ± 4*† | 72 ± 2‡ |
| Body mass index, kg/m2 | 22 ± 1 | 22 ± 1 | 26 ± 1*† | 23 ± 0‡ |
| Body fat, % | 22 ± 3 | 12 ± 2* | 30 ± 3† | 17 ± 2‡ |
| V̇o2max, ml·kg−1·min−1 | 46 ± 3 | 60 ± 3* | 37 ± 2*† | 55 ± 3‡ |
| Heart rate at rest, beats/min | 58 ± 3 | 51 ± 3 | 59 ± 2 | 49 ± 2*‡ |
| Systolic BP at rest, mmHg | 112 ± 2 | 119 ± 3 | 121 ± 3 | 119 ± 3 |
| Mean BP at rest, mmHg | 80 ± 2 | 84 ± 1 | 90 ± 2* | 89 ± 2* |
| Diastolic BP at rest, mmHg | 64 ± 2 | 64 ± 2 | 74 ± 3*† | 71 ± 2 |
| Total cholesterol, mmol/l | 3.6 ± 0.2 | 3.7 ± 0.3 | 4.9 ± 0.2*† | 4.9 ± 0.3*† |
| LDL cholesterol, mmol/l | 2.2 ± 0.2 | 2.0 ± 0.2 | 3.0 ± 0.3† | 3.0 ± 0.2† |
| HDL cholesterol, mmol/l | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.3 | 1.5 ± 0.2 |
Values are means ± SE. V̇o2max, maximal oxygen consumption; BP, blood pressure.
P < 0.05 vs. young sedentary;
P < 0.05 vs. young endurance trained;
P < 0.05 vs. middle-aged sedentary (one-way ANOVA with Bonferroni post hoc tests).
Procedures.
For premenopausal women, measures of vascular function were performed during the early follicular phase of their menstrual cycle to control for the effects of estrogen (10). Before the first testing session, all subjects were >4-h fasted and abstained from caffeine. Body composition was measured by dual-energy X-ray absorptiometry (Lunar DPX, General Electric Medical Systems, Fairfield, CT), and brachial arterial blood pressure at rest was measured by the oscillometric technique (STBP-780, Colin Medical, San Antonio, TX). Maximal oxygen consumption was measured during a modified Balke incremental treadmill exercise test (1% grade increase per minute at individualized treadmill speed) as previously described (56). Oxygen consumption (indirect calorimetry via respiratory gas measurements; Physio-Dyne, Quogue, NY), heart rate, and ratings of perceived exertion (the original Borg scale) were measured throughout the protocol.
For 72 h before the primary testing session, subjects followed and recorded a nitrate- and nitrite-free diet adapted from the National Heart, Blood, and Lung Institute (41) to reduce the effects of diet on circulating nitrite and nitrate concentrations, thereby isolating this marker of endogenous NO production (60). This diet did not allow the ingestion of foods containing nitrates or nitrites, including vegetables or vegetable products, legumes, cured or processed meats, cheese, seafood or fish, alcoholic beverages, strawberries, melons, bananas, or potatoes. Adherence to the diet was verified by dietary records, which were subsequently analyzed by a registered dietician. Total caloric intake was higher in young and middle-aged endurance-trained subjects compared with young sedentary subjects, and young endurance-trained subjects consumed a greater percentage of their calories from carbohydrates and lower percentage of calories from fat compared with the middle-aged sedentary group (data not presented).
