Abstract
In the peripheral circulation, nitric oxide (NO) is released in response to shear stress across vascular endothelial cells. We sought to assess the degree to which NO contributes to exercise-induced vasodilation in the brachial artery (BA) and to determine the potential of this approach to noninvasively evaluate NO bioavailability. In eight young (25 ± 1 yr) healthy volunteers, we used ultrasound Doppler to examine BA vasodilation in response to handgrip exercise (4, 8, 12, 16, 20, and 24 kg) with and without endothelial NO synthase blockade [intra-arterial NG-monomethyl-l-arginine (l-NMMA), 0.48 mg·dl−1·min−1]. Higher exercise intensities evoked significant BA vasodilation (4–12%) that was positively correlated with the hyperemic stimulus (r = 0.98 ± 0.003, slope = 0.005 ± 0.001). During NO blockade, BA vasodilation at the highest exercise intensity was reduced by ∼70% despite similar exercise-induced increases in shear rate (control, +224 ± 30 s−1; l-NMMA, +259 ± 46 s−1). The relationship and slope of BA vasodilation with increasing shear rate was likewise reduced (r = 0.48 ± 0.1, slope = 0.0007 ± 0.0005). We conclude that endothelial NO synthase inhibition with l-NMMA abolishes the relationship between shear stress and BA vasodilation during handgrip exercise, providing clear evidence of NO-dependent vasodilation in this experimental model. These results support this paradigm as a novel and valid approach for a noninvasive assessment of NO-dependent vasodilation in humans.
Keywords: endothelium, endothelial nitric oxide synthase, NG-monomethyl-l-arginine
measurement of brachial artery (BA) flow-mediated vasodilation (FMD) following ischemic cuff occlusion, first described by Celermajer et al. (1), has been widely used in recent years as a noninvasive means of evaluating endothelial function in a research setting (10, 15, 17, 20, 36). The assessment of endothelial function via FMD has been proposed to represent a functional bioassay for endothelium-derived nitric oxide (NO) bioavailability in humans (7, 16), though there is new evidence challenging the view that FMD is a reliable and selective index of endothelial NO function (23). Earlier work demonstrated a positive correlation between endothelium-dependent vasodilation of the brachial and coronary arteries (32), a finding that has fueled the ongoing interest in a noninvasive evaluation of NO bioactivity in humans.
The uncertainty surrounding conventional FMD testing has raised the question of whether a more robust and comprehensive experimental paradigm might be adopted for the noninvasive determination of vascular health. One such approach is dynamic handgrip exercise, first described by Shoemaker et al. (29), which elevates shear stress through the BA and produces a subsequent vasodilation. We have recently identified the intensity-dependent nature of this response (25) and also demonstrated a decline in BA vasodilation in response to handgrip exercise in the elderly (6), a group known to suffer from impaired endothelial function (30). However, the fundamental question of whether BA vasodilation during handgrip exercise is NO mediated has not been addressed.
Therefore, in the present study we tested the hypotheses that 1) the BA would vasodilate in an intensity-dependent manner during incremental handgrip exercise, effectively producing a stepwise series of FMD values in response to sustained shear rate and that 2) this BA vasodilation would be due, at least in part, to endothelial-derived NO release. To test these hypotheses, dynamic handgrip exercise at six different intensities was performed in the absence and presence of high-dose endothelial NO synthase (eNOS) inhibition achieved by continuous intra-arterial NG-monomethyl-l-arginine (l-NMMA) infusion.
METHODS
Subjects.
Eight young (n = 6 men, n = 2 women, 25 ± 1 yr) healthy subjects were enrolled in the present study. All subjects were nonsmokers, normotensive (<140/90 mmHg), and were not participating in any regular exercise program. Subjects were not taking any prescription medication and were free of overt cardiovascular disease, as indicated by a health history and a physical exam. Women were studied within the first 5 days of their menstrual cycle, since BA reactivity has been documented to fluctuate with the menstrual phase (34). Protocol approval and written informed consent were obtained according to the University of Utah Institutional Review Board requirements, in accordance with the principles outlined in the Declaration of Helsinki. All data collection took place at the Veteran Affairs Salt Lake City Geriatric, Research, Education, and Clinical Center Vascular Research Laboratory. Subjects remained supine in a thermoneutral environment for the duration of the study.
