Abstract
Arachidonic acid (AA) metabolites function as EDHFs in arteries of many species. They mediate cyclooxygenase (COX)- and nitric oxide (NO)-independent relaxations to acetylcholine (ACh). However, the role of AA metabolites as relaxing factors in mouse arteries remains incompletely defined. ACh caused concentration-dependent relaxations of the mouse thoracic and abdominal aorta and carotid, femoral, and mesentery arteries (maximal relaxation: 57 ± 4%, 72 ± 4%, 82 ± 3%, 80 ± 3%, and 85 ± 3%, respectively). The NO synthase inhibitor nitro-l-arginine (l-NA; 30 μM) blocked relaxations in the thoracic aorta, and l-NA plus the COX inhibitor indomethacin (10 μM) inhibited relaxations in the abdominal aorta and carotid, femoral, and mesenteric arteries (maximal relaxation: 31 ± 10%, 33 ± 5%, 41 ± 8%, and 73 ± 3%, respectively). In mesenteric arteries, NO- and COX-independent relaxations to ACh were inhibited by the lipoxygenase (LO) inhibitors nordihydroguaiaretic acid (NDGA; 10 μM) and BW-755C (200 μM), the K+ channel inhibitor apamin (1 μM), and 60 mM KCl and eliminated by endothelium removal. They were not altered by the cytochrome P-450 inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (20 μM) or the epoxyeicosatrienoic acid antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (10 μM). AA relaxations were attenuated by NDGA or apamin and eliminated by 60 mM KCl. Reverse-phase HPLC analysis revealed arterial [14C]AA metabolites that comigrated with prostaglandins, trihydroxyeicosatrienoic acids (THETAs), hydroxyepoxyeicosatrienoic acids (HEETAs), and hydroxyeicosatetraenoic acids (HETEs). Epoxyeicosatrienoic acids were not observed. Mass spectrometry confirmed the identity of 6-keto-PGF1α, PGE2, 12-HETE, 15-HETE, HEETAs, 11,12,15-THETA, and 11,14,15-THETA. AA metabolism was blocked by NDGA and endothelium removal. 11(R),12(S),15(S)-THETA relaxations (maximal relaxation: 73 ± 3%) were endothelium independent and blocked by 60 mM KCl. Western immunoblot analysis and RT-PCR of the aorta and mesenteric arteries demonstrated protein and mRNA expression of leukocyte-type 12/15-LO. Thus, in mouse resistance arteries, 12/15-LO AA metabolites mediate endothelium-dependent relaxations to ACh and AA.
Keywords: endothelium-derived hypolarizing factor, endothelium, mass spectrometry, potassium channels, trihydroxyeicosatrienoic acids, 12/15-lipoxygenase
acetylcholine (ACh) stimulates the release of soluble mediators from the vascular endothelium that diffuse to the underlying smooth muscle layer and cause relaxation. The major relaxing factors include nitric oxide (NO), prostacyclin, a cyclooxygenase (COX) metabolite of arachidonic acid (AA), and a group of compounds collectively called EDHFs (11, 29, 41). Relaxations resistant to NO synthase (NOS) and COX inhibition are attributed to EDHFs. EDHFs cause vascular relaxation through the activation of smooth muscle membrane K+ channels to cause hyperpolarization (for reviews, see Refs. 4 and 22). Alternatively, the EDHF response can be mediated through the activation of endothelial cell K+ channels and the spread of hyperpolarizing current to the smooth muscle via myoendothelial gap junctions (15, 37). The chemical identity of the mediators of EDHF-dependent relaxation varies with the vascular bed and species. In coronary arteries from numerous species, including humans, cytochrome P-450 metabolites of AA, the epoxyeicosatrienoic acids (EETs), function as EDHFs (5, 23, 36). In rat hepatic arteries, K+ functions as an EDHF (16), and in murine and human mesenteric arteries or human coronary arteries, this function is also mediated by H2O2 (35, 38, 39).
The contribution of lipoxygenase (LO) metabolites to vascular relaxation was initially noted by Furchgott and Zawadzki in the rabbit thoracic aorta (24). Relaxation of the rabbit aorta to ACh was reversed by LO inhibition, inhibited by high extracellular K+, and eliminated by endothelium removal. Other investigators have documented the contribution of LO metabolites to vascular relaxation/dilation through the use of pharmacological inhibitors in human coronary (54), rat mesenteric (40, 58), rat basilar (21), canine basilar (33), and canine femoral (13, 32) arteries and skeletal arteries from apolipoprotein E and LDL receptor knockout mice (51). In the rabbit aorta and mesenteric arteries, EDHF-dependent relaxations to ACh are mediated by 15-LO metabolites of AA. The relaxations were dependent on an intact endothelium, resistant to NOS and COX inhibition, reduced by LO inhibitors, and blocked high extracellular K+ or K+ channel inhibitors (6, 45, 57). ACh increases the release of the 15-LO metabolite 11,12,15-trihydroxyeicosatrienoic acid (THETA) from the rabbit aorta (6). Decreasing rabbit endothelial 15-LO expression with antisense oligonucleotides reduces ACh relaxations (53), whereas increasing in vivo 15-LO expression by adenoviral transduction enhances ACh relaxations (2). In rabbit arteries, hydroxyepoxyeicosatrienoic acids (HEETAs) and THETAs function as EDHFs. They activate smooth muscle cell K+ channels to cause relaxation (8, 26, 57).
The present study is the first thorough investigation of AA metabolism in mouse arteries. Our results indicate that the mouse vascular endothelium metabolizes AA via a 12/15-LO pathway to numerous metabolites including THETAs, HEETAs, and hydroxyeicosatetraenoic acids (HETEs). In mouse mesenteric arteries, LO metabolites contribute to NO- and COX-resistant relaxations to ACh and AA.
MATERIALS AND METHODS
Animals.
Male ICR or male and female C57BL6 mice (24–28 g) (The Jackson Laboratory, Bar Harbor, ME) were used. Animal protocols were approved by the Animal Care Committee of the Medical College of Wisconsin, and procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). Arteries were dissected, placed in HEPES buffer containing (in mM) 10 HEPES, 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 6 glucose (pH 7.4), and cleaned of connective tissue.
Isometric tension recording.
Arterial sections from male C57BL6 mice (1.5–1.8 mm in length) were mounted in a four-chamber wire myograph (model 610M, Danish Myo Technology) as previously described (57). Arteries were maintained at 37°C in physiological saline solution containing (in mM) 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1.17 MgSO4, 24 NaHCO3, 1.18 KH2PO4, 0.026 EDTA, and 5.5 glucose and gassed with 95% O2-5% CO2. Mesenteric, femoral, and carotid arteries were stretched to a resting tension of 0.25 mN, and aortic sections were stretched to a resting tension of 1.0 mN. Mesenteric arteries were used with relative diameters of 150–300 μm. Resting tension was determined by a length-tension relationship with KCl (60 mM) plus the thromboxane mimetic U-46619 (100 nM). Arteries were stimulated two to three times with KCl plus U-46619 for 8–10 min at 10-min intervals. Arteries were contracted with submaximal concentrations of U-46619 (50–300 nM) to 50–90% of their maximum KCl + U-46619 challenge. Where indicated, the endothelium was removed by gently rubbing the arterial intimal surface with a human hair. The endothelium was considered intact if ACh (1 μM) caused >50% relaxation and effectively removed if ACh induced <10% relaxation. Cumulative concentrations of ACh (0.1 nM–10 μM) or AA (100 nM–30 μM) were added. Responses were obtained under control conditions or with arteries pretreated with the NOS inhibitor nitro-l-arginine (l-NA; 30 μM), the COX inhibitor indomethacin (Indo; 10 μM), the LO inhibitors nordihydroguaiaretic acid (NDGA; 10 μM) or BW-755C (100–200 μM), the hydroperoxide isomerase inhibitor clotrimazole (20 μM), the cytochrome P-450 inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH; 20 μM), the EET antagonist 14,15-epoxyeicosa-5(Z)enoic acid (14,15-EEZE; 10 μM), 60 mM KCl, or the K+ channel inhibitor apamin (1 μM) either separately or in combination. High concentrations of extracellular K+ reduces the electrochemical gradient for K+ efflux, thereby neutralizing the effect of K+ channel activation. Inhibitors were added 10 min before the initiation of U-46619 contraction. 11,12,15-THETA isomer (10 nM–3 μM) responses were obtained in arteries pretreated with l-NA and Indo. Results are expressed as percentage of relaxation of the U-46619-preconstricted arteries with 100% representing basal tension. l-NA, ACh, Indo, NDGA, clotrimazole, and apamin were purchased from Sigma (St. Louis, MO). AA was purchased from Nu-Chek Prep (Elysian, MN). U-46619 was obtained from Cayman Chemical (Ann Arbor, MI). BW-755C was a kind gift from Burroughs Wellcome. MS-PPOH, 14,15-EEZE, and 11,12,15-THETA stereoisomers were synthesized by J. R. Falck as previously described (19, 20).