For the primary testing session, subjects reported to the laboratory in the morning after fasting >10 h, not taking medications for >24 h, and abstaining from low-intensity exercise >12 h and moderate- to high-intensity exercise >24 h. Brachial blood pressure was measured two to three times after subjects had rested in the supine position >10 min in a quiet, dimly lit, temperature-controlled (23 to 26°C) laboratory room. Endothelial-dependent vasodilation of the brachial artery was assessed by FMD in the right arm before and 15, 30, and 45 min after 20 min of lower-arm cuff occlusion using an ultrasound machine (iE33, Philips Medical, Bothel, WA), equipped with a high-resolution linear-array transducer as previously described (14). These time points were specifically selected based on previous studies using this model that measured at 15- to 30-min intervals and demonstrated that endothelial function recovers within 60 min after this form of injury (6, 26, 27, 42, 52) to allow for the recovery of the brachial artery between measures of FMD and to provide adequate time for reperfusion injury to occur in the initial 15 min after injury, a form of injury that is thought to cause greater damage than ischemia alone (18). At each time point, longitudinal images of baseline brachial artery diameters were recorded proximal to the forearm cuff for an average of 90 s and blood velocities were measured at <60° for 30 s before forearm occlusion with a rapid cuff inflator (E20 Inflator, Hokanson, Bellevue, WA) set to >100 mmHg suprasystolic pressure. To ensure arm stability and transducer placement, a customized arm rest and transducer holder device cradled the arm and locked the transducer 2 to 8 cm proximal to the antecubital fossa. Ten seconds before the occlusion cuff was released, blood velocity recordings were commenced and continued until 20 s after cuff deflation. B-mode images of the brachial artery were recorded from 20 s to 3 min after cuff release. Endothelial-independent vasodilation, the response of arterial smooth muscle cells to pharmacological doses of NO donors, was not measured because it is not associated with aging (9, 55) or exercise training in healthy adults (4, 47), administration of sublingual nitroglycerin would interfere with the repeated measures of FMD and plasma nitrate and nitrite concentrations, and previous studies using the forearm I/R injury model have shown that endothelial-independent vasodilation is not affected by 20 min of forearm occlusion (8, 26, 34, 38, 43).
Ultrasound images were transferred to digital-viewing software (Brachial Analyzer, Vascular Tools, Version 5, Medical Imaging Applications, Coralville, IA) where all diameters and velocities were analyzed by the same investigator (A. E. DeVan). For each time point, an average of 44 ± 3 end-diastolic diameters was analyzed before cuff occlusion (baseline diameter), and the three highest consecutive end-diastolic diameters after cuff release (peak diameters) were used to calculate FMD using the following equation: [(peak diameter − baseline diameter)/baseline diameter] × 100 (11). In a subgroup analysis of five young sedentary, six young endurance-trained, nine middle-aged sedentary, and six middle-aged endurance-trained subjects, the area under the curve of blood velocities was analyzed during the first 15 s after cuff deflation (20, 46). After I/R injury, statistical analyses revealed no significant differences between or within groups for postocclusion blood velocities at all time points (P < 0.05; repeated-measures ANOVA with Bonferroni post hoc tests). Accordingly, FMD was not normalized for shear rate (3, 20).
Blood samples were obtained from the left antecubital vein using a closed intravenous catheter system (Saf-T-Intima, BD Medical, Sandy, UT) before and 15, 30, and 45 min after the 20-min cuff was released for various circulating biomarkers. At baseline, total cholesterol, LDL cholesterol, and HDL cholesterol were measured in fresh serum (Table 1) using a multianalyte chemistry analyzer. All other blood samples were stored at −80°C for later analyses. Serum concentrations of total nitrite and endogenous nitrite were assessed in duplicate by colorimetric detection of nitrite as an azo dye product of the Griess Reaction (Parameter Total NO/Nitrite/Nitrate Assay, R&D Systems, Minneapolis, MN) after deproteinization by filtration (Millipore, Billerica, MA). Nitrate was calculated as the difference between total nitrite and endogenous nitrite. Serum concentrations of TNF-α, IL-6, IL-8, VCAM-1, and ICAM-1 were measured in duplicate using a cytometric bead array (High Sensitivity Human Cytokine Kit, Millipore, St. Charles, MO) read by lasers (Bio-Plex Luminex 200 System, Bio-Rad, Hercules, CA). Concentrations of reduced glutathione (GSH) were calculated by colorimetric reaction rates read over 3 min at 412 nM in duplicate by a spectrophotometer (DU640, Beckman Coulter, Brea, CA) in purified whole blood (Bioxytech GSH/GSSG-412, Percipio Biosciences, Portland, OR).