Protocols.
Subjects reported to the laboratory at 8:00 am on the experimental day. Using sterile technique, an arterial catheter (Arrow, 18-gauge, 20 cm) was placed in the BA ∼10 cm distal to the axilla and advanced 6–8 cm in the retrograde direction. The catheter was placed in this region of the upper arm to ensure that l-NMMA entered the artery upstream (∼15 cm) to the ultrasound Doppler sample volume, allowing assessment of drug effects on BA diameter and blood velocity.
After a 30-min recovery from the catheter placement, baseline measurements were made and three maximal voluntary contractions (MVCs) were performed. Subjects then performed dynamic handgrip exercise (1 Hz) using a commercially available handgrip dynamometer (TSD121C, Biopac Systems, Goleta, CA), interfaced with an analog-to-digital conversion box. Cadence was provided via metronome, accompanied by real-time visual feedback of dynamometer force. Subjects were encouraged to perform rapid contractions with the goal of limiting contraction time to <25% of the duty cycle. Subjects exercised at 4, 8, 12, 16, 20, and 24 kg. Each exercise stage was performed for 3 min to achieve a hemodynamic steady state, and 1-min breaks were given between each work rate to limit fatigue. After 60 min of recovery to allow the restoration of baseline resting BA blood flow, a dose response for l-NMMA (0.6, 0.12, 0.24, and 0.48 mg/dl arm volume, 5 min per dose) was performed. Handgrip exercise commenced immediately thereafter as described above, but with continuous high-dose (0.48 mg/dl) l-NMMA infusion.
l-NMMA infusion.
Lower and upper arm volumes were determined anthropometrically and then used for the calculation of drug dosing. Total arm volume receiving infusate was calculated as follows: Total volume (dl) = forearm volume + (upper arm × 0.5). A portion of the upper arm was included in this calculation because of the proximal location of the arterial catheter.
l-NMMA (Bachem, Germany) was diluted from 250 mg lypholyzed powder in normal saline to a concentration of 2.5 mg/ml. The drug was infused intra-arterially at 0.6, 0.12, 0.24, and 0.48 mg/dl arm volume (3 min each dose) for the dose-response protocol, and the highest dose (0.48 mg/dl, 2.9–4.4 ml/min) was administered for 5 min before and continuously during the handgrip exercise protocol.
Measurements.
The primary outcome measures for the present study were BA diameter, BA blood velocity, heart rate (HR), and arterial blood pressure. Simultaneous measurements of BA blood velocity and vessel diameter were performed in both the infused and control arms using Logiq 7 and Logic e ultrasound Doppler systems (GE Medical Systems, Milwaukee, WI) operating in duplex mode. The Logic 7 and Logic e were equipped with linear array transducers operating at imaging frequencies of 14 and 12 MHz, respectively. Blood velocity was obtained using the same transducers with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel on the basis of real-time ultrasound visualization. Mean velocity values (Vmean, angle-corrected and intensity-weighted area under the curve) were automatically calculated using commercially available software (Logic 7 and Logic e). End-diastolic, ECG R-wave-gated images were collected via video output from the Logic 7 and Logic e for off-line analysis of BA vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA). With the use of arterial diameter and Vmean, BA blood flow [Vmeanπ(vessel diameter/2)2·60], and shear rate (8Vmean/BA diameter) were calculated.
HR was monitored from a standard three-lead ECG. Arterial blood pressure measurements were collected continually from within the BA, with the pressure transducer placed at the level of the catheter (Transpac IV, Abbot Laboratories). Mean arterial pressure (MAP) was calculated using the time integral of the directly measured arterial waveform. BA vascular conductance (VC) was then calculated as BA blood flow/MAP. Stroke volume (SV) and cardiac output (CO) were determined with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). SV was calculated using the Modelflow method, a validated model (31) that uses an algorithm to compute the aortic flow waveform from an arterial blood pressure pulsation by simulating a nonlinear, self-adaptive (3-element Windkessel) model of the aortic input impedance (Beatscope, version 1.1; Finapres Medical Systems) (5). CO was then calculated as the product of HR and SV.
Data analysis.