Western immunoblot analysis.
Arterial sections were cleaned of adherent connective tissue and homogenized in lysis buffer containing 50 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, and protease inhibitor cocktail (Roche Molecular Biochemicals) and further incubated on ice in lysis buffer for 10 min. Homogenates were vortexed, sonicated five times for 30 s each, and then centrifuged for 10 min at 2,000 g. The supernatant was stored at −80°C. Equal amounts of supernatant protein (30 μg) were loaded in each lane and separated by SDS-PAGE using a 10% resolving gel and 4% stacking gel. Proteins were transferred to nitrocellulose membranes. Nonspecific binding was blocked with Tris-buffered saline (TBS) buffer containing 20 mM Tris base, 150 mM NaCl, 0.1% sodium azide, and 3% BSA overnight at 4°C. Membranes were probed with an antibody generated in our laboratory (18) or a commercial antibody (Cayman Chemical) against 12/15-LO (1:2,000 in blocking buffer) for 1 h at room temperature. Membranes were rinsed with TBS buffer containing 0.1% Tween 20, incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000) for 1 h at room temperature, and washed with TBS buffer. Immunoreactive bands were identified using the Renaissance chemiluminescence detection kit and Kodak BioMax ML film.
RT-PCR.
Total RNA was isolated from the aorta and mesenteric arteries using TRIzol reagent (Life Technologies). DNA was digested with amplification grade DNase-1 (Invitrogen), and cDNA was synthesized from total RNA using the SuperScript III first-strand synthesis system for quantitative RT-PCR (Invitrogen). For controls, reaction mixtures without reverse transcriptase or without RNA were prepared. cDNA for leukocyte 12/15-LO and GAPDH were amplified using a Bio-Rad iCycler. The reaction mixture (25 μl) contained 0.2 μg cDNA, 0.2 μM primers, and RT2 SYBR Green/Fluorescein qPCR Master Mix (Super Array Bioscience Technologies). Primers for leukocyte 12/15-LO (forward: 5′-GCGACGCTGCCCAATCCTAATC-3′ and reverse: 5′-CATATGGCCACGCTGTTTTCTACC-3′) were designed from the mouse leukocyte-type 12/15-LO cDNA sequence and primers for epidermal 12-LO (forward: 5′-AGTGACACCGATGTGAAGGAG-3′ and reverse: 5′-CTCTCAGATGGTCACACTG-3′) were designed for the mouse epidermal 12-LO cDNA sequence and were synthesized by Operon (Huntsville, AL). GAPDH primers were supplied by SABiosciences (Frederick, MD). The program for the iCycler was 94°C for 30 s, 58°C for 1 min, and 72°C for 1.5 min, repeated 40 times, followed by final extension at 72°C for 7 min. Amplified PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining to confirm the presence of a single amplified product (2).
[14C]AA metabolism.
Arteries were incubated for 15 min at 37°C in HEPES buffer containing [14C]AA (0.05 μCi, 50 μM, Perkin Elmer Life Sciences, Boston MA) and 0.1 μM AA. A-23187 (20 μM, Sigma) was added, and arteries were incubated for an additional 5 min. In some instances, Indo (10 μM) or NDGA (10 μM) was added 10 min before the addition of AA. Incubations were stopped by the addition of ethanol to a final concentration of 15%. The buffer was collected, acidified (pH <3.5) with glacial acetic acid, and extracted on ODS solid-phase extraction columns as previously described (8, 47). Extracted metabolites were evaporated to dryness under a stream of nitrogen and stored at −40°C until analysis by reverse- or normal-phase HPLC.
Reverse-phase HPLC analysis.
[14C]AA metabolites were resolved by reverse-phase HPLC using a Nucleosil-C18 column (5 μ, 4.6 × 250 mm). The following solvent systems were used. In solvent system I, solvent A was water and solvent B was acetonitrile containing 0.1% glacial acetic acid. The program consisted of a 40-min linear gradient from 50% solvent B in solvent A to 100% solvent B. The flow rate was 1 ml/min. Column eluate was collected in 0.2-ml fractions (8, 47). In solvent system II, solvent A was water containing 0.1% glacial acetic acid and solvent B was acetonitrile. The program consisted of a 5-min isocratic phase with 35% solvent B in solvent A followed by a 35-min linear gradient to 85% solvent B. The flow rate was 1 ml/min. Column eluate was collected in 0.2-ml fractions (47). In solvent system III, solvent A was water containing 0.025 M phosphoric acid and solvent B was acetonitrile. The program consisted of a 40-min isocratic phase with 31% solvent B in solvent A, a 20-min linear gradient to 100% solvent B, and a 10-min isocratic phase with 100% solvent B. The flow rate was 1 ml/min. Column eluate was collected in 0.5-ml fractions (46). For all solvent systems, absorbance was monitored at 205 nm, and column fraction radioactivity was determined by liquid scintillation spectrometry. All solvents were HPLC grade and purchased from Burdick and Jackson or Sigma.
Isolation of AA metabolites.
From reverse-phase HPLC solvent system I, fractions corresponding to THETAs (5–8 min) and HETEs (17–22.6 min) were collected, acidified, and extracted with cyclohexane-ethyl acetate (50:50). Samples were dried under a stream of nitrogen, and the residue was redissolved in the HPLC mobile phase. THETA fractions were rechromatographed on reverse-phase HPLC using solvent system II, analyzed by liquid chromotography-tandem mass spectrometry (LC-MS/MS), or derivatized for gas chromotography (GC)-MS analysis. HETE fractions were further analyzed by LC-MS/MS. For COX metabolites, aortae were incubated with [14C]AA without Indo, and metabolites were extracted as described above. Prostaglandin (PG) metabolites were separated using reverse-phase HPLC solvent system III.
LC-MS/MS analysis of PGs and THETA and HETE metabolites.
PG and HETE fractions were collected from solvent system I and analyzed using LC-MS/MS using a Waters-MicroMass Quattro tandem quadrupole mass analyzer (56). Fractions were chromatographed using a Kromasil C-18 (5 μm, 2 × 250 mm) column. For PGs, the program consisted of a 10-min linear gradient (flow rate of 0.2 ml/min) from 15% solvent B (acetonitrile) in solvent A (deionized water with 0.005% glacial acetic acid) to 60% solvent B and a 10-min linear gradient of 60–80% solvent B in solvent A followed by an additional 5-min gradient of 80–100% solvent B in solvent A. Desolvation and cone gas flow were 700 and 100 l/h, respectively. For collision-induced dissociation MS/MS, the MS conditions were as follows: cone voltage, 1 or 30 V; collision energy, 11.0 or 22.0 eV; capillary voltage, 3.0 kV; source temperature, 90°C; and desolvation temperature, 350°C. For HETEs, the program consisted of a 20-min linear gradient (flow rate of 0.2 ml/min) from 60% solvent B (acetonitrile with 0.005% glacial acetic acid) in solvent A (deionized water with 0.005% glacial acetic acid) to 100% solvent B. Capillary voltage was 3.5 kV, cone voltage was 20 V, source temperature was 130°C, desolvation temperature was 350°C, and collision energy was 25.0 eV. Desolvation and cone gas flow were 600 and 50 l/h, respectively. THETA fractions were analyzed using electrospray ionization with a Fourier transform ion cyclotron resonance mass spectrometer (ESI-FT/MS, IonSpec 7.0-tesla FTICR high-resolution mass spectrometer with a Waters Z spray ESI source) (12). Fractions were chromatographed using a Kromasil C-18 (5 μm, 1 × 150 mm) column. The program consisted of a 40-min linear gradient (flow rate of 0.1 ml/min) from 25% solvent B (acetonitrile) in solvent A (deionized water with 0.005% glacial acetic acid) to 44% solvent B followed by a 5-min linear gradient of 44–100% solvent B in solvent A. The source temperature was 80°C. The cone voltage was 45 V, and the probe high voltage was 3,500 V. Precursor ions were isolated and fragmented with sustained off-resonance irradiation collision-induced dissociation using N2 as the collision gas with an offset frequency of 4,563 Hz, amplitude of 13.25 V, and gas pulse of 20 ms. All ions were monitored in the negative ion mode.