Statistical analyses.
One-way ANOVA with Bonferroni post hoc tests was used to identify significant differences in descriptive variables, ultrasound-derived measures at the brachial artery within groups, and circulating biomarkers (Tables 1, 2, and 4). ANOVA with repeated measures and Bonferroni post hoc tests were used for determining changes in FMD (Fig. 1) and ultrasound-derived vascular measures at the brachial artery between groups (Table 2). A general linear model and multivariate analysis covarying for FMD before I/R injury was used to verify significant changes in FMD after I/R injury were independent of initial differences in FMD before I/R injury. Univariate correlation (two tailed) and stepwise multiple regression analyses were used to identify significant determinants of brachial artery FMD (Table 3). Significance was set at P < 0.05 for all statistical analyses. All data are expressed as means ± SE.
Table 2.
Ultrasound-derived vascular measures of the brachial artery
| Before | 15 min Post | 30 min Post | 45 min Post | Before | 15 min Post | 30 min Post | 45 min Post | |
|---|---|---|---|---|---|---|---|---|
| Young sedentary | Young endurance trained | |||||||
| n | 10 | 9 | ||||||
| Baseline arterial diameter, mm | 3.51 ± 0.17 | 3.62 ± 0.15 | 3.58 ± 0.14 | 3.57 ± 0.14 | 3.73 ± 0.12 | 3.79 ± 0.14 | 3.84 ± 0.12 | 3.85 ± 0.14 |
| Peak arterial diameter, mm | 3.70 ± 0.18 | 3.74 ± 0.15 | 3.75 ± 0.15 | 3.75 ± 0.14 | 3.98 ± 0.13 | 3.95 ± 0.13 | 4.04 ± 0.13¶ | 4.07 ± 0.14¶ |
| ΔArterial diameter, mm | 0.19 ± 0.03 | 0.12 ± 0.02 | 0.16 ± 0.03 | 0.18 ± 0.03¶ | 0.25 ± 0.03 | 0.15 ± 0.02‡ | 0.21 ± 0.04 | 0.22 ± 0.02¶ |
| Middle-aged sedentary | Middle-aged endurance trained | |||||||
| n | 9 | 9 | ||||||
| Baseline arterial diameter, mm | 4.12 ± 0.18 | 4.22 ± 0.17 | 4.18 ± 0.16 | 4.17 ± 0.16 | 4.15 ± 0.21 | 4.21 ± 0.23 | 4.24 ± 0.25 | 4.23 ± 0.25 |
| Peak arterial diameter, mm | 4.28 ± 0.18 | 4.27 ± 0.16 | 4.23 ± 0.15 | 4.23 ± 0.16 | 4.35 ± 0.20 | 4.32 ± 0.22 | 4.36 ± 0.25 | 4.33 ± 0.25 |
| ΔArterial diameter, mm | 0.17 ± 0.03 | 0.05 ± 0.01†‡ | 0.05 ± 0.03†‡ | 0.06 ± 0.02*†‡ | 0.20 ± 0.03 | 0.11 ± 0.02‡ | 0.12 ± 0.02 | 0.11 ± 0.03†‡ |
Values are means ± SE; n, number of subjects.
P < 0.05 vs. young sedentary at same time point;
P < 0.05 vs. young endurance trained at same time point;
P < 0.05 vs. before within group;
P < 0.05 vs. 15 min Post within group;
P < 0.05 vs. 30 min Post within group (one-way ANOVA with Bonferroni post hoc tests for within group; repeated-measures ANOVA with Bonferroni post hoc tests for between groups).
Table 4.