Ultrasound images and Doppler velocity spectra were recorded continuously at rest and during each exercise stage. During the last 60 s of each ultrasound Doppler segment, Vmean was averaged across four 15-s intervals, which were matched with intima-to-intima BA diameter measurements evaluated during diastole. Linear regression analysis was performed on an individual data, with r values and slope determined to evaluate BA vasodilation in response to shear rate before and after l-NMMA infusion. Statistics were performed with the use of commercially available software (SigmaPlot 11.0, Systat Software, Point Richmond, CA). A two-way repeated-measure analysis of variance was used to identify significant changes in measured variables within and between drug conditions and across exercise intensities, with the Bonferroni test used for post hoc analysis when a significant main effect was found. A paired t-test was used to compare the effect of drug on slope and y-intercept values from linear regression analysis. All group data are expressed as means ± SE. Significance was established at P < 0.05.
RESULTS
Subject characteristics are presented in Table 1.
Table 1.
Stature, grip strength, and blood characteristics
| Age, yr | 25 ± 1 |
| Height, cm | 179 ± 4 |
| Weight, kg | 78 ± 4 |
| Body mass index, kg/m2 | 24 ± 1 |
| Arm volume, dl | 18 ± 2 |
| Maximal voluntary contraction, kg | 50 ± 4 |
| Glucose, mg/dl | 81 ± 5 |
| Sodium, mmol/l | 140 ± 1 |
| Potassium, mmol/l | 3.9 ± 0.1 |
| Chloride, mmol/l | 103 ± 1 |
| Calcium, mg/dl | 8.9 ± 0.2 |
| Creatine, mg/dl | 0.9 ± 0.06 |
| Cholesterol, mg/dl | 156 ± 18 |
| Triglycerides, mg/dl | 51 ± 8 |
| HDL, mg/dl | 52 ± 3 |
| LDL, mg/dl | 85 ± 9 |
Values are means ± SE.
l-NMMA dose response.
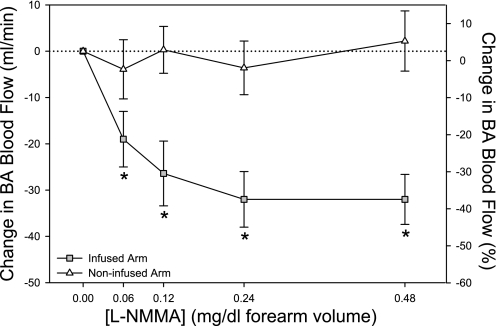
During the l-NMMA dose response, BA blood flow decreased significantly at all doses, with a plateau in vasoconstriction at the highest dose (0.48 mg/dl) (Fig. 1). This dose of l-NMMA also reduced BA shear rate (86 ± 13 to 59 ± 8 s−1) and BA mean blood velocity (9 ± 1 to 4 ± 1 cm/s), whereas BA diameter, HR, SV, CO, and MAP were unchanged. Additionally, in the contralateral arm, there were no changes in BA diameter, mean blood velocity, or blood flow (Fig. 1) during any dose of l-NMMA, confirming that the drug remained localized to the vasculature of the infused arm.
Fig. 1.
Changes in brachial artery (BA) blood flow during continuous infusion if NG-nitro-l-arginine methyl ester (l-NMMA) in the infused (gray squares) and noninfused (white triangles) limb. Significant vasoconstriction was observed at all doses of the infused limb, with a clear plateau at the highest doses, confirming a saturating effect of the drug on blood flow. Noninfused (control) forearm blood flow was unchanged during infusion of the contra lateral arm, indicating a lack of systemic hemodynamic effect for l-NMMA. Percent changes (right axis) are approximated for reference purposes only. *P < 0.05, significantly different than preinfusion.
l-NMMA at rest.