GC/MS analysis.
The THETA fraction was dissolved in acetonitrile (120 μl) and treated with ethereal diazomethane for 6 min at 0°C to form the methyl ester. The reacted sample was evaporated to dryness under nitrogen. Hydroxyl groups were converted to the trimethylsilyl (TMS) ethers by incubation with bis-TMS-trifluoroacetamide (15 μl) at 37°C for 60 min. GC/MS was performed using a Hewlett-Packard 5989A mass spectrometer coupled to a 5890 Series 2 gas chromatograph. Chemical ionization of the samples was performed at 65–70 eV using methane as the reagent gas. Ions were measured in the positive ion mode as total ion current. The sample was resolved using a 14-m capillary DB-5 column with a linear gradient from 100 to 300°C (25).
Trapping and MS identification of HEETAs.
Aortic rings were incubated with [14C]AA as described above in the presence or absence of the soluble epoxide hydrolase (sEH) inhibitor 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA; 1 μM, provided by Dr. Bruce Hammock, University of California, Davis, CA). Incubation buffer was immediately transferred to 25 volumes of methanol acidified with glacial acetic acid to pH 3.5 (8). The methanol solution was incubated at room temperature for 30 min. Excess methanol was removed by rotary evaporation under vacuum. The remaining incubation medium was extracted on ODS extraction columns as described above. The metabolites were resolved using reverse-phase HPLC solvent system I. Fractions containing HEETA methanol derivatives, the methoxydihydroxyeicosatrienoic acids (MDHEs; 8.2–11 min), were collected, acidified, and extracted with cyclohexane-ethyl acetate (50:50). The solvent was dried under a stream of N2 and stored at −40°C until MS analysis. MDHE fractions were analyzed by ESI-FT/MS as described above. Ions with a mass-to-charge (m/z) of 367 were isolated and fragmented. Daughter ions were detected with a m/z range from 25 to 500. Ions were measured in negative ion mode (8).
Data analysis.
Data are presented as means ± SE. Significant differences between mean values were evaluated by Student t-test or ANOVA followed by the Student-Newman-Keuls multiple-comparison test. P values of <0.05 was considered statistically significant.
RESULTS
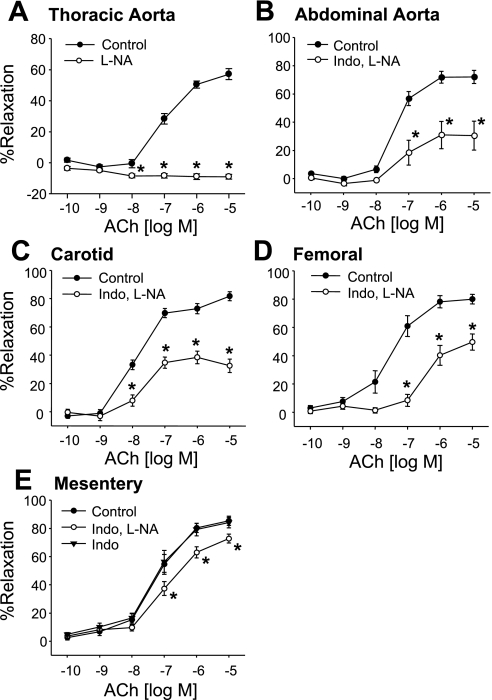
In preconstricted thoracic aortic, abdominal aortic, carotid, mesenteric, and femoral arterial rings from C57BL6 male mice, ACh (100 pM–10 μM) caused concentration-related relaxations (Fig. 1). NOS inhibition by l-NA (30 μM) blocked relaxations in the thoracic aorta (Fig. 1A). COX inhibition with Indo (10 μM) plus l-NA inhibited, but did not block, ACh-induced relaxations in the abdominal aorta and carotid, mesenteric, and femoral arteries (Fig. 1, B–E). In mesenteric arteries, ACh-induced relaxations were not altered by Indo alone (Fig. 1E). l-NA- and Indo-resistant relaxations to ACh were greater in mesenteric arteries than in the abdominal aorta and carotid and femoral arteries (maximal relaxation: 73 ± 3%, 31 ± 10%, 38 ± 4%, and 50 ± 8%, respectively). For this reason, experiments on the role of endothelial AA metabolites in mediating EDHF activity were performed on mesenteric arteries.
Fig. 1.
ACh-induced relaxations of the mouse thoracic (A) and abdominal aorta (B) and carotid (C), femoral (D), and mesenteric (E) arteries. Relaxations were performed on arterial rings pretreated with vehicle, nitro-l-arginine (l-NA; 30 μM), indomethacin (Indo; 10 μM), or the combination of l-NA plus Indo. Values are means ± SE; n = 6–41. *P < 0.05 compared with control.
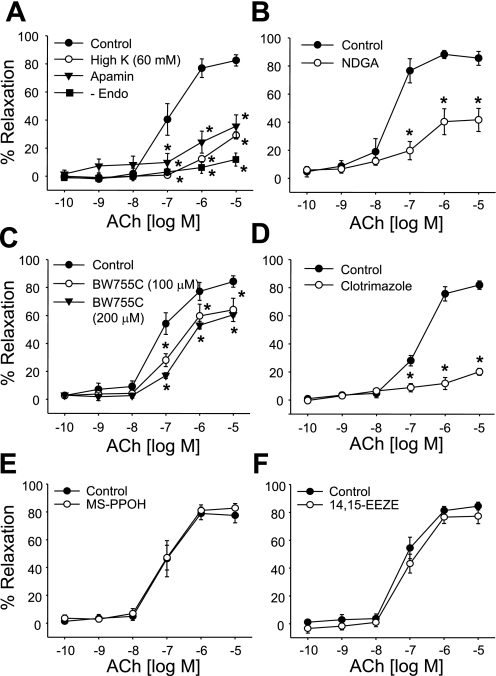
In l-NA- and Indo-treated mesenteric arteries, concentration-related relaxations to ACh were abolished by endothelium removal and nearly eliminated by high K+ (60 mM KCl) or the small-conductance Ca2+-activated K+ (SKCa) channel inhibitor apamin (1 μM; Fig. 2A). Indo- and l-NA-resistant relaxations were also inhibited by the LO inhibitors BW-755C and NDGA (10 μM; Fig. 2, B and C) or the hydroperoxide isomerase inhibitor clotrimazole (20 μM; Fig. 2D). They were not altered by the cytochrome P-450 inhibitor MS-PPOH (20 μM; Fig. 2E) or the EET antagonist 14,15-EEZE (10 μM; Fig. 2F).
Fig. 2.
ACh-induced relaxations of mouse mesenteric arteries pretreated with l-NA (30 μM) and Indo (10 μM). A: effects of endothelial cell removal, high extracellular K+ (60 mM), and the small-conductance Ca2+-activated K+ (SKCa) channel inhibitor apamin (1 μM). B and C: effect of the lipoxygenase (LO) inhibitors nordihydroguaiaretic acid (NDGA; 10 μM; B) and BW-755C (100 and 200 μM; C). D: effect of the hydroperoxide isomerase inhibitor clotrimazole (20 μM). E: effect of the cytochrome P-450 inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH; 20 μM). F: effect of the epoxyeicosatrienoic acid antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE; 10 μM). Values are means ± SE; n = 4–10. *P < 0.05 compared with control.
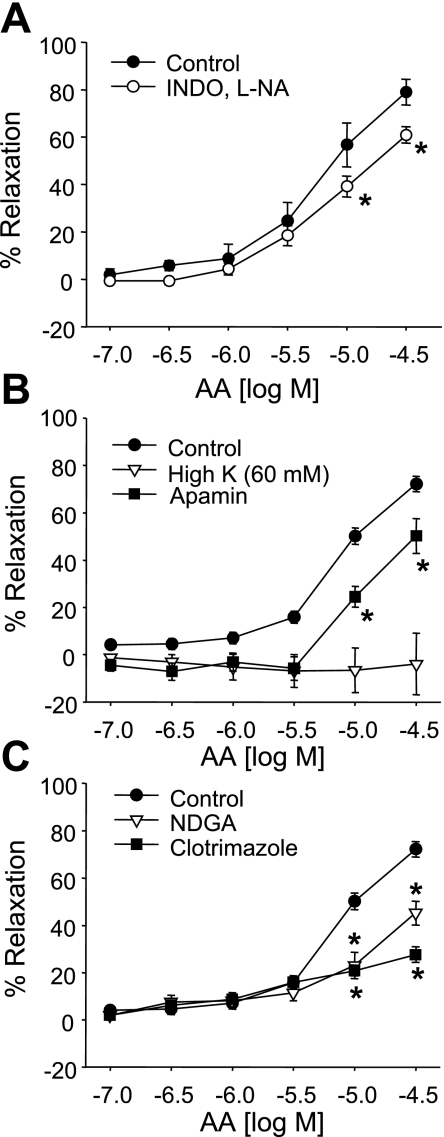
Similarly, AA caused concentration-related relaxations of mesenteric arteries (Fig. 3). Relaxations to AA were slightly diminished by Indo and l-NA (Fig. 3A). In the presence of Indo and l-NA, relaxations to AA were abolished by high K+ and inhibited by apamin, NDGA, and clotrimazole (Fig. 3, B and C). These results suggest that endothelial LO metabolites of AA function as EDHFs and contribute to ACh and AA relaxations of mouse mesenteric arteries.
