Abstract
Arginase 1, via competing with nitric oxide (NO) synthase for the substrate l-arginine, may interfere with NO-mediated vascular responses. We tested the hypothesis that arginase 1 contributes to coronary vasomotor dysfunction in patients with diabetes mellitus (DM). Coronary arterioles were dissected from the right atrial appendages of 41 consecutive patients with or without DM (the 2 groups suffered from similar comorbidities), and agonist-induced changes in diameter were measured with videomicroscopy. We found that the endothelium-dependent agonist ACh elicited a diminished vasodilation and caused constriction to the highest ACh concentration (0.1 μM) with a similar magnitude in patients with (18 ± 8%) and without (17 ± 9%) DM. Responses to ACh were not significantly affected by the inhibition of NO synthesis with NG-nitro-l-arginine methyl ester in either group. The NO donor sodium nitroprusside-dependent dilations were not different in patients with or without DM. Interestingly, we found that the presence of NG-hydroxy-l-arginine (10 μM), a selective inhibitor of arginase or application of l-arginine (3 mM), restored ACh-induced coronary dilations only in patients with DM (to 47 ± 6% and to 40 ± 19%, respectively) but not in subjects without DM. Correspondingly, the protein expression of arginase 1 was increased in coronary arterioles of patients with DM compared with subjects without diabetes. Moreover, using immunocytochemistry, we detected an abundant immunostaining of arginase 1 in coronary endothelial cells of patients with DM, which was colocalized with NO synthase. Collectively, we provided evidence for a distinct upregulation of arginase 1 in coronary arterioles of patients with DM, which contributes to a reduced NO production and consequently diminished vasodilation.
Keywords: coronary microvessel, endothelium, endothelial nitric oxide synthase
patients with diabetes exhibit endothelial dysfunction, which is characterized by an impaired flow- and acetylcholine (ACh)-induced, endothelium-dependent relaxation of brachial artery (8) and forearm resistance vessels (36). Kaneda et al. (17) performed a study in which 165 patients underwent intracoronary injection of ACh and found that diabetes was the strongest predictor for ACh-induced vasospasm, a response that indicates coronary endothelial dysfunction. This and other studies indicated that diabetes is also associated with an impaired dilator function of coronary arteries, and this is manifested and measured as a reduced vasodilator or even vasoconstrictor response to ACh (5, 23). Our previous studies have demonstrated that coronary arterioles isolated from animals with experimental diabetes also exhibit impaired ACh-induced dilation, which is primarily due to the reduced synthesis and/or availability of nitric oxide (NO) (1, 2, 9, 16). The exact mechanism(s) responsible for the diminished NO production in human diabetes is still incompletely understood.
l-Arginine, the substrate for NO synthase, is the precursor for NO synthesis in the vascular endothelium. Experimental and also human studies have indicated that the administration of l-arginine could improve NO bioavailability in coronary arteries (12). Intracoronary infusion of l-arginine in patients with coronary artery disease attenuated the vasoconstrictor response to intracoronary ACh and increased coronary blood flow (6). Lerman et al. (19) studied the effect of the long-term administration of l-arginine (9 g/day) on patients with nonobstructive coronary disease and found a markedly improved coronary vasodilator response to ACh. It is known that the Km of NO synthase for l-arginine is 2.9 μM and the intracellular concentrations of l-arginine range from 0.1 to 1.0 mM; thus in these studies it was surprising that the administration of l-arginine is associated with an increased bioavailability of NO, the arginine paradox. Interestingly, recent studies suggested an attractive hypothesis, namely, that arginase 1, the focal enzyme of the urea cycle hydrolyzing l-arginine, interferes with NO synthesis and may contribute to the development of vascular disease (7). Recently, Zhang et al. (40) demonstrated that in pigs with experimental hypertension, NO-mediated dilation of coronary arterioles is reduced because of an increased arginase-1 activity, which limited l-arginine availability to NO synthase. A study by Romero et al. (31) showed that increased arginase-1 activity contributes to a diminished NO-mediated response in coronary arteries of diabetic rats. Experimental evidence supporting the contribution of arginase 1 to diminished coronary dilation is lacking in human diabetes.