Circulating markers of nitric oxide breakdown products, antioxidant capacity, and inflammation
| Young Sedentary | Middle-Aged Sedentary | Middle-Aged Endurance Trained | |
|---|---|---|---|
| Nitrite, μmol/l | 1.0 ± 0.1 | 0.6 ± 0.1* | 0.8 ± 0.1 |
| n | 9 | 8 | 9 |
| Nitrate, μmol/l | 40.4 ± 11.6 | 37.6 ± 7.8 | 26.1 ± 9.5 |
| n | 9 | 8 | 9 |
| Tumor necrosis factor-α, pg/ml | 6.6 ± 1.3 | 5.8 ± 1.0 | 7.3 ± 1.0 |
| n | 7 | 5 | 7 |
| Interleukin-6, pg/ml | 2.9 ± 0.5 | 8.5 ± 5.7 | 4.1 ± 0.9 |
| n | 10 | 7 | 6 |
| Interleukin-8, pg/ml | 3.5 ± 0.3 | 5.0 ± 1.4 | 3.6 ± 0.4 |
| n | 10 | 8 | 9 |
| VCAM-1, ng/ml | 1,300 ± 52 | 1,380 ± 68 | 1,329 ± 94 |
| n | 10 | 9 | 9 |
| ICAM-1, ng/ml | 133 ± 14 | 137 ± 13 | 140 ± 15 |
| n | 10 | 9 | 9 |
| GSH, μM | 808 ± 54 | 784 ± 40 | 817 ± 46 |
| n | 8 | 9 | 9 |
Values are means ± SE; n, number of subjects. VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1; GSH, reduced glutathione.
P < 0.05 vs. young sedentary (one-way ANOVA with Bonferroni post hoc tests).
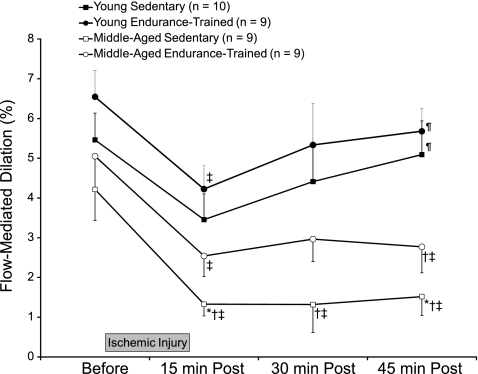
Fig. 1.
Flow-mediated dilation before and after (15, 30, and 45 min Post) ischemia-reperfusion injury. Values are means ± SE; n, number of subjects. *P < 0.05 vs. young sedentary; †P < 0.05 vs. young endurance-trained; ‡P < 0.05 vs. before; ¶P < 0.05 vs. 15 min Post (repeated-measures ANOVA with Bonferroni post hoc tests).
Table 3.
Selected physiological correlates (Pearson correlation coefficients) of changes in flow-mediated dilation before and after ischemia-reperfusion injury
| Before to 15 min Post | Before to 30 min Post | Before to 45 min Post | |
|---|---|---|---|
| n | 37 | 37 | 37 |
| Age | NS | NS | −0.47 |
| Body mass index | NS | NS | NS |
| Body fat percentage | NS | NS | NS |
| V̇o2max | NS | NS | NS |
| Systolic BP | NS | NS | NS |
| Diastolic BP | NS | NS | NS |
| Total cholesterol | NS | −0.36 | −0.39 |
| HDL cholesterol | NS | −0.43 | −0.37 |
| LDL cholesterol | NS | NS | NS |
n, Number of subjects. NS, not significant.
RESULTS
All subjects were nonobese, normolipidemic, and normotensive (Table 1). Middle-aged sedentary subjects had higher body mass, BMI, diastolic blood pressure, and total cholesterol and lower maximal oxygen consumption than young sedentary and endurance-trained subjects. As expected, middle-aged endurance-trained subjects had lower body mass, BMI, body fat percentage, and heart rate at rest and higher maximal oxygen consumption than their age-matched sedentary counterparts.