Fig. 3.
Arachidonic acid (AA) relaxations of mouse mesenteric arteries. A: effect of Indo (10 μM) plus l-NA (30 μM). B: effect of high extracellular K+ (60 mM) and the SKCa channel inhibitor apamin (1 μM) in arteries pretreated with Indo plus l-NA. C: effect of the LO inhibitor NDGA (10 μM) or the hydroperoxide isomerase inhibitor clotrimazole (20 μM) in arteries pretreated with Indo plus l-NA. Values are means ± SE; n = 4–30. *P < 0.05 compared with control.
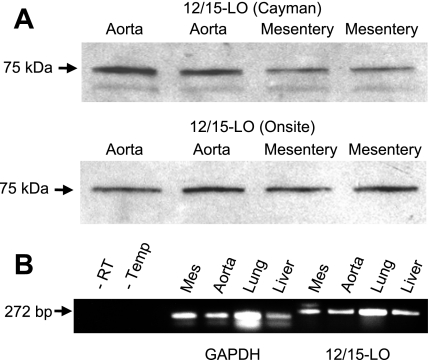
Western immunoblot analysis and RT-PCR were performed to confirm the presence of 12/15-LO in mouse arteries. Two different leukocyte-type 12/15-LO antibodies, our antibody (18) and a commercial antibody, were used to probe mesenteric and aortic protein lysates. A 75-kDa immunoreactive band consistent with leukocyte-type 12/15-LO was observed with both antibodies (Fig. 4A). Agarose gel separation of RT-PCR products from mesenteric arteries and aortic RNA using specific leukocyte 12/15-LO primers showed a single band at the expected size of 272 bp (Fig. 4B). Primers specific for mouse epidermal 12/15-LO, a 570-bp product, did not detect epidermal 12/15-LO in mouse arteries (data not shown). As a control, primers specific for GAPDH showed a single band of the expected size of 175 bp in RT-PCR products from both the mouse aorta and mesenteric arteries. Bands were not observed in lanes loaded from RT-PCRs without reverse transcriptase or primer template. These data demonstrate that leukocyte-type 12/15-LO mRNA and protein are present in mouse arteries.
Fig. 4.
Expression of leukocyte-type 12/15-LO protein and RNA in mouse arteries. A: Western immunoblot for leukocyte-type 12/15-LO (75 kDa) in lysates (30 μg protein/lane) prepared from the mouse aorta and mesenteric arteries with a commercial antibody (Cayman Chemical) and an author-generated (Onsite) antibody. B: RT-PCR products amplified using primers specific for leukocyte-type 12/15-LO (272 bp) and GAPDH (175 bp) probing cDNA prepared from the mouse mesenteric arteries, aorta, lung, and liver.
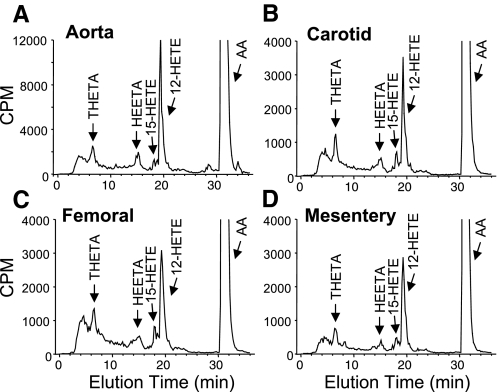
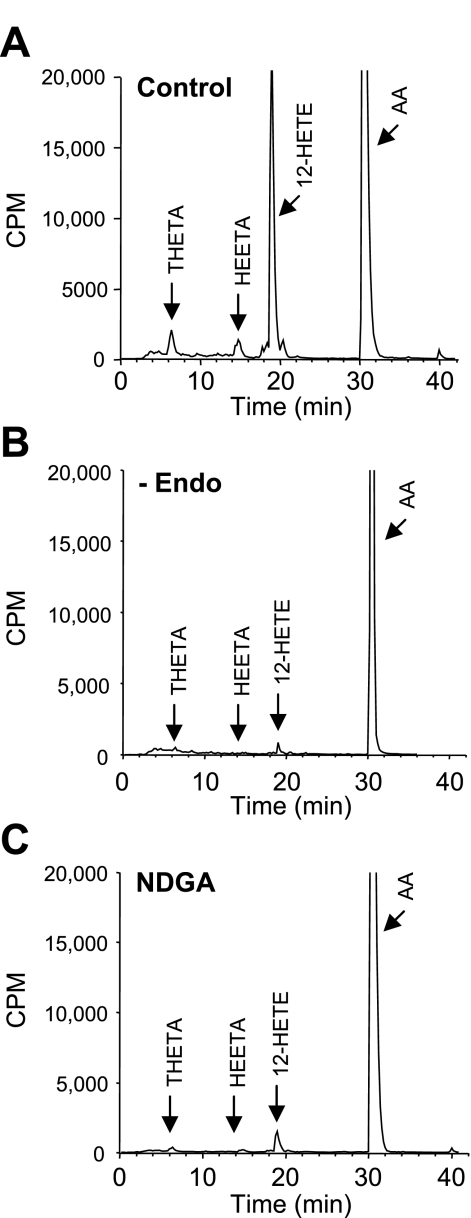
Arterial sections of the aorta and carotid, mesenteric, and femoral arteries from C57BL6 male mice were incubated with [14C]AA in the presence of Indo. Metabolites were extracted and resolved by reverse-phase HPLC solvent system I. The retention times of radioactive metabolites were compared with known standards for THETAs, HEETAs, and HETEs (Fig. 5). Similar metabolite profiles were observed in all four mouse arteries. Major metabolites comigrated with THETAs, HEETAs, 15-HETE, and 12-HETE. The predominant HETE observed was 12-HETE. Interestingly, cytochrome P-450 epoxygenase metabolites of AA, the EETs (elution time: 22–24 min), or their metabolites, the dihydroxyeicosatrienoic acids (DHETs; elution time 12.0–14.5 min), were not observed. The [14C]AA metabolic profile of the aorta was similar in incubations from male C57BL6 (Fig. 5A) and from male ICR and female C57BL6 mice (data not shown). [14C]AA metabolism was abolished by endothelium removal and nearly eliminated by NDGA (10 μM; Fig. 6). These results indicate that an endothelial LO pathway is responsible for the observed AA metabolism in mouse arteries.
Fig. 5.
AA metabolism in the mouse aorta (A) and carotid (B), femoral (C), and mesenteric (D) arteries. Arterial segments were incubated with [14C]AA in the presence of Indo (10 μM). Metabolites were extracted and resolved by reverse-phase HPLC solvent system I. Migration times of known standards are indicated. CPM, counts per minute; THETA, trihydroxyeicosatrienoic acid; HEETA, hydroxyepoxyeicosatrienoic acid; HETA, hydroxyeicosatetraenoic acid.
Fig. 6.
Effects of endothelial cell removal and LO inhibition on AA metabolism in the mouse aorta. Aortic segments were incubated with [14C]AA in the presence of Indo (10 μM) under control conditions (A), with endothelial removal (−Endo; B), or in the presence of the LO inhibitor NDGA (10 μM; C). Metabolites were extracted and resolved by reverse-phase HPLC solvent system I. Migration times of known standards are indicated. Representative chromatographs of 3 experiments are shown.