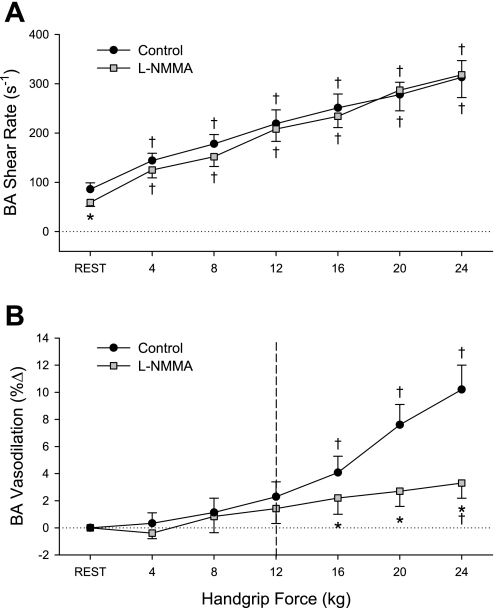
At rest, l-NMMA reduced resting BA mean blood velocity, blood flow, shear rate, and VC by ∼40%, whereas BA diameter remained unchanged during the drug infusion (Table 2 and Fig. 2). Similarly, HR, SV, CO, and MAP were not affected by l-NMMA (Table 2), supporting the regional effect of the drug without measureable systemic effects.
Table 2.
Cardiovascular variables at rest and during progressive handgrip exercise (1 Hz)
| Exercise Intensity |
|||||||
|---|---|---|---|---|---|---|---|
| Absolute, kg | Rest | 4 | 8 | 12 | 16 | 20 | 24 |
| Relative, %MVC | — | 8 ± 1 | 17 ± 1 | 25 ± 2 | 33 ± 2 | 42 ± 3 | 50 ± 3 |
| Control | |||||||
| HR, beats/min | 59 ± 2 | 61 ± 1 | 63 ± 2† | 62 ± 1† | 63 ± 1† | 65 ± 1† | 68 ± 1† |
| CO, l/min | 5.4 ± 0.3 | 5.6 ± 0.4 | 6.0 ± 0.4† | 6.1 ± 0.4† | 6.0 ± 0.4† | 6.1 ± 0.4† | 6.5 ± 0.5† |
| SV, ml/min | 93 ± 6 | 94 ± 6 | 94 ± 5 | 98 ± 6 | 96 ± 6 | 94 ± 6 | 98 ± 6 |
| BA diameter, mm | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2† | 4.7 ± 0.2† | 4.8 ± 0.2† |
| BA vasodilation, %Δ | - | 0.3 ± 0.8 | 1.1 ± 1.1 | 2.2 ± 1.1 | 4.0 ± 1.3† | 7.6 ± 1.7† | 10.2 ± 1.9† |
| BA velocity, cm/s | 10 ± 2 | 16 ± 2† | 20 ± 2† | 24 ± 3† | 28 ± 3† | 33 ± 3† | 37 ± 3† |
| MAP, mmHg | 92 ± 3 | 93 ± 3 | 93 ± 2 | 96 ± 4 | 98 ± 3† | 98 ± 4† | 101 ± 3† |
| BA VC | 1.1 ± 0.3 | 1.6 ± 0.3† | 2.0 ± 0.3† | 2.5 ± 0.4† | 2.9 ± 0.4† | 3.7 ± 0.6† | 4.1 ± 0.5† |
| l-NMMA | |||||||
| HR, beats/min | 59 ± 1 | 61 ± 2 | 61 ± 1 | 63 ± 1† | 63 ± 1† | 63 ± 1† | 66 ± 1† |
| CO, l/min | 5.7 ± 0.3 | 5.9 ± 0.4 | 6.2 ± 0.3 | 6.3 ± 0.3† | 6.2 ± 0.3† | 6.2 ± 0.4† | 6.7 ± 0.4† |
| SV, ml/min | 98 ± 8 | 98 ± 7 | 101 ± 6 | 102 ± 6 | 101 ± 6 | 98 ± 6 | 105 ± 6 |
| BA diameter, mm | 4.3 ± 0.2 | 4.3 ± 0.2 | 4.3 ± 0.2 | 4.3 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.2* | 4.4 ± 0.2* |
| BA vasodilation, %Δ | — | −0.4 ± 1 | 0.8 ± 1.2 | 1.4 ± 1.1 | 2.2 ± 1.2* | 2.7 ± 1.4* | 3.3 ± 1*† |
| BA velocity, cm/s | 6 ± 1* | 13 ± 2† | 16 ± 2† | 21 ± 2† | 25 ± 2† | 29 ± 3† | 33 ± 3† |
| MAP, mmHg | 93 ± 4 | 95 ± 3 | 94 ± 3 | 97 ± 3 | 98 ± 3† | 100 ± 3† | 105 ± 4† |
| BA VC | 0.6 ± 0.1* | 1.2 ± 0.2† | 1.5 ± 0.3† | 1.9 ± 0.3† | 2.3 ± 0.3*† | 2.6 ± 0.3*† | 2.9 ± 0.3*† |
Values are means ± SE.