Before I/R injury, brachial artery FMD was highest in the young endurance-trained (6.55 ± 0.66%), followed by the young sedentary (5.46 ± 0.68%) then middle-aged endurance-trained (5.05 ± 0.81%) and, finally, the middle-aged sedentary (4.21 ± 0.78%) group, though these differences were not statistically significant (Fig. 1; one-way ANOVA with Bonferroni post hoc tests). From before to 15 min after injury (15 min Post), FMD fell 37 and 35% in young sedentary and young endurance-trained subjects, respectively, but recovered within 45 min. In contrast, FMD in middle-aged sedentary subjects decreased by 68% and remained significantly lower than before I/R injury in subsequent measures. When compared with the young sedentary group, middle-aged sedentary subjects had significantly lower FMD at 15, 30, and 45 min after I/R injury, whereas the middle-aged endurance-trained group was not significantly different from the young sedentary group at any time point. Middle-aged endurance-trained subjects demonstrated a 50% decline in FMD 15 min after I/R injury and did not recover within 45 min. Even when covarying for FMD before I/R injury (general linear model and multivariate analysis), the young endurance-trained group remained significantly different from middle-aged sedentary 15 and 45 min after injury and young sedentary adults were significantly different from middle-aged sedentary adults at 45 min after I/R injury. In the pooled sample (two tailed), FMD before I/R injury was significantly correlated (r = −0.65) with the magnitude of reduction in FMD 15 min after I/R injury (15 min Post FMD − FMD before I/R injury).
As shown in Table 2, there were no significant differences between groups for baseline or peak diameters at any time point, though the baseline diameters in young sedentary compared with the middle-aged sedentary (P = 0.095) and young sedentary compared with the middle-aged endurance-trained (P = 0.071) groups were approaching significance. Within some groups, peak diameter and absolute change in diameter (peak diameter − baseline diameter) were significantly different. Between the middle-aged sedentary group and young endurance-trained group, the absolute change in diameter was significantly lower 15, 30, and 45 min after injury. Forty-five minutes after injury, the absolute change in diameter was lower in the middle-aged sedentary compared with the young sedentary group and lower in the middle-aged versus young endurance-trained group. Age, total cholesterol, and HDL cholesterol were significantly correlated with the change in FMD after I/R injury (Table 3). Multiple regression analyses revealed that the significant independent predictors were HDL cholesterol (standardized β = −0.43) for FMD from before to 30 min and age (standardized β = −0.47) for FMD from before to 45 min after I/R injury, accounting for 19 and 22% of the variance, respectively. There was no significant association between maximal oxygen consumption and the response to endothelial I/R injury.
Circulating markers of cytokines and adhesion molecules were not different between groups before (Table 4) or after (data not shown) I/R injury. Baseline concentrations of nitrite were significantly lower in middle-aged sedentary than in young sedentary subjects. GSH concentrations were not different between groups.
DISCUSSION
The primary findings of this study are as follows. First, brachial FMD decreased significantly after 20 min of forearm ischemia, suggesting that this surrogate model of the coronary arteries produces significant I/R injury to the endothelium of the brachial artery. Second, young subjects recovered more quickly from I/R injury than older subjects, regardless of their exercise status. Specifically, 30 and 45 min after injury, FMD in young sedentary and young endurance-trained subjects was not significantly different from baseline values, whereas middle-aged groups had blunted FMD, suggesting that aging is associated with a greater magnitude and delayed recovery from endothelial I/R injury. Though the middle-aged endurance-trained group had delayed recovery from I/R injury compared with younger groups, the magnitude of injury was slightly less than their age-matched sedentary counterparts, perhaps indicating a possible partial protection of habitual endurance exercise against endothelial I/R injury with advancing age.
During ischemia, energy depletion and reactive oxygen species production occur from inadequate blood flow to tissues. While the best treatment for ischemia is immediate reperfusion of the tissue with blood, reentry of the blood into the ischemic tissues also causes damage through calcium loading, inflammation, and a surge of free radical production. With reperfusion, oxygen levels drastically rise with a simultaneous increase in pH levels, leading to a burst of reactive oxygen species formation (21, 44). Within the vasculature, reactive oxygen species produced by the mitochondria, endothelial NO synthase (eNOS) uncoupling, xanthine oxidoreductase, and NADPH oxidase (40) interact with NO to produce peroxynitrite, thereby reducing the bioavailability of NO and degrading tetrahydrobiopterin, an essential cofactor of eNOS, lessening the production of NO (61). In the present study, 20 min of forearm ischemia and 15 min of reperfusion caused a significant fall in endothelial function, as assessed by FMD of the brachial artery. After I/R injury, this decline and recovery of brachial artery FMD within 60 min in the young groups is in agreement with previous studies in young healthy populations (26, 35, 37, 62).