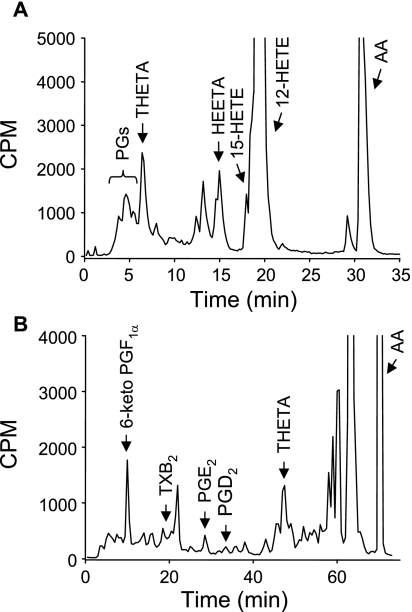
Our next goal was to isolate and identify the various AA metabolites. [14C]AA incubations of aortic rings were resolved using reverse-phase HPLC solvent system I. A polar radioactive peak comigrating with PGs was detected in addition to THETAs, HEETAs, and HETEs (Fig. 7A). To isolate individual COX metabolites, mouse aortae were incubated with [14C]AA, and metabolites were resolved by reverse-phase HPLC solvent system III. Using this system, 14C-labeled metabolites comigrated with 6-keto-PGF1α, the metabolite of PGI2, and PGE2 (Fig. 7B). The identities of these metabolites were confirmed by LC-MS/MS. Two major products were detected with HPLC elution times of 13.1 and 15.5 min that comigrated with standards for 6-keto-PGF1α and PGE2, respectively (Table 1). For the 13.1-min product, MS analysis indicated m/z [M-H] 369, consistent with 6-keto-PGF1α. Fragmentation produced daughter ions of m/z 351 ([M-H]-H2O), 333 ([M-H]-2H2O), 315 ([M-H]-3H2O), 289 ([M-H]-2H2O-CO2), and 271 ([M-H]-3H2O-CO2). These ions and the most abundant daughter ion m/z 163 confirmed the synthesis of 6-keto-PGF1α (41). Identical fragmentation occurred with the 6-keto-PGF1α standard. For the 15.5-min product, MS analysis indicated m/z 351 [M-H], consistent with PGE2. Fragmentation produced daughter ions of m/z 333 ([M-H]-H2O), 315 ([M-H]-2H2O) (most abundant), 271 ([M-H]-2H2O-CO2), 233 ([M-H]-H2O-C6H12O), and 189 (233-CO2). Identical fragmentation occurred with the PGE2 standard. These data confirm the production of 6-keto-PGF1α and PGE2 and COX metabolism of AA in mouse arteries.
Fig. 7.
Analysis of AA cyclooxygenase metabolites in the mouse aorta. Aortae were incubated with [14C]AA, and samples were extracted and resolved by reverse-phase HPLC solvent systems I (A) or III (B). Migration times of known standards are indicated. PG, prostaglandin; TXB2, thromboxane B2.
Table 1.
LC-MS/MS analysis of prostaglandins and HETEs
| Sample | Retention Time, min | Major Ion [M-H], m/z | MS/MS Daughter Ions, m/z |
|---|---|---|---|
| 6-Keto-PGF1α | |||
| Standard | 13.2 | 369 | 351 ([M-H]-H2O), 333 ([M-H]-2H2O), 315 ([M-H]-3H2O), 289 ([M-H]-2H2O-CO2), 271 ([M-H]-3H2O-CO2), 245, 207, 163* |
| Biological | 13.1 | 369 | 351 ([M-H]-H2O), 333 ([M-H]-2H2O), 315 ([M-H]-3H2O), 289 ([M-H]-2H2O-CO2), 271 ([M-H]-3H2O-CO2), 245, 207, 163* |
| PGE2 | |||
| Standard | 15.5 | 351 | 333 ([M-H]-H2O), 315* ([M-H]-2H2O), 271([M-H]-2H2O-CO2), 233 ([M-H]-H2O-C6H120), 189 (233-CO2) |
| Biological | 15.5 | 351 | 333 ([M-H]-H2O), 315* ([M-H]-2H2O), 271 ([M-H]-2H2O-CO2), 233 ([M-H]-H2O-C6H120), 189 (233-CO2) |
| 12-HETE | |||
| Standard | 15.7 | 319 | 301 ([M-H]-H2O), 257 ([M-H]-H2O-CO2), 208 ([M-H]-C8H15), 179* ([M-H]-C9H16O), 135 (179-CO2) |
| Biological | 15.7 | 319 | 301 ([M-H]-H2O), 257 ([M-H]-H2O-CO2), 208 ([M-H]-C8H15), 179* ([M-H]-C9H16O), 135 (179-CO2) |
| 15-HETE | |||
| Standard | 14.4 | 319 | 301* ([M-H]-H2O), 257 ([M-H]-H2O-CO2), 219 ([M-H]-C6H12O), 175, (219-CO2) |
| Biological | 14.4 | 319 | 301* ([M-H]-H2O), 257 ([M-H]-H2O-CO2), 219 ([M-H]-C6H12O), 175, (219-CO2) |
LC, liquid chromotography; MS, mass spectroscopy; m/z, mass-to-charge ratio; HETE, hydroxyeicosatetraenoic acid.
Daughter ion of greatest abundance.
To confirm the identity of HETE metabolites, HETE fractions (17–22.6 min; Fig. 7A) were also analyzed by LC-MS/MS. Two major products (HPLC elution times of 14.4 and 15.7 min; Table 1) and two minor products (HPLC elution times of 15.0 and 16.4 min) were detected. The major products comigrated with 15-HETE and 12-HETE standards, and the minor products comigrated with 11-HETE and 5-HETE standards. For the four products, MS analysis indicated m/z 319 [M-H] as the major ion, which is consistent with the HETE structure. Fragmentation produced common daughter ions of m/z 301 ([M-H]-H2O), 275 ([M-H]-CO2), and 257 ([M-H]-CO2-H2O). Additionally, ions of m/z 219, 167, 179, and 115 occurred with the 14.4-, 15.0-, 15.7-, and 16.4-min products, respectively, and represent carboxylate anions from cleavage adjacent to the hydroxyl group for 15-HETE, 11-HETE, 12-HETE, and 5-HETE, respectively (12, 42). Identical fragmentation patterns occurred with the respective HETE standards. This confirmed the production of 15-HETE, 11-HETE, 12-HETE, and 5-HETE by mouse arteries.
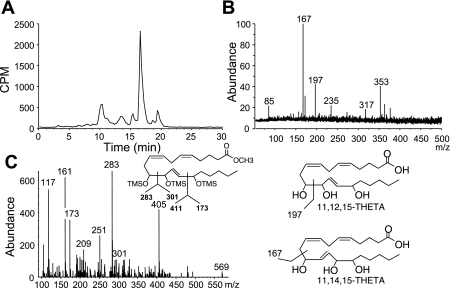
From aortic incubations resolved using reverse-phase HPLC solvent system I (Fig. 7A), the THETA peak (5–8 min) was collected and further resolved using reverse-phase HPLC solvent system II (Fig. 8A). The reverse-phase HPLC solvent system II chromatogram showed a major peak with an elution time of 16–17 min, which is similar to THETAs isolated from the rabbit aorta (47). The THETA peak was further analyzed by LC-MS/MS (Fig. 8B) or derivatized to the methyl ester, TMS ether, and analyzed by GC-MS (Fig. 8C). LC-MS/MS analysis revealed m/z 353 [M-H] as the major ion, which corresponds to the molecular mass of THETA (structure shown in Fig. 8D). The major daughter ions of m/z 235, 197, 167, and 85 correspond to the presence of 11,12,15-THETA and 11,14,15-THETA regioisomers (12). GC-MS analysis of the methyl ester, TMS ether THETA derivative revealed a product with an elution time of 13.52 min and a molecular weight of 584 [M+]. Fragmentation of this product revealed ions of m/z 569 ([M+-15]; loss of CH3), 405 [[M+-179]; loss of (CH3)3SiOH and (CH3)3SiO], 301 {[M+-283]; loss of [(CH3)3SiO]-CH-(CH2-CH=CH)2-(CH2)3-COOCH3}, 283 {[M+-301]; loss of [(CH3)3SiO]-CH-CH=CH-CH[(CH3)3SiO]-(CH2)4-CH3}, and 173 ([M+-411]; loss of {CH=CH-CH[(CH3)3SiO]-CH[(CH3)3SiO]-(CH2-CH=CH)2-(CH2)3-COOH3}). The greater abundance of m/z 283 versus 173 (ratio of 283/173 = 1.9) indicates the presence of 11,12,15-THETA (47). Another product with an elution time of 13.64 min was observed with a similar fragmentation pattern (data not shown) but with greater abundance of the m/z 173 fragment (ratio of 283/173 = 0.1), indicating the presence of 11,14,15-THETA. These results demonstrate that mouse arteries produce both 11,12,15-THETA and 11,14,15-THETA; however, the latter may arise from the acid hydrolysis of HEETA during extraction (8).