MVC, maximal voluntary contraction; HR, heart rate; CO, cardiac output; SV, stroke volume; BA, brachial artery; MAP, mean arterial pressure; VC, vascular conductance; l-NMMA, NG-monomethyl-l-arginine.
P < 0.05, significantly different than control;
P < 0.05, significantly different than rest.
Fig. 2.
BA shear rate (A) and percent vasodilation (B) during progressive handgrip exercise in the control (black circles) and nitric oxide (NO)-blocked (gray squares) state. Dashed line represent the point after which significant vasodilation occurred. †P < 0.05, significantly different than rest; *P < 0.05, significant difference between control and l-NMMA trials.
l-NMMA during exercise.
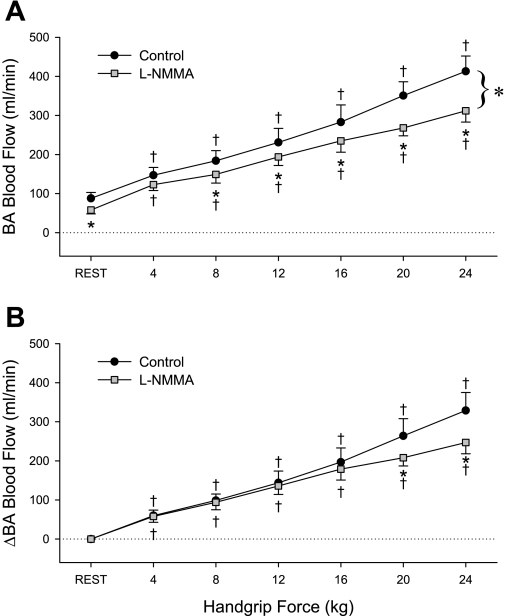
Before l-NMMA infusion, handgrip exercise resulted in an immediate and intensity-dependent increase in BA mean blood velocity (Table 2), VC (Table 2), shear rate (Fig. 2A), and blood flow (Fig. 3A). No measureable increase in BA diameter was evident at the lower (4, 8, and 12 kg) exercise intensities, but significant BA vasodilation was present at 16, 20, and 24 kg (Table 2 and Fig. 2B). Likewise, at the higher exercise intensities, significant exercise-dependent increases in HR, CO, MAP, and VC were also observed (Table 2). After l-NMMA, blood flow was reduced by 15–25% for any given exercise intensity, and the slope of BA blood flow across all exercise levels was significantly reduced (slope = 84 ± 14 to 66 ± 13, control vs. l-NMMA, P = 0.036) (Fig. 3A). When expressed as a change from baseline, blood flow was significantly reduced at the highest two exercise intensities (Fig. 3B). Despite similar exercise-induced increases in BA shear rate before and after l-NMMA (Fig. 2A), exercise-induced BA vasodilation was significantly blunted at the higher exercise intensities in the presence of l-NMMA; at the highest (24 kg) work rate, l-NMMA administration reduced exercised-induced BA vasodilation by ∼70% (Fig. 2B). As during the control exercise trial, significant exercise-dependent increases in HR, CO, MAP, and VC were observed during l-NMMA infusion, but these responses were not different from the control trial (Table 2). When BA diameter is viewed in relation to shear rate during the exercise intensities where vasodilation was observed (16, 20, and 24 kg) (Fig. 4), a strong relationship was observed for all individuals in the control exercise trial (r = 0.98 ± 0.003), and this relationship was significantly attenuated following l-NMMA (r = 0.48 ± 0.1). Likewise, the slope of BA vasodilation across shear rate was effectively ablated after l-NMMA (0.005 ± 0.001 to 0.0007 ± 0.0005, control vs. l-NMMA) (Fig. 4).
Fig. 3.