Accordingly, in this study we set out to characterize alterations in endothelium-dependent coronary arteriolar responses and the contribution of arginase 1 in this process in patients with diabetes mellitus (DM). Thus changes in the diameter of coronary arterioles were monitored before and after the pharmacological-interfering l-arginine/NO pathway and arginase 1. Protein expression and localization of arginase 1 was also detected to furnish further evidence for an upregulated arginase 1 in human coronaries.
METHODS
Patient characteristics.
All protocols were approved by the Institutional Review Board at New York Medical College, Valhalla, New York. Consecutive patients who underwent cardiac surgery were enrolled into this study. Patients were divided into two groups with or without documented diabetes.
Isolation of coronary arterioles.
With the use of microsurgical instruments and a stereomicroscope (Nikon SMZ 1000; Nikon Instruments, Melville, NY), the coronary arteriole (∼1 mm in length) from the right atrial appendage was isolated and cannulated, as previously described (34). Briefly, the cannulated arteriole was connected with silicone tubing to a pressure servo control system (Living Systems Instrumentation, Burlington, VT) to set the intraluminal pressure to 80 mmHg. Changes in arteriolar diameter were continuously recorded with a CCD camera (CFW1310; Scion, Frederick, MD), connected to a microscope (Eclipse 80i; Nikon Instruments, Melville, NY) and measured with a microangiometer (Texas Instruments, Dallas, TX).
Experimental protocols.
During an incubation period of 1 h, a spontaneous myogenic tone developed in the isolated coronary arteriole in response to the intraluminal pressure of 80 mmHg. In the first series of experiments, cumulative concentrations of the endothelium-dependent agonist ACh (0.1 nmol/l–0.1 μmol/l) and the endothelium-independent dilator sodium nitroprusside (1 nmol/l–1 μmol/l) were administered to the arterioles and changes in diameter were measured. The same protocols were repeated in the presence of NG-nitro-l-arginine methyl ester (l-NAME, 10 μmol/l for 30 min), an inhibitor of the NO synthase. Arterioles were also incubated in the presence of NG-hydroxy-l-arginine (l-NOHA), a selective inhibitor of arginase (10 μmol/l for 30 min) (14) or l-arginine (3 mmol/l for 30 min), and agonist-induced arteriolar responses were obtained again.
Immunoblots.
Coronary arterioles, same-sized microvessels as used in the functional experiments, were dissected from the hearts of patients with (n = 5) and without (n = 5) diabetes, cleared of connective tissue, and briefly rinsed in ice-cold physiological salt solution. Protein electrophoresis and immunoblot analysis were carried out as previously described (16). Briefly, single coronary arterioles were homogenized in 20 μl of radioimmunoprecipitation assay buffer and mixed with an equal amount of Laemmli sample buffer. The total amount of homogenates was then loaded to the gel for electrophoresis, without further quantifying the actual protein concentrations. After blotting, a goat polyclonal antibody was used for the detection of the protein expression of arginase 1 (dilution, 1:1,000). Membranes were also reprobed with anti-β-actin IgG (dilution, 1:5,000) to normalize for loading variations in protein concentrations. Corresponding horseradish peroxidase-labeled secondary antibodies were used, and enhanced chemiluminescence was visualized autoradiographically. The optical density of the bands was measured and normalized for β-actin by using National Institutes of Health (NIH) ImageJ software.
Immunocytochemistry for colocalization.
To visualize individual endothelial cells, after longitudinal cutting, en face coronary arterioles from patients with or without diabetes (n = 4 in each group) were prepared. Acetone-fixed preparations were simultaneously immunolabeled with a monoclonal mouse anti-arginase-1 primary antibody (dilution, 1:100) and a polyclonal rabbit endothelial NO synthase (eNOS) primary antibody (dilution, 1:100). Subsequent fluorescent labeling was performed with Alexa 488-labeled anti-rabbit or Alexa 597-labeled anti-mouse secondary antibodies. 4,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. For nonspecific binding, the primary antibody was omitted. With the use of a ×100 oil immersion objective (numerical aperature, 1.4), individual endothelial cells were visualized in the en face vascular preparation. Images were collected from multiple endothelial cells (at least 5 cells from 3 different regions from each vessels) with an electron-multiplying CCD camera (LucaEM-S, Andor, Belfast, UK), connected to an Olympus BX61 microscope (Olympus America, Center Valley, PA). Merged RGB images were generated with NIH ImageJ software, and representative images are shown.