Older age is the most important nonmodifiable predictor of recovery from myocardial infarction in humans (63), and animal studies demonstrate that aging is associated with increased susceptibility to myocardial (5, 23) and endothelial (50) I/R injury. In the present study, we compared the decline and recovery in brachial artery FMD in response to endothelial I/R injury between young and middle-aged sedentary adults who were free of overt cardiovascular disease. In this model of “primary” aging, middle-aged individuals had a 31% greater fall in FMD and delayed recovery compared with younger individuals. The differences in FMD after I/R injury between young and older subjects remained statistically significant even when covarying for FMD before I/R injury. To our knowledge, this is the first study to demonstrate an age-associated decline in endothelial function after I/R injury using a human model.
With advancing age, the fall in endothelial function is most strongly associated with increases in the expression of NADPH oxidase and nuclear factor-κB in endothelial cells (15) and lower levels of tetrahydrobiopterin (16, 54). Previous studies using this human model support the role of oxidative stress in endothelial I/R injury. Indeed, an intra-arterial infusion of vitamin C (43) or tetrahydrobiopterins (cofactors of eNOS and antioxidants) (38) each prevented the fall in endothelial function after 20 min of forearm ischemia, and patients with a genetic disease leading to an inactivation of NADPH oxidase were protected from endothelial I/R injury (36). Similarly, a study measuring a circulating biomarker of oxidative stress before and 20 min after forearm ischemia found that older subjects had a greater increase and duration of elevated F2-isoprostane concentrations compared with young adults (13), which was attenuated by 2 wk of tart cherry juice consumption, a drink containing high levels of antioxidants (59). Given these results, we attempted to provide insight into the mechanisms behind the age-associated decline in endothelial function with I/R injury by measuring baseline circulating markers of antioxidant capacity, inflammation, and NO bioavailability. Because glutathione is the major thiol-disulfide redox buffer of cells (25, 57) and the constant interaction of whole blood with endothelial cells, GSH was measured in whole blood as an indicator of antioxidant capacity. Contrary to our hypothesis, GSH and serum inflammatory cytokine concentrations at baseline did not indicate lower antioxidant capacity or increased inflammation in the middle-aged sedentary population and these markers were not correlated with changes in FMD after I/R injury. It is possible that the measurement of systemic instead of local concentrations, the use of a healthy population without overt cardiovascular disease, or the small sample size may explain these findings.
Circulating concentrations of nitrite are considered to be a noninvasive measure of NO bioavailability as nitrite is an oxidative product of NO metabolism. At baseline, middle-aged sedentary subjects had significantly lower serum nitrite levels than young sedentary subjects, whereas middle-aged endurance-trained subjects had higher circulating nitrite levels than their sedentary age-matched counterparts (though not statistically significant), suggesting that middle-aged sedentary subjects had lower levels of NO bioavailability. This is in agreement with Kleinbongard et al. (28) who found a significant negative correlation between plasma nitrite concentrations and age. Reduced NO bioavailability, possibly because of increased oxidative stress, is thought to be the primary factor causing impaired endothelial function with aging in humans. In contrast, no differences were found between groups in serum nitrate concentrations, which is plausible as studies in both humans and mammals indicate that circulating nitrite, not nitrate, reflects endothelial-dependent NO production (28, 29).