BA blood flow (A) and change in blood flow (B) during progressive handgrip exercise in the control (black circles) and NO-blocked (gray squares) state. †P < 0.05, significantly different than rest; *P < 0.05, significant difference between control and l-NMMA trials.
Fig. 4.
Relationship between changes in BA shear rate and the associated change in BA vasodilation during the final 3 stages (16, 20, and 24 kg) of handgrip exercise in the control (black) and NO-blocked (gray) state. Large symbols are mean ± SE values, small symbols indicate individual data. A significant reduction in slope was evident during NO blockade compared with control, which may represent NO-mediated vasodilation. *P < 0.05, significantly different than control. m, Slope of linear regression.
DISCUSSION
The present study has identified several new findings regarding NO-mediated vasodilation and the handgrip exercise model. First, we have further characterized the intensity-dependent increase in BA shear rate and vessel diameter across a wide range of forearm handgrip exercise levels, offering additional support for the use of this exercise modality in providing a wide-ranging shear stimulus for a comprehensive, noninvasive assessment of BA vasodilatory capacity. This incremental, sustained shear stress stimulus during exercise correlated well with the vasodilatory response, a crucial component in demonstrating the validity of this experimental approach for the assessment of vascular function. Second, during the inhibition of eNOS with l-NMMA, BA vasodilation was reduced by ∼70% and the relationship between shear rate and vasodilation was ablated at higher exercise intensities, implicating NO as a major contributor to shear-induced BA vasodilation during progressive handgrip exercise. In addition, l-NMMA reduced blood flow at rest and during all exercise intensities and blunted the overall hyperemic response (i.e., slope of blood flow across exercise intensities), indicating a significant role for NO in determining exercise hyperemia. Together, these findings confirm that graded handgrip exercise provides sustained, wide-ranging BA shear rates that allow a comprehensive characterization of BA reactivity and also demonstrate a clear NO-mediated component to this vasodilatory response. These observations thus support the assessment of BA vasodilation during handgrip exercise as a robust, noninvasive tool to assess NO bioavailability in humans.
Determination of BA vasodilation during handgrip exercise.
The present study sought to evaluate the potential of our established handgrip exercise paradigm (6, 25) to noninvasively assess NO-mediated vasodilation in humans. The first step toward achieving this goal was to carefully characterize the stimulus-response relationship between exercise hyperemia and the subsequent vasodilation, which is illustrated in Fig. 2 (control). By using six separate exercise intensities, we were able to provoke a linear, fivefold increase in limb blood flow (Fig. 3A) and associated shear rate (Fig. 2A), exposing the BA to a wide range of sustained shear force that resulted in a significant (∼10%) vasodilation at the higher exercise intensities (Fig. 2B). These data extend recent work from our group using a similar handgrip exercise paradigm but with considerably lower work rates (3, 6, and 9 kg) and cadence (0.5 Hz) (6, 25), offering additional support for the use of this exercise modality in providing a wide-ranging shear stimulus for a comprehensive, noninvasive assessment of BA vasodilatory capacity.
Impact of eNOS blockade on BA vasodilation.
Of equal importance to the evaluation of the stimulus-response relationship is identifying what portion of the shear-induced BA vasodilation is attributable to NO. To address this, we first determined the dose-response relationship for l-NMMA to evaluate the efficacy of the regional eNOS blockade. As can be seen in Fig. 1, a clear plateau was reached in BA blood flow in the infused arm, such that a doubling dose of the drug did not elicit further vasoconstriction. Importantly, the performance of this l-NMMA dose response did not produce a change in BA blood flow in the contralateral (noninfused) arm or alter arterial blood pressure, supporting an absence of measurable systemic effects during the local drug infusion. The highest dose of l-NMMA was then administered during handgrip exercise to provide regional eNOS blockade. This intra-arterial dose of l-NMMA (8.6 ± 0.9 mg/min) exceeds dosing reported elsewhere (1–5 mg/min) as efficacious in achieving eNOS blockade during handgrip exercise (5, 8, 26).