Drugs and chemicals.
All drugs, chemicals, and antibodies were purchased from Sigma (St. Louis, MO) with the exception of anti-β-actin IgG (Abcam, Cambridge, UK), polyclonal rabbit eNOS primary antibody (BD Bioscience; Rockville, MD), Alexa 488-labeled anti-rabbit and Alexa 597-labeled anti-mouse secondary antibodies (Invitrogen; Carlsbad, CA).
Data analysis.
Statistical analyses were performed using GraphPad Prism Software (version 5.00 for Macintosh, San Diego, CA). All pertinent risk factors were examined by Fisher exact test, and continuous variables were examined by Student's t-test between the two patient groups. Agonist-induced arteriolar responses were expressed as changes in arteriolar diameter as a percentage of the maximal dilation, defined as the passive diameter of the vessel at 80-mmHg intraluminal pressure in a Ca2+-free medium, as previously described (34). Statistical analysis of agonist-induced vascular responses was performed by repeated-measures ANOVA, followed by Tukey's post hoc test. P < 0.05 was considered statistically significant. Data are expressed as means ± SE.
RESULTS
Clinical data.
Clinical data are presented in Table 1. The mean age was 58 ± 12 yr in patients without diabetes and 64 ± 10 yr in patients with diabetes. As analyzed by Fisher exact test, there was no significant difference in sex, other comorbidities, and the surgical procedures between the two groups (Table 1). All patients with DM (including 5 patients with type 1 and 15 patients with type 2 DM) were on antidiabetic medication (insulin and/or oral antidiabetic drugs, Table 1).
Table 1.
Patient demographics, diseases, and medications
| DM− | DM+ | |
|---|---|---|
| n | 21 | 20 |
| Men | 14 | 10 |
| Age, yr | 58 ± 12 | 64 ± 10 |
| Underlying disease, n | ||
| Type 1 diabetes | 0 | 5 |
| Type 2 diabetes | 0 | 15 |
| Hypertension | 17 | 18 |
| Hypercholesterolemia | 13 | 10 |
| Coronary artery disease | 9 | 16 |
| Angina | 10 | 15 |
| Premyocardial infarction | 2 | 7 |
| Peripheral vascular disease | 0 | 3 |
| Heart failure | 0 | 4 |
| Valve disease | 13 | 6 |
| Medications, n | ||
| Aspirin | 11 | 13 |
| Lipid lowering | 15 | 13 |
| Insulin | 0 | 8 |
| Oral antidiabetics | 0 | 15 |
| β-Blockers | 16 | 16 |
| ACE inhibitor | 13 | 18 |
| Diuretics | 10 | 10 |
| Anticoagulants | 13 | 10 |
| Calcium-channel blockers | 6 | 4 |
| Surgical procedures, % | ||
| Coronary artery bypass graft | 7 | 11 |
| Valve replacement | 13 | 7 |
| Other causes | 1 | 2 |
Data are means ± SD; n, number of patients studied without (−) or with (+) diabetes mellitus (DM). ACE, angiotensin-converting enzyme.
Vasomotor responses of human coronary arterioles.
In isolated coronary arterioles, a spontaneous tone developed in response to 80 mmHg intraluminal pressure, and there were no significant differences between the active (non-DM, 106 ± 9 μm; and DM, 97 ± 12 μm) and passive (in Ca2+-free solution: non-DM, 131 ± 11 μm; and DM, 133 ± 17 μm) diameters.