Fig. 8.
Isolation and identification of 11,12,15-THETA and 11,14,15-THETA from the mouse aorta. Aortae were incubated with [14C]AA in the presence of Indo (10 μM), and samples were extracted and resolved using reverse-phase HPLC solvent system I. THETA fractions were collected, further resolved using solvent system II, and analyzed by liquid chromatography-tandem mass spectroscopy (MS; A) or derivatized and analyzed by gas chromatography/MS (B and C). C, inset: structure of derivatized 11,12,15-THETA. D: structures of 11,12,15-THETA and 11,14,15-THETA and major fragmentation sites. m/z, mass-to-charge ratio.
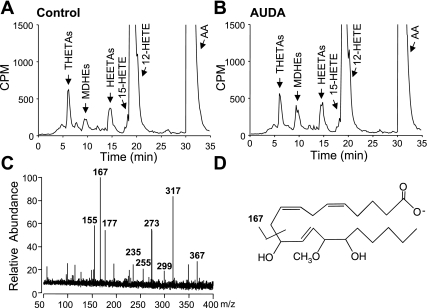
Isolation and identification of HEETA metabolites is complicated by their lability and acid hydrolysis (8). Therefore, we incubated aortic tissue in the presence and absence of the sEH inhibitor AUDA to inhibit HEETA metabolism and added 25-fold excess of methanol to the buffer immediately after incubation to capture acid-sensitive HEETA as MDHE derivatives (8). Using reverse-phase HPLC solvent system I, radioactive metabolites comigrating with THETAs, HEETAs, and HETEs were observed (Fig. 9, A and B). Additional products eluting at 8.2–11 min comigrated with MDHEs. The presence of AUDA increased the formation of MDHEs. MDHE fractions (8.2–11 min) were collected and analyzed by high-resolution ESI-FT/MS (Fig. 9C). ESI-FT/MS analysis revealed m/z 367 [M-H] as the major ion, which corresponds to the molecular mass of MDHEs (structure shown in Fig. 9D). Fragmentation revealed daughter ions of m/z 317 [[M-H]-(H2O)-(CH3OH)], 299 [[M-H]-2(H2O)-(CH3OH)], 273 [[M-H]-(H2O)-(CH3OH)-(CO2)], 255 [[M-H]-2(H2O)-(CH3OH)-(CO2)], 235 [[M-H]-132; loss of CH(OH)-(CH2)4CH3 and CH3OH], and 167 [[M-H]-200-CH=CH-CH2-CH=CH-(CH2)3COO]. This pattern is consistent with a mixture of 12-methoxy-11,15-dihydroxy- and 14-methoxy-11,15-dihydroxy-eicosatrienoic acid, which indicates the presence of 15-H-11,12-EETA, the metabolic precursor of 11,12,15-THETA (8).
Fig. 9.
Isolation and identification of HEETA from the mouse aorta. Aortae were incubated with [14C]AA in the presence of Indo (10 μM) and the presence and absence of the soluble epoxide hydrolase inhibitor 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA; 1 μM). A and B: samples were acidified in 25-fold excess of methanol, extracted, and analyzed by reverse-phase HPLC solvent system I. Migration times of known standards are indicated. C: methoxydihydroxyeicosatrienoic acid (MDHE) fractions were collected and analyzed by electrospray ionization with a Fourier transform ion cyclotron resonance MS. D: structure of MDHE with the major fragmentation site.
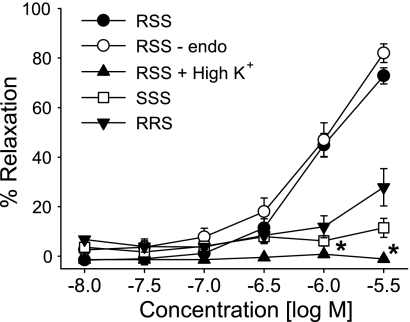
Three stereoisomers of 11,12,15-THETA were tested for their ability to relax U-46619-preconstriced mouse mesenteric arteries. 11(R),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid caused concentration-dependent relaxations with a maximal relaxation of 73 ± 3% at 3 μM (Fig. 10). Relaxations were not altered by endothelium removal but were blocked by high extracellular K+ (60 mM). In contrast to the RSS-isomer, 11(S),12(S),15(S)-THETA and 11(R),12(R),15(S)-THETA caused slight relaxations (maximal relaxation: 11 ± 4 and 28 ± 8%, respectively). Thus, in mouse mesenteric arteries, a specific stereochemical configuration of 11,12,15-THETA is required to induce relaxations, and these relaxations are dependent on smooth muscle K+ channel activation.
Fig. 10.
Relaxations of mouse mesenteric arteries by 11,12,15-THETA stereoisomers. Relaxations of 11(R),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid (RSS), 11(S),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid (SSS), and 11(R),12(R),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid (RRS) were compared. Relaxations to 11(R),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid were repeated in arteries with the endothelium removed or in the presence of high extracellular K+ (60 mM). Arteries were pretreated with Indo (10 μM) and l-NA (30 μM). Values are means ± SE; n = 5–8. *P < 0.05 compared with control.
DISCUSSION
This is the first study to extensively investigate AA metabolism in mouse arteries and to relate the metabolism to vascular relaxation. Our findings indicate that 12/15-LO is the predominant enzymatic pathway of AA metabolism in mouse arteries and that these metabolites contribute to ACh and AA relaxations.
All mouse arteries tested demonstrated concentration-dependent relaxations to ACh. In the thoracic aorta, the relaxations are mediated by NO and were eliminated by pretreatment with the NOS inhibitor l-NA. Similar blockade of mouse aortic ACh relaxations by NOS inhibitors has been previously described (7, 39). In contrast, the abdominal aorta and carotid, femoral, and mesenteric arteries demonstrated ACh relaxations that were resistant to NOS and COX inhibition. The greater role of NO in mediating ACh relaxations of the thoracic aorta is not surprising since the relative contribution of NO to endothelium-dependent relaxation is greatest in conduit arteries, whereas that of EDHF increases as vessel size decreases (39, 50).
Mesenteric arteries demonstrated the highest level of l-NA- and Indo-resistant relaxation to ACh. ACh-induced relaxations of the mouse mesenteric arteries were blocked by endothelial removal and nearly eliminated by high extracellular K+ or the SKCa channel inhibitor apamin. These findings indicated that l-NA- and Indo-resistant relaxations to ACh were mediated by K+ channel activation, revealing the role of an EDHF. l-NA- and Indo-resistant ACh relaxations were also reduced by LO inhibitors. Similarly, Indo-resistant relaxations to AA were blocked by high extracellular K+ and inhibited by apamin and the LO inhibitor NDGA. In contrast, in mesenteric arteries from apolipoproteinE-deficient or db/+ control mice, apamin alone did not alter EDHF-dependent relaxations to ACh (14, 44). However, the combination of apamin plus the intermediate-conductance Ca2+-activated K+ channel inhibitor charybdotoxin eliminated or reduced the relaxations (14, 17, 27, 44, 52). Thus, the contribution of apamin-sensitive channels in EDHF-mediated relaxations to ACh in mouse mesenteric arteries may differ in normal and pathological conditions. Further studies clarifying the role of specific K+ channels in EDHF activity under various conditions are required.
Two structurally unrelated LO inhibitors, BW-755C and NDGA, were used. We cannot disregard possible interactions of these inhibitors with other enzymatic pathways. BW-755C inhibits COX and 12-LO in rabbit platelets (28). NDGA inhibits human platelet 12-LO and COX activity at low micromolar concentrations (<5 μM) (3) but also inhibits monooxygenase activity at concentrations of >40 μM (1). The effect of NDGA and BW-755C in the present study was due to LO inhibition because 1) COX inhibition with Indo had minor effects on vascular relaxation, 2) NDGA used at the concentration of 10 μM imparts specificity toward COX and LO inhibition, and 3) NDGA was tested in l-NA- and Indo-treated arteries. In addition, the cytochrome P-450 epoxygenase inhibitor MS-PPOH and the EET antagonist 14,15-EEZE did not alter Indo- and l-NA-resistant relaxations to ACh in mouse mesenteric arteries. Taken together, our results indicate that ACh-induced relaxations of mouse mesenteric arteries are mediated by endothelial LO metabolite(s) of AA.