By design, l-NMMA was infused into the BA proximal to the portion of the vessel that was imaged, producing eNOS blockade in all downstream vascular beds of the arm. The site of drug administration was an important experimental consideration, ensuring that ultrasound measurements of BA diameter were taken from a region fully exposed to the drug. Indeed, several previous studies have infused NO synthase (NOS)-blocking drugs in the arm distal to the site where blood velocity and diameter are measured, and while these studies have been quite informative regarding the degree to which NO regulates exercise-induced hyperemia in the forearm (5, 9, 12, 14, 27, 28), they could not provide insight into exercise-induced BA vasodilation or the effect of NOS inhibition on this vasodilation.
With this approach, to our knowledge this is the first study to directly determine the contribution of NO to exercise-induced vasodilation of the BA in humans. The BA vasodilation present in the control trial (∼10%) was greatly reduced (∼3%) following l-NMMA, with only a minimal increase in BA diameter at the highest exercise intensity (Fig. 2B), thus providing evidence that NO is a major contributor (∼70%) to BA vasodilation in this exercise paradigm.
The role of NO in exercise hyperemia.
In addition to assessing the role of NO in conduit artery vasodilation during exercise, the present study also afforded the opportunity to examine the contribution of NO to overall exercise hyperemia. Previous studies using NOS inhibition during handgrip (9, 12–14, 27, 28) and knee-extensor (11, 24) exercise have collectively demonstrated a 10–20% reduction in skeletal muscle blood flow in the presence of NOS blockade. Findings from these studies have led to the prevailing view that the reduced hyperemic response in the presence of NOS blockade represents a downward shift with a similar overall change in blood flow from rest to maximal exercise, such that NO-mediated vasodilation is of little functional consequence to hyperemia in the exercising limb (33).
The present data differ from these former studies, identifying a 20–25% reduction in BA blood flow in the presence of l-NMMA, accompanied by a reduced slope for blood flow across exercise intensities in the presence of l-NMMA (Fig. 3A). We contend that these responses represent more than simply a downward shift with similar magnitude of change in blood flow; indeed, when the hyperemic response is viewed as changes in blood flow, which take into account baseline differences between trials, a significant difference remains at the higher exercise intensities (Fig. 3B). This disparity between previous studies and the present data are likely attributable to differences in the range of exercise intensities and the method by which blood flow is determined. Indeed, the majority of former studies examining eNOS blockade during handgrip exercise did not examine the intensity-dependent nature of the response, relying instead on a single handgrip intensity that was often only 10% MVC (27, 28). In contrast, six exercise intensities (ranging from 6 to 64% MVC, Table 2) were used in the present study, producing BA blood flows as high as 600 ml/min in some individuals at the highest exercise intensity. Other previous studies have examined the effects of l-NMMA on exercise hyperemia using strain-gauge plethysmography (9, 12, 13), which requires the cessation of exercise for the determination of blood flow. The present study thus builds on these previous studies through continuous ultrasound Doppler measurements and the inclusion of wide-ranging exercise intensities to more fully characterize the role of NO during exercise.
This pattern of reduced perfusion during l-NMMA infusion at any given absolute exercise intensity (Fig. 3, A and B) could be viewed as evidence for the importance of NO to the vasodilatory response for a given metabolic demand. The physiological consequence of reduced perfusion for a given O2 consumption during exercise may not be trivial; indeed, it may be likened to that seen in the elderly during exercise, when a 20–30% reduction in exercising leg blood flow must be met with an increase in arterial-to-venous O2 difference across the exercising muscle to ensure adequate O2 availability (18, 22). In this light, NO may indeed act as an important player in the cast of vasodilators that collectively ensure proper matching of perfusion and metabolism during exercise.
Implications.
Because of the potential prognostic and diagnostic value of in vivo determinations of endothelial health in preventative cardiology (19), the overall goal of the present study was to examine the potential of handgrip exercise for a noninvasive assessment of NO-dependent vasodilation. The need for complimentary approaches has become increasingly apparent as FMD testing has been refined over the past ten years (2, 15), a process that has brought to the forefront several noteworthy confounding influences, including variance in the hyperemic response, the singular nature of results from FMD testing, and uncertainty regarding whether the test truly reflects NO bioavailability (23). The present study addresses each of these concerns by provoking sustained, stepwise increases in shear stimulus to the BA that results in vasodilation.