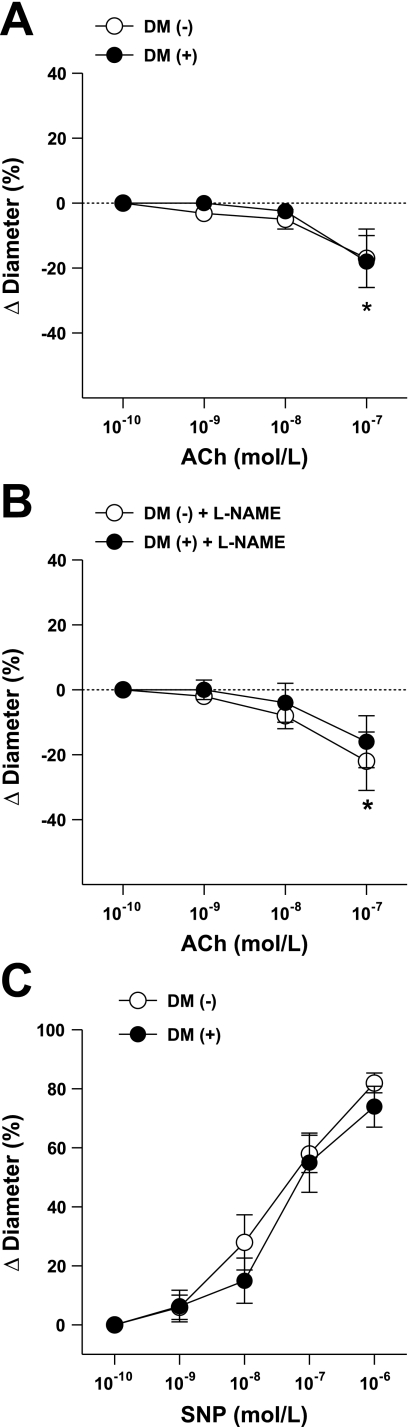
We found that in coronary arterioles, ACh (1 nM–0.1 μM) elicited diminished dilations, and it caused significant constrictions in response to the highest (0.1 μM) concentrations of ACh with similar magnitude in patients with or without DM (Fig. 1A). The NO synthesis inhibitor l-NAME had no effect on these responses in either group of arterioles (Fig. 1B). The NO donor sodium nitroprusside-induced dilations were also similar in magnitude in coronary arterioles isolated from patients with or without DM (Fig. 1C).
Fig. 1.
Changes in diameter of coronary arterioles isolated from patients without diabetes mellitus (DM−, n = 11) and patients with DM (DM+, n = 10) in response to cumulative concentrations of ACh in the absence (A) or in the presence (B, n = 6 in each group) of nitric oxide synthesis inhibitor NG-nitro-l-arginine methyl ester (l-NAME). Changes in diameter of coronary arterioles isolated from subjects without (DM−, n = 9) and with diabetes (DM+, n = 8) in response to cumulative concentrations of sodium nitroprusside (SNP; C) are shown. Data are means ± SE. *P < 0.05, significant changes in diameter from the baseline diameters on A and B.
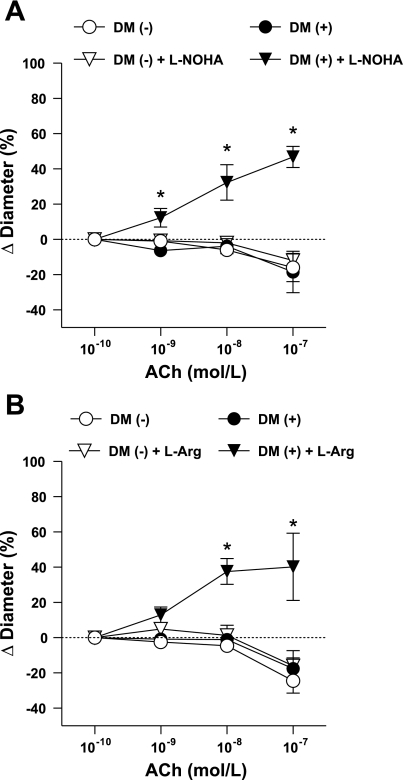
In separate experiments coronary arterioles were incubated with NG-hydroxy-l-arginine (l-NOHA, 10 μM), a selective inhibitor of arginase. l-NOHA had no significant effect on the basal diameter of arterioles (after incubation with l-NOHA, arteriolar diameter was 98 ± 10 and 91 ± 7 μm in patients without and with DM, respectively). We have found that in the presence of l-NOHA, ACh elicited significant dilation in coronary arterioles of patients with DM, but it had no effect on diminished coronary responses in patients without DM (Fig. 2A). Similarly, we found that in arterioles of patients with DM, the application of l-arginine (3 mM) restored vasodilation to ACh, but it had no affect on the diminished responses of vessels obtained from patients without diabetes (Fig. 2B). l-Arginine incubation increased basal diameters in both groups (after incubation with l-arginine, arteriolar diameter was 112 ± 9 and 113 ± 14 μm in patients without and with DM, respectively).