[14C]AA metabolism by the mouse aorta and carotid, femoral, and mesenteric arteries showed similar profiles with major metabolites comigrating with THETAs, HEETAs, and HETEs. There was no evidence of EET or DHET production. Identical results were obtained in aortas from two different mouse strains (ICR and C57BL6) and in aortas from male and female C57BL6 mice. Thus, these results are not species or sex specific but may be generalized to mouse arteries. Through further HPLC and MS analysis, we confirmed the presence of 11,12,15-THETA, 11,14,15-THETA, 15-H-11,12-EETA, 5-HETE ,12-HETE, 11-HETE, and 5-HETE. THETAs, HEETAs, and HETEs are products of endothelial cell LO metabolism because removal of the endothelium or the presence of a LO inhibitor blocked their production. Similar profiles of LO metabolites have been produced by arteries isolated from rabbits, rats, pigs, dogs, and humans (9). Western immunoblot analysis and RT-PCR demonstrated vascular protein and RNA for leukocyte-type 12/15-LO. 12/15-LO expression and the profile of AA metabolites demonstrated that a functional enzymatic pathway is present for the vascular production of vasoactive LO metabolites.
An enzymatic pathway of AA metabolism by 15-LO to vasodilatory metabolites has been proposed in rabbit arteries (8, 9, 47, 48, 53). In the rabbit vascular endothelium, 15-LO metabolizes AA to 15(S)-hydroperoxyeicosatetraenoic acid (HPETE). 15(S)-HPETE is rearranged to 15-H-11,12-EETA by hydroperoxide isomerase or reduced to 15-HETE by a peroxidase. sEH converts 15-H-11,12-EETA to 11,12,15-THETA. A similar enzymatic pathway involving 12/15-LO metabolism of AA could occur in the mouse vascular endothelium. A similar metabolite profile was observed in mouse arteries as in rabbit arteries (47). An obvious difference between the two species is that 12-HETE is the predominant HETE regioisomer in the mouse, whereas 15-HETE is the predominant HETE in the rabbit. Vascular LO enzymes of rabbits and mice are both classified 12/15-LOs, but they differ in the ratio of 12-HETE and 15-HETE synthesized (10, 53). The ratio of 12-HETE-to-15-HETE synthesis for recombinant mouse leukocyte-type 12/15-LO is 3:1 (10), whereas the ratio for recombinant rabbit 12/15-LO and the rabbit vascular 15-LO is 1:12 (34, 53). In the present study, the approximate 12-HETE-to-15-HETE ratio observed in mouse arterial AA metabolism was 12:1 (see Fig. 5). The reason for the discrepancy in 12-HETE-to-15-HETE ratios between mouse arteries and recombinant mouse 12/15-LO is probably related to the further metabolism of 15(S)-HPETE to HEETAs and THETAs thereby decreasing the amount of 15-HETE without changing 12-HETE concentrations. In support of this suggestion, if we group 15-HPETE metabolites (i.e., THETA and HEETA) with 15-HETE, the 12-HETE-to-15-HETE ratio approaches 2:1, which is similar to the enzymatic activity of recombinant leukocyte-type 12/15-LO.
Vascular relaxations to ACh and AA were decreased by clotrimazole. Clotrimazole has several documented actions. First, it inhibits cytochrome P-450s (43). However, the vascular effects of clotrimazole appear to be unrelated to cytochrome P-450 blockade because another cytochrome P-450 epoxygenase inhibitor, MS-PPOH, was without effect. Second, clotrimazole inhibits K+ channel activity, specifically, large- and intermediate-conductance Ca2+-activated K+ channels but not SKCa channels (30, 31, 37). Since apamin-sensitive SKCa channels mediate the relaxations to ACh and AA, clotrimazole must be acting by a mechanism other than K+ channel inhibition. Finally, clotrimazole is a hydroperoxide isomerase inhibitor blocking HEETA and THETA production from 15-HPETE in rat liver microsomes (55) and rabbit aortic microsomes (48). Inhibition of hydroperoxide isomerase and decreasing HEETA and THETA synthesis are the most likely explanations for clotrimazole's inhibition of ACh and AA relaxations.
In studies of mouse aortic AA metabolism, the synthesis of acid-labile HEETA metabolite(s) captured as MDHEs was increased by the sEH inhibitor AUDA. This suggests that HEETAs are metabolized by sEH. In the rabbit aorta, biologically isolated HEETA metabolites cause vascular relaxation (47), and a HEETA metabolite was identified as 15-H-11,12-EETA (8). Similarly, we identified 15-H-11,12-EETA in the mouse vasculature. However, the vasoactivity of 15-H-11,12-EETA and its contribution to EDHF activity in mouse arteries remain to be determined.
To verify the role of 11,12,15-THETA as a possible mediator of vascular relaxation, we determined the effect of 11(R),12(S),15(S)-THETA, 11(R),12(R),15(S)-THETA, and 11(S),12(S),15(S)-THETA on the vascular tone of mouse mesenteric arteries. 11(R),12(S),15(S)-THETA relaxed arteries, whereas 11(R),12(R),15(S)-THETA and 11(S),12(S),15(S)-THETA showed minimal effects. 11(R),12(S),15(S)-THETA-induced relaxations were independent of the endothelium and involved smooth muscle K+ channels. In rabbit arteries, 11(R),12(S),15(S)-THETA comigrated with biological 11,12,15-THETA, activated smooth muscle apamin-sensitive K+ channels, and was the only 11,12,15-THETA stereoisomer to cause relaxation (25). The stereochemical configuration of mouse vascular 11,12,15-THETA has not been defined. Further studies are required to determine the contribution of specific AA metabolites in mediating the EDHF response in mouse arteries.
In mouse arteries, we have also demonstrated that AA is metabolized to 6-keto-PGF1α, the metabolite of PGI2, and PGE2. We did not observe a metabolite that comigrated with the vasoconstrictor thromboxane. In mesenteric arteries, COX inhibition with Indo did not alter relaxations to ACh. However, the combination of Indo plus l-NA slightly inhibited AA relaxations. This suggests that COX metabolites may play a minor role in AA relaxations but do not contribute to ACh-induced relaxations of mouse mesenteric arteries.
The present study is the first report of LO metabolites contributing to EDHF activity in mouse arteries. H2O2 has also been shown to function as an EDHF in mouse mesenteric arteries (27, 39, 52). In some arteries, EDHF activity is mediated by two independent factors. In human coronary arteries, EETs and H2O2 both function as EDHFs (35), and in rabbit mesenteric arteries, 15-LO metabolites of AA and K+ function as EDHFs (57). In mouse mesenteric arteries in the present study, residual relaxations to ACh were observed in the presence of NOS, COX, and LO inhibitors. It is possible that an additional EDHF contributes to the ACh-induced relaxations. Further studies are required to determine if a similar phenomenon occurs in mouse mesenteric arteries with both H2O2 and 12/15-LO metabolites functioning as EDHFs.
In summary, the present study indicate that mouse arteries metabolize AA primarily to 12/15-LO metabolites. LO metabolites were identified as 15-H-11,12-EETA, 11,12,15-THETA, 12-HETE, and 15-HETE. Although LO metabolites contribute to l-NA- and Indo-resistant relaxations to ACh and AA, it is unclear which specific metabolite(s) mediates this activity.
GRANTS
This work was supported by a American Heart Association (AHA) (Greater Midwest Affiliate) grant-in-aid (to K. M. Gauthier), by National Institutes of Health Grants HL-37981 (to W. B. Campbell) and GM-31278 (J. R. Falck), and by the Robert A. Welch Foundation (to J. R. Falck). Y. Chawengsub and N. T. Aggarwal were supported by predoctoral fellowships from American Heart Association (Greater Midwest Affiliate). Support for the FTICR-MS was provided by the Kern Family Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Gretchen Barg for secretarial assistance and Marilyn Isbell for technical assistance with the LC/MS analyses.