The linear relationship between these variables, and the degree to which NO contributes to this relationship, is illustrated in Fig. 4. In the control condition, an excellent correlation between the shear stimulus and subsequent vasodilation is exhibited in all individuals, with r values ranging from 0.97 to 0.99. This relationship is significantly diminished after l-NMMA administration, and the slope of the vasodilatory response is likewise reduced by approximately two-thirds. In practical terms, the slope calculated from this array of stimulus-response values produced during a short bout of handgrip exercise provides a numeric index of NO-dependent vasodilation, such that a reduced slope may be associated with impaired endothelial function. This index (slope value) may be viewed as similar to the percent vasodilation value reported with conventional FMD testing, but with the added advantage of including several serial measurements in the same vessel across wide-ranging shear rates. Accordingly, we propose that handgrip exercise does indeed represent a valid approach to noninvasively probe endothelial health and NO bioactivity in humans. Certainly, further studies are required to evaluate the predictive capacity of this paradigm for cardiovascular disease and outcomes, as has been established for traditional FMD (37).
Experimental considerations.
Because of the lasting effects of l-NMMA, the protocol was ordered such that the control trial was always performed first. However, our group (35) and others (3) have previously reported a high reproducibility in the overall hyperemic response when multiple exercise bouts are performed sequentially during infusion-based studies. We also acknowledge that the efficacy of l-NMMA to inhibit eNOS was not evaluated in the present study. However, this concern is somewhat mitigated by the observed plateau in vasoconstriction at the highest doses of l-NMMA (Fig. 1) and support from previous work (4) reporting successful blockade at doses well below those administered in the present study. It is also possible that the level of eNOS blockade present at rest was altered as a consequence of the high-flow conditions during handgrip exercise. To minimize this risk, l-NMMA was maintained at the same high dose used for drug loading (0.48 mg/dl) throughout the exercise protocol, rather than the more conventional one-fifth dose for maintenance (5, 8). We cannot exclude the possibility that BA hemodynamics were affected by the placement of the intra-arterial catheter proximal to the ultrasound Doppler sample volume. However, this approach is not without precedent; indeed, previous studies have used a similar anatomical orientation (i.e., infusion of l-NMMA in the BA with measurements in the radial artery) to examine the role of NO on FMD (7, 23). Interobserver variability was not evaluated in the present study, but we have previously reported a coefficient of variation ∼20% when measuring BA vasodilation in the same subject on multiple visits to the laboratory (21). Finally, we wish to acknowledge that we did not determine blood flow during the recovery period following exercise, which has been reported to differ following l-NMMA administration (13).
Summary.
We have further characterized the handgrip exercise model as capable of producing the requisite incremental and sustained shear stress and associated vasodilation needed to validate this exercise modality as appropriate for the assessment of BA vascular function. Additionally, we have identified NO as a significant mechanism by which exercise-induced BA vasodilation is achieved. As such, these findings lend credence to the use of handgrip exercise for a noninvasive determination of NO-dependent vasodilatory capacity in humans.
GRANTS
This work was supported by National Institutes of Health Grants PO1-HL-091830 (to R. S. Richardson) and AG-028403 (to M. A. Supiano), Tobacco-Related Disease Research Program Grant 15RT-0100 (to R. S. Richardson), Veterans Affairs Rehabilitation Research and Development Service Grant E6910R (to R. S. Richardson), and American Heart Association Grant 0835209N (D. W. Wray).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298: H671–H678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001 [PubMed] [Google Scholar]
- 8. Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol 92: 2019–2025, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation 90: 2886–2890, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Eskurza I, Monahan KD, Robertson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frandsenn U, Bangsbo J, Sander M, Höffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation 90: 2853–2858, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med 7: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Kimura Y, Matsumoto M, Den YB, Iwai K, Munehira J, Hattori H, Hoshino T, Yamada K, Kawanishi K, Tsuchiya H. Impaired endothelial function in hypertensive elderly patients evaluated by high resolution ultrasonography. Can J Cardiol 15: 563–568, 1999 [PubMed] [Google Scholar]
- 18. Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest 127: 2254–2263, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol 98: 1251–1257, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovasc Res 35: 125–131, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci (Lond) 106: 371–376, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol 28: 460–466, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tschakovsky ME, Joyner MJ. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Metab 33: 151–161, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86: 5389–5395, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident carodiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]