Fig. 2.
Changes in diameter of coronary arterioles isolated from patients without (DM−) and with (DM+) diabetes in response to cumulative concentrations of ACh before and after incubation with NG-hydroxy-l-arginine (l-NOHA, n = 5–5; A) or with l-arginine (l-Arg, n = 6–6; B). Data are means ± SE. *P < 0.05, significant differences in the diameter changes before and after incubation with l-NOHA or l-Arg.
Expression of arginase 1 in human coronary arterioles.
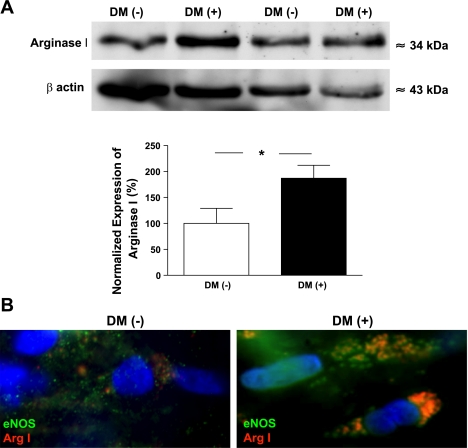
Protein expression of arginase 1 was detected by Western blot analysis in coronary arterioles isolated from the atrial appendages of patients with and without DM. We have found an increased arginase-1 expression in the coronary arterioles of patients with DM compared with subjects without DM (Fig. 3A).
Fig. 3.
A, top: Western blot analysis of the expression of arginase 1 (Arg 1) in coronary arterioles isolated from the atrial appendage of patients with (n = 5) and without (n = 5) DM. Anti-β-actin was used to normalize for loading variations. A, bottom: summary of normalized densitometric ratios. Data are means ± SE. B: representative images of individual endothelial cells (×100, 1.4 numerical aperature objective) on acetone-fixed, en face coronary arteries obtained from patients without (DM−, n = 4; left) or with (DM+, n = 4; right) DM. Cells were immunolabeled with primary antibodies against endothelial nitric oxide synthase (eNOS) and Arg 1. Fluorescent labeling of anti-eNOS was performed with Alexa 488 secondary antibody (shown in green) and Alexa 597 antibody (shown in red) for anti-Arg 1. 4,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining (shown in blue). Coimmunostaining is shown in orange/yellow in the merged images; cells represent 5 cells from 3 different regions in each patient.
With the use of fluorescence microscopy and immunocytochemistry approaches, the protein expression of eNOS and arginase 1 was also detected in endothelial cells in coronary arterioles of patients with or without DM. Arginase-1 immunostaining was prominent in the coronary endothelium of patients with DM, and arginase 1 was less abundantly expressed in those arterioles of subjects without DM. Importantly, we have found that arginase-1 immunostaining was colocalized with eNOS in the endothelial cells of coronary arterioles of patients with DM but not in subjects without DM (Fig. 3B).
DISCUSSION
This study demonstrates that in the coronary arterioles of patients with diabetes, arginase 1 is upregulated, which interferes with NO-mediated vasomotor responses. This conclusion is supported by the findings that in coronary arterioles dissected from the heart of patients with diabetes, ACh elicits a diminished vasodilation, which is not affected by the inhibition of NO synthesis, but it is restored by prior incubation with an arginase inhibitor or l-arginine. When compared with those of subjects without diabetes, coronary arterioles from patients with diabetes exhibit an increased protein expression of arginase 1, which is colocalized with eNOS in endothelial cells.