REFERENCES
- 1. Agarwal R, Wang ZY, Bik DP, Mukhtar H. Nordihydroguaiaretic acid, an inhibitor of lipoxygenase, also inhibits cytochrome P-450-mediated monooxygenase activity in rat epidermal and hepatic microsomes. Drug Metab Dispos 19: 620–624, 1991 [PubMed] [Google Scholar]
- 2. Aggarwal NT, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension 51: 246–251, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Argentieri DC, Ritchie DM, Ferro MP, Kirchner T, Wachter MP, Anderson DW, Rosenthale ME, Capetola RJ. Tepoxalin: a dual cyclooxygenase/5-lipoxygenase inhibitor of arachidonic acid metabolism with potent anti-inflammatory activity and a favorable gastrointestinal profile. J Pharmacol Exp Ther 271: 1399–1408, 1994 [PubMed] [Google Scholar]
- 4. Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-Trihydroxyeicosatrienoic acid mediates acetylcholine-induced relaxations in the rabbit aorta. Am J Physiol Heart Circ Physiol 285: H2648–H2656, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chataigneau T, Feletou M, Huang PL, Fishman MC, Duhault J, Vanhoutte PM. Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br J Pharmacol 126: 219–226, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chawengsub Y, Aggarwal NT, Nithipatikom K, Gauthier KM, Anjaiah S, Hammock BD, Falck JR, Campbell WB. Identification of 15-hydroxy-11,12-epoxyeicosatrienoic acid as a vasoactive 15-lipoxygenase metabolite in rabbit aorta. Am J Physiol Heart Circ Physiol 294: H1348–H1356, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 297: H495–H507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen XS, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J Biol Chem 269: 13979–13987, 1994 [PubMed] [Google Scholar]
- 11. Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: beyond nitric oxide and cyclic GMP. Circulation 92: 3337–3349, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Cui L, Isbell M, Chawengsub Y, Falck JR, Campbell WB, Nithipatikom K. Structural characterization of monohydroxyeicosatetraenoic acids and dihydroxy- and trihydroxyeicosatrienoic acids by ESI-FTICR. J Am Soc Mass Spectrom 19: 569–585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Mey JG, Claeys M, Vanhoutte PM. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther 222: 166–173, 1982 [PubMed] [Google Scholar]
- 14. Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, Triggle C. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol 146: 1110–1118, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res 40: 480–490, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Ellis A, Cheng ZJ, Li Y, Jiang YF, Yang J, Pannirselvam M, Ding H, Hollenberg MD, Triggle CR. Effects of a Western diet versus high glucose on endothelium-dependent relaxation in murine micro- and macro-vasculature. Eur J Pharmacol 601: 111–117, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Endsley MP, Aggarwal N, Isbell MA, Wheelock CE, Hammock BD, Falck JR, Campbell WB, Nithipatikom K. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: location, hydrolysis and 12-lipoxygenase metabolism. Int J Cancer 121: 984–991, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falck JR, Barma DK, Mohapatra B, Bandyopadhyay A, Reddy KM, Qi J, Campbell WB. Asymmetric synthesis of the stereoisomers of 11,12,15(S)-trihydroxy-5(Z),8(Z),13(Z)-trienoic acid, a potent endothelium-derived vasodilator. Bioorganic Med Chem Lett 14: 4987–4990, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Falck JR, Manna S, Viala J, Siddhanta AK. Arachidonate epoxygenase: inhibitors and metabolite analogs. Tetrahedron Lett 26: 2287–2290 [Google Scholar]
- 21. Faraci FM, Sobey CG, Chrissobolis S, Lund DD, Heistad DD, Weintraub NL. Arachidonate dilates basilar artery by lipoxygenase-dependent mechanism and activation of K+ channels. Am J Physiol Regul Integr Comp Physiol 281: R246–R253, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor. Where are we now? Arterioscler Thromb Vasc Biol 23: 729–736, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Furchgott RF, Zawadzki JW. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 25. Gauthier KM, Chawengsub Y, Goldman DH, Conrow RE, Anjaiah S, Falck JR, Campbell WB. 11(R),12(S),15(S)-trihydroxyeicosa-5(Z),8(Z),13(E)-trienoic acid: an endothelium-derived 15-lipoxygenase metabolite that relaxes rabbit aorta. Am J Physiol Heart Circ Physiol 294: H1467–H1472, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension 43: 413–419, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves AC, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 29: 54–60, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Hidaka T, Takeo K, Hosoe K, Katasumi I, Yamashita T, Watanabe K. Inhibition of polymorphonuclear leukocyte 5-lipoxygenase and platelet cyclooxygenase by α-(3,5-di-tert-butyl-4-hydroxybenzylidene)-γ-butyrolactone (KME-4), a new anti-inflammatory drug. Jpn J Pharmacol 38: 267–272, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651–11656, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 1: 113–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaiser L, Hull SS, Jr, Sparks HV., Jr Methylene blue and ETYA block flow-dependent dilation in canine femoral artery. Am J Physiol Heart Circ Physiol 250: H974–H981, 1986 [DOI] [PubMed] [Google Scholar]
- 33. Kanamaru K, Waga S, Kojima T, Fujimoto K, Itoh H. Endothelium-dependent relaxation of canine basilar arteries. Part 1: difference between acetylcholine- and A23187-induced relaxation and involvement of lipoxygenase metabolite(s). Stroke 18: 932–937, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Kuhn H, Thiele BJ, Ostareck-Lederer A, Stender H, Suzuki H, Yoshimoto T, Yamamoto S. Bacterial expression, purification and partial characterization of recombinant rabbit reticulocyte 15-lipoxygenase. Biochim Biophys Acta 1168: 73–78, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome P450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res 102: 59–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKCa channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology 21: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 209: 909–913, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller AW, Katakam PV, Lee HC, Tulbert CD, Busija DW, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. J Pharmacol Exp Ther 304: 139–44, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 30: 293–331, 1978 [PubMed] [Google Scholar]
- 42. Murphy RC, Barkley RM, Berry KZ, Hankin J, Harrison K, Johnston C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem 346: 1–42, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Ong CE, Coulter S, Birkett DJ, Bhasker CR, Miners JO. The xenobiotic inhibitor profile of cytochrome P4502C8. Br J Clin Pharmacol 50: 573–580, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pannirselvam M, Ding H, Anderson TJ, Triggle CR. Pharmacological characteristics of endothelium-derived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur J Pharmacol 551: 98–107, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Pfister SL, Campbell WB. Arachidonic acid- and acetylcholine-induced relaxations of rabbit aorta. Hypertension 20: 682–689, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Pfister SL, Spitzbarth N, Edgemond W, Campbell WB. Vasorelaxation by an endothelium-derived metabolite of arachidonic acid. Am J Physiol Heart Circ Physiol 270: H1021–H1030, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem 273: 30879–30887, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Pfister SL, Spitzbarth N, Zeldin DC, Lafite P, Mansuy D, Campbell WB. Rabbit aorta converts 15-HPETE to trihydroxyeicosatrienoic acids; potential role of cytochrome P450 as endothelium-derived relaxing factors of rabbit aorta. Arch Biochem Biophys 420: 142–152, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Rittenhouse AR, Parker C, Brugnara C, Morgan KG, Alper SL. Inhibition of maxi-K currents in ferret portal vein smooth muscle cells by the antifungal clotrimazole. Am J Physiol Cell Physiol 273: C45–C56, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Shimokawa H, Matoba T. Does EDHF contribute to the control of peripheral resistance? Dialog Cardiovasc Med 6: 235–239, 2001 [Google Scholar]
- 51. Stapleton PA, Goodwill AG, James ME, Frisbee JC. Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia. Am J Physiol Regul Integr Comp Physiol 293: R1110–R1119, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Takaki A, Morikawa K, Tsutsui M, Murayama Y, Tekes E, Yamagishi H, Ohashi J, Yada T, Yanagihara N, Shimokawa H. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J Exp Med 205: 2053–2063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-I is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol 26: 78–84, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Toda N. Mechanism of histamine actions in human coronary arteries. Circ Res 61: 280–286, 1987 [DOI] [PubMed] [Google Scholar]
- 55. Weiss RH, Arnold JL, Estabrook RW. Transformation of an arachidonic acid hydroperoxide into epoxyhydroxy and trihydroxy fatty acids by liver microsomal cytochrome P-450. Arch Biochem Biophys 252: 334–338, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Yi XY, Gauthier KM, Cui L, Nithipatikom K, Falck JR, Campbell WB. Metabolism of adrenic acid to vasodilatory 1α,1β-dihomo-epoxyeicosatrienoic acids by bovine coronary arteries. Am J Physiol Heart Circ Physiol 292: H2265–H2274, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. Acetylcholine-induced relaxations of rabbit small mesenteric arteries: role of arachidonic acid metabolites and K+. Am J Physiol Heart Circ Physiol 293: H152–H159, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Zhou W, Wang XL, Kaduce TL, Spector AA, Lee HC. Impaired arachidonic acid-mediated dilation of small mesenteric arteries in Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288: H2210–H2218, 2005 [DOI] [PubMed] [Google Scholar]