Because lifestyle modifications remain the first-line approach for the prevention of age-associated cardiovascular disease (17, 51), we determined the role of habitual endurance exercise in the protection against endothelial I/R injury in young and middle-aged adults. In the present study, middle-aged endurance-trained subjects demonstrated a partial protection from the magnitude, but not duration, of endothelial I/R injury during the 45 min after forearm ischemia. This may suggest that habitual exercise training attenuates the initial reduction in endothelial function after an acute stress (such as 20 min of forearm ischemia), but the role of habitual exercise training in the recovery from endothelial I/R injury in middle-aged adults is still unclear. Measurements of endothelial function were continued until 45 min after ischemia based on previous studies suggesting a full recovery from endothelial I/R injury within 60 min (6, 26, 27, 42, 52); however, it appears that middle-aged endurance-trained and sedentary subjects need a longer period of time to recover. Future studies need to assess endothelial function for a longer period of time after injury to determine whether habitual exercise training lessens the time to full recovery in older adults. In animals, endurance exercise training protects against myocardial I/R injury (45) and attenuates endothelial dysfunction from disease in the vasculature (24). In humans, habitual endurance exercise attenuates the age-related impairment of endothelial function and alleviates decreases in endothelial function induced by cardiovascular disease, perhaps by increasing NO bioavailability, lowering oxidative stress, and reducing inflammation (19, 32, 49, 51). Given the findings in the present study, further research into the mechanisms underlying the possible protection of habitual endurance exercise training on endothelial I/R injury is warranted.
Though it is increasingly common to adjust FMD for shear rate or shear stress using a number of different indexes (e.g., peak, mean, area under the curve to peak dilation, etc.), we chose not to normalize FMD for shear rate in the present study for many reasons. First, it has not been clearly established that conventionally measured shear rate is the stimuli for NO production and FMD. Indeed, blood vessels of eNOS knockout mice still experience FMD by responding to shear stress (53), and the contribution of NO to endothelial function may be reduced with advancing age (3, 39). Second, our data do not satisfy some of the statistical requirements for ratio normalization (1). For example, like many other studies, we have not observed a consistent or strong association between shear rate and FMD (3, 22, 58). Such discrepancies in the literature may be due, at least in part, to a failure of Poiseuille's Law to accurately capture in vivo shear rate/stress. Poiseuille's Law is applicable only to long, straight, and rigid tubes with steady laminar flow, Newtonian fluid, and a parabolic velocity profile. However, these conditions are never realized in the in vivo condition in which FMD is measured. In the present study, a subgroup analysis of the area under the curve of blood velocity revealed no significant differences between or within groups at any time point despite significant differences between groups in FMD. Finally, the peak brachial FMD response, not normalized FMD, has been shown to predict cardiovascular risk and future events (7, 64).
In conclusion, 20 min of limb ischemia is associated with a transient fall in endothelial function, but this is not associated with circulating markers of oxidative stress or inflammation at baseline. Middle-aged sedentary subjects demonstrate greater injury and delayed recovery from endothelial I/R injury compared with younger subjects. Habitual endurance exercise training may provide partial protection against the magnitude of endothelial I/R injury with advancing age.
GRANTS
This study was supported in part by National Institutes of Health Awards AG-20966 and DA-018431. D. Umpierre was supported by a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Phil Stanforth, Shawn Sommerlad, Jill Barnes, Nantinee Nualnim, Jill Tanaka, Erica Royder, Derek Rowan, Roy Cantu, and Rick Garcia for technical assistance.