Although acute and chronic ischemic syndromes are commonly due to coronary flow-limiting atherosclerotic plaques in epicardial coronary arteries, about 10 to 20% of patients with prominent cardiac symptoms, and therefore undergoing cardiac catheterization, are found to have normal coronary angiograms (4). Nitenberg et al. (25) have demonstrated that coronary dilation is impaired in patients with diabetes with angiographically normal coronaries (25). The coronary flow reserve, as defined by the ratio of coronary flow under maximal drug-induced vasodilation to coronary flow under resting conditions, is reduced in patients with diabetes in the absence of significant stenosis of epicardial coronary arteries (26). Thus it is clear that epicardial atherosclerosis may not be the only underlying mechanism resulting in disturbed myocardial perfusion in patients with diabetes, but an important pathological role for functional alterations of coronary microvessels can also be assigned (24, 26). The underlying mechanism(s) of coronary arteriolar vasomotor dysfunction remains incompletely understood in patients with diabetes, so that effective preventive therapeutic strategies cannot be adopted in patients with DM.
The vascular endothelium produces and secretes numerous compounds that regulate a variety of physiological functions, including vasomotor tone, coagulation, inflammation, permeability, and cell adhesion (37). Among others, NO is considered to be one of the key molecules in maintaining normal vascular homeostasis, and it is a major contributor to maintain adequate coronary microvascular tone (20). Solid experimental evidence indicates that diabetes is associated with impaired bioavailability of NO both in conduit vessels and resistance arteries (10, 13, 30, 33). In this study we have found that the inhibition of NO synthesis did not affect ACh-induced coronary vasomotor responses both in patients with or without diabetes. In this context, earlier we have found that coronary arterioles obtained from a small number of young individuals (undergoing only valve surgery and had no sign of coronary artery disease, such as patients with Marfan syndrome) develop vasodilation (maximum, ∼60%) in response to ACh (10−7 M), a response that was diminished by a subsequent inhibition of NO synthesis with l-NAME (Z. Bagi, unpublished observation). Collectively, these aforementioned indicate that the coronary arterioles of patients involved in the current study lack NO mediation of ACh-induced vasomotor responses and suggest that patients with various other comorbidities already exhibit a compromised availability of NO, regardless of the presence or the absence of diabetes. On the other hand, our finding that the NO donor sodium nitroprusside-induced dilations were similar in the two groups indicates an intact downstream, soluble guanylate cyclase and cGMP-dependent signaling and shows a maintained ability of coronary vessels to dilate in response to the endothelium-independent agonist in both patient groups.
Oxidative stress has been proposed to be responsible for the diminished availability of NO in diabetes (5). In patients with diabetes, the administration of the antioxidant vitamin C prevented the decreases in methacholine-induced brachial artery dilations (3). However, other studies failed to demonstrate any significant beneficial effect of antioxidant therapy in the prevention of diabetes-induced vascular complications (21, 22). For example, vitamin-E supplementation for 8 wk did not improve the reduced ACh- and bradykinin-induced dilations of brachial arteries in patients with diabetes (11). We have also found earlier that the administration of the superoxide dismutase did not prevent the dilation of skeletal muscle arterioles isolated from diabetic rats (1). These aforementioned observations raised questions about the direct and only role for oxidative stress in diabetes-related vascular dysfunction. It is known that an adequate level of substrates and cofactors for NO syntheses, such as l-arginine (7) and tetrahydrobiopterin (BH4), is essential for NO synthesis (35, 38) and NO-mediated vasodilation (1). Diabetes has been shown to interfere with the availability of these cofactors, thereby leading to a diminished NO synthesis. To provide experimental evidence for this scenario, Ihlemann et al. (15) demonstrated that in healthy humans, oral glucose challenge-induced reduction in forearm blood flow is restored by pretreatment with BH4 (15). A coinfusion of BH4 and l-arginine into the forearm of patients with diabetes prevented ischemia-reperfusion-induced endothelial dysfunction in the brachial artery (32). Oral l-arginine administration alone also improved brachial artery relaxation in women with diabetes (29). Thus it seems established that diabetes leads to impaired NO synthesis via interfering with the availability of eNOS cofactor BH4. Furthermore, the availability of the NO synthase substrate l-arginine seems also compromised in diabetes, which has been demonstrated in early investigations in diabetic animals (27, 28).
However, the beneficial effect of l-arginine supplementation seemed controversial in light of the known Km value of NO synthase for l-arginine, which is 2.9 μM, and the intracellular concentrations of l-arginine can range from 0.1 to 1.0 mM, the arginine paradox. Interestingly, recent studies suggested that arginase, the focal enzyme of the urea cycle, may reduce l-arginine concentration in the close proximity of NO synthase, hence limiting NO synthesis (7). In this context, Romero et al. (31) demonstrated that increased arginase-1 activity leads to a reduced availability of l-arginine to NO synthase in coronary arteries of diabetic rats. In agreement with this observation and extending those toward human diabetes, in this study we have demonstrated that diabetes is associated with an increased expression of arginase 1 in the coronary arteriolar wall. Arginase-1 expression was abundant in endothelial cells and was colocalized with eNOS in coronary vessels of patients with diabetes but not in subjects without diabetes. To reveal the functional consequence(s) of the upregulated arginase 1, we demonstrated that the inhibition of arginase with l-NOHA caused a restoration of endothelium-dependent agonist-induced dilation in coronary arterioles of patients with diabetes. It should be noted that the arginase inhibitor l-NOHA does not have selectivity toward arginase isoforms (arginase 1 vs. arginase 2). Thus the functional role for arginase 2 in the development of coronary vasomotor dysfunction in patients with diabetes cannot be entirely excluded. In this regard, a previous study has found that an increased expression of arginase 2 may lead to a decreased NO synthesis in pulmonary endothelial cells of patients with pulmonary arterial hypertension (39). The pathological role of arginase isoforms in diabetes-related coronary microvasular dysfunction has yet to be elucidated.
Moreover, the underlying mechanism(s) leading to selective upregulation of arginase 1 in coronary arterioles in patients with diabetes also remains unclear. It is known that insulin suppresses the expression and activity of enzymes of the urea synthesis pathway. Since insulin signaling can be impaired in diabetes (a majority of patients in this study had type 2 diabetes and likely exhibit insulin resistance), it is possible that the failure of insulin regulatory action contributes to the upregulations of arginase 1. Because of the limited number of patients with type 1 diabetes in this study, we have not analyzed the difference in the vasomotor function and arginase-1 expression between type 1 and type 2 diabetes, and we were also not able to statistically distinguish between responses that were obtained in patients receiving insulin supplementation or were on insulin sensitizer medication. Further studies are warranted to ascertain the role of insulin, insulin sensitization, and other pathological factors that could contribute to increased arginase-1 expression in diabetes. Interestingly, in patients without diabetes but suffering from other cardiovascular diseases and other comorbidities, arginase inhibition and also l-arginine supplementation failed to improve coronary dilation, suggesting that mechanism(s) other than the upregulated arginase can be responsible for the diminished ACh-induced response. We could only speculate whether in patients without diabetes an increased level of endogenous NO synthase inhibitor asymmetric dimethylarginine (18) or perhaps a decreased level of other NO synthase cofactors, such as BH4 (15), plays also, if not even a more significant role in this process. Nevertheless, based on our present observation, clinical studies may reveal whether l-arginine supplementation and/or arginase inhibition are beneficial in the prevention and treatment of coronary microvascular complications that are associated with diabetes and insulin resistance. In conclusion, this study is the first to provide experimental evidence for the pathological role of vascular arginase 1, which contributes to the diminished, NO-mediated coronary arteriolar dilation in patients with diabetes.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-104126 and PO-HL-43023 and British Heart Foundation Grant RE/08/004.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Marta Balogh in performing Western blot and immunocytochemistry experiments.