REFERENCES
- 1. Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 19: 644–652, 1995 [PubMed] [Google Scholar]
- 2. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Atkinson G, Batterham AM, Black MA, Cable NT, Hopkins ND, Dawson EA, Thijssen DH, Jones H, Tinken TM, Green DJ. Is the ratio of flow-mediated dilation and shear rate a statistically sound approach to normalization in cross-sectional studies on endothelial function? J Appl Physiol 107: 1893–1899, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res 83: 247–261, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Bohm F, Settergren M, Gonon AT, Pernow J. The endothelin-1 receptor antagonist bosentan protects against ischaemia/reperfusion-induced endothelial dysfunction in humans. Clin Sci (Lond) 108: 357–363, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 108: 2093–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation 110: 2077–2082, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 24: 1468–1474, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Chan NN, MacAllister RJ, Colhoun HM, Vallance P, Hingorani AD. Changes in endothelium-dependent vasodilatation and alpha-adrenergic responses in resistance vessels during the menstrual cycle in healthy women. J Clin Endocrinol Metab 86: 2499–2504, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol 105: 1333–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies SS, Traustadottir T, Stock AA, Ye F, Shyr Y, Harman SM, Roberts LJ., 2nd Ischemia/reperfusion unveils impaired capacity of older adults to restrain oxidative insult. Free Radic Biol Med 47: 1014–1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhindsa M, Sommerlad SM, DeVan AE, Barnes JN, Sugawara J, Ley O, Tanaka H. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol 105: 427–432, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fries JF. Measuring and monitoring success in compressing morbidity. Ann Intern Med 139: 455–459, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gori T, Lisi M, Forconi S. Ischemia and reperfusion: the endothelial perspective. A radical view. Clin Hemorheol Microcirc 35: 31–34, 2006 [PubMed] [Google Scholar]
- 19. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann NY Acad Sci 1047: 248–258, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol 103: 2120–2128, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc 38: 445–454, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348: 93–112, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 103: 1624–1630, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kilian JG, Nakhla S, Griffith K, Harmer J, Skilton M, Celermajer DS. Reperfusion injury in the human forearm is mild and not attenuated by short-term ischaemic preconditioning. Clin Exp Pharmacol Physiol 32: 86–90, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40: 295–302, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35: 790–796, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 7: 29–49, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Laughlin MH. Joseph B Wolfe Memorial lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc 36: 352–362, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 46: 450–456, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia-reperfusion injury in the human forearm. Circulation 113: 1015–1019, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, Deanfield JE. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 121: 2310–2316, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 116: 1386–1395, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Mayahi L, Heales S, Owen D, Casas JP, Harris J, MacAllister RJ, Hingorani AD. (6R)-5,6,7,8-tetrahydro-l-biopterin and its stereoisomer prevent ischemia reperfusion injury in human forearm. Arterioscler Thromb Vasc Biol 27: 1334–1339, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Mingorance C, Herrera MD, Alvarez De Sotomayor M. Mechanism involved in aged-related endothelial dysfunction. Med Clin (Barc) 132: 62–69, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005 [DOI] [PubMed] [Google Scholar]
- 41. National Heart, Blood, and Lung Institute Low Nitrate Diet [Online]. 7 East Cardiology Unit; National Heart, Blood, and Lung Institute; http://www.nhlbi.nih.gov/labs/7east/lowNdiet.htm [29 Mar 2007] [Google Scholar]
- 42. Pernow J, Bohm F, Beltran E, Gonon A. l-Arginine protects from ischemia-reperfusion-induced endothelial dysfunction in humans in vivo. J Appl Physiol 95: 2218–2222, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Pleiner J, Schaller G, Mittermayer F, Marsik C, MacAllister RJ, Kapiotis S, Ziegler S, Ferlitsch A, Wolzt M. Intra-arterial vitamin C prevents endothelial dysfunction caused by ischemia-reperfusion. Atherosclerosis 197: 383–391, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Powers SK, Murlasits Z, Wu M, Kavazis AN. Ischemia-reperfusion-induced cardiac injury: a brief review. Med Sci Sports Exerc 39: 1529–1536, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Powers SK, Quindry J, Hamilton K. Aging, exercise, and cardioprotection. Ann NY Acad Sci 1019: 462–470, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol 88: 761–766, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8: 226–238, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Rush JW, Denniss SG, Graham DA. Vascular nitric oxide and oxidative stress: determinants of endothelial adaptations to cardiovascular disease and to physical activity. Can J Appl Physiol 30: 442–474, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Seal JB, Gewertz BL. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg 19: 572–584, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 105: 1323–1332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. l-Arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 204: 73–78, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res 85: 288–293, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol 83: 1947–1953, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol 286: R431–R444, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol 296: H57–H64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Traustadottir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, 2nd, Harman SM. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr 139: 1896–1900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res 39: 797–815, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733–739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Woo KS, White HD. Factors affecting outcome after recovery from myocardial infarction. Annu Rev Med 45: 325–339, 1994 [DOI] [PubMed] [Google Scholar]
- 64. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]