REFERENCES
- 1. Bagi Z, Koller A. Lack of NO-mediation of flow-dependent arteriolar dilation in diabetes is restored by sepiapterin. J Vasc Res 40: 47–57, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bagi Z, Koller A, K G. Peroxisome proliferator-activated receptor-γ activation, by reducing oxidative stress Increases NO bioavailability in coronary arterioles in type 2 diabetes. Am J Physiol Heart Circ Physiol 286: H742–H748, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 103: 1618–1623, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Cannon RO, 3rd, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation 85: 883–892, 1992 [DOI] [PubMed] [Google Scholar]
- 5. De Vriese AS, Verbeuren TJ, Van De Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubois-Rande JL, Zelinsky R, Roudot F, Chabrier PE, Castaigne A, Geschwind H, Adnot S. Effects of infusion of l-arginine into the left anterior descending coronary artery on acetylcholine-induced vasoconstriction of human atheromatous coronary arteries. Am J Cardiol 70: 1269–1275, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enderle MD, Benda N, Schmuelling RM, Haering HU, Pfohl M. Preserved endothelial function in IDDM patients, but not in NIDDM patients, compared with healthy subjects. Diabetes Care 21: 271–277, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol 291: H2107–H2115, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 281: H1304–H1311, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Gazis A, White DJ, Page SR, Cockcroft JR. Effect of oral vitamin E (alpha-tocopherol) supplementation on vascular endothelial function in Type 2 diabetes mellitus. Diabet Med 16: 304–311, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Gornik HL, Creager MA. Arginine and endothelial and vascular health. J Nutr 134: 2880S–2887S, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol 97: 1159–1168, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Huynh NN, Harris EE, Chin-Dusting JF, Andrews KL. The vascular effects of different arginase inhibitors in rat isolated aorta and mesenteric arteries. Br J Pharmacol 156: 84–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Kober L, Torp-Pedersen C. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol 285: H875–H882, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Jebelovszki E, Kiraly C, Erdei N, Feher A, Pasztor ET, Rutkai I, Forster T, Edes I, Koller A, Bagi Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: role of soluble guanylate cyclase activation. Am J Physiol Heart Circ Physiol 294: H2558–H2564, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kaneda H, Taguchi J, Kuwada Y, Hangaishi M, Aizawa T, Yamakado M, Ogasawara K, Ohno M. Coronary artery spasm and the polymorphisms of the endothelial nitric oxide synthase gene. Circ J 70: 409–413, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care 30: 1834–1839, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr Long-term l-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 97: 2123–2128, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis 38: 87–104, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Marchioli R, Schweiger C, Levantesi G, Tavazzi L, Valagussa F. Antioxidant vitamins and prevention of cardiovascular disease: epidemiological and clinical trial data. Lipids 36: S53–S63, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Maxwell S, Greig L. Anti-oxidants—a protective role in cardiovascular disease? Expert Opin Pharmacother 2: 1737–1750, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Miller FJ, Jr, Dellsperger KC, Gutterman DD. Pharmacologic activation of the human coronary microcirculation in vitro: endothelium-dependent dilation and differential responses to acetylcholine. Cardiovasc Res 38: 744–750, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Nahser PJ, Jr, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation 91: 635–640, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Nitenberg A, Paycha F, Ledoux S, Sachs R, Attali JR, Valensi P. Coronary artery responses to physiological stimuli are improved by deferoxamine but not by l-arginine in non-insulin-dependent diabetic patients with angiographically normal coronary arteries and no other risk factors. Circulation 97: 736–743, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 42: 1017–1025, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Pieper GM, Dondlinger LA. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J Pharmacol Exp Ther 283: 684–691, 1997 [PubMed] [Google Scholar]
- 28. Pieper GM, Siebeneich W, Moore-Hilton G, Roza AM. Reversal by l-arginine of a dysfunctional arginine/nitric oxide pathway in the endothelium of the genetic diabetic BB rat. Diabetologia 40: 910–915, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, Oliver-Pickett CK, Reusch JE, Weil JV. Oral l-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med 8: 169–175, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Roberts CK, Vaziri ND, Wang XQ, Barnard RJ. Enhanced NO inactivation and hypertension induced by a high-fat, refined-carbohydrate diet. Hypertension 36: 423–429, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102: 95–102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. l-Arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 204: 73–78, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res 34: 55–68, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Szerafin T, Erdei N, Fulop T, Pasztor ET, Edes I, Koller A, Bagi Z. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res 99: e12–e17, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347: 1–16, 2000 [PMC free article] [PubMed] [Google Scholar]
- 36. Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 97: 22–28, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med 323: 27–36, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 44: 935–943, 2004 [DOI] [PubMed] [Google Scholar]