Abstract
We previously reported that small mesenteric arteries from hypertensive rats have increased NOS-derived H2O2 and reduced NO/cGMP signaling. We hypothesized that antihypertensive therapy lowers blood pressure through a tetrahydrobiopterin (BH4)-dependent mechanism restoring NO/cGMP signaling and endothelial NOS (NOS3; eNOS) phosphorylation in small arteries. To test this hypothesis, small mesenteric arteries from normotensive rats (NORM), angiotensin II-infused rats (ANG), ANG rats with triple therapy (reserperine, hydrochlorothiazide, and hydralazine), or ANG rats with oral BH4 therapy were studied. Both triple therapy and oral BH4 therapy attenuated the rise in systolic blood pressure in ANG rats and restored NO/cGMP signaling in small arteries similarly. Triple therapy significantly increased vascular BH4 levels and BH4-to-BH2 ratio similar to ANG rats with BH4 supplementation. Furthermore, triple therapy (but not oral BH4 therapy) significantly increased GTP cyclohydrolase I (GTPCH I) activity in small arteries without a change in expression. NOS3 phosphorylation at Ser1177 was reduced in small arteries from ANG compared with NORM, while NOS3 phosphorylation at Ser633 and Thr495 were similar in ANG and NORM. NOS3 phosphorylation at Ser1177 was restored with triple therapy or oral BH4 in ANG rats. In conclusion, antihypertensive therapy regulates NO/cGMP signaling in small arteries through increasing BH4 levels and NOS3 phosphorylation at Ser1177.
Keywords: hypertension, nitric oxide synthase, small mesenteric arteries, triple therapy
nitric oxide (no)/cyclic guanosine monophosphate (cGMP) signaling regulates a variety of vascular functions. Reduced NO production and/or bioavailability contributes to vascular dysfunction in many cardiovascular diseases, including hypertension. Loss of nitric oxide synthase (NOS)-mediated vasorelaxation is associated with reduced NO bioavailability and reduced NO/cGMP signaling in conduit arteries studied from animal models of hypertension (23, 27). In contrast, we (20, 34) previously reported that NOS-mediated vasorelaxation was maintained in small arteries from hypertensive rats through activation of NOS-derived H2O2, yet NO/cGMP signaling was still reduced in the small arteries. This finding is despite the fact that we and others have previously shown that endothelial NOS (NOS3; eNOS) protein expression is unchanged or even increased in the vasculature in hypertensive animal models such as angiotensin II-infused hypertensive rats (ANG; Ref. 38), DOCA-salt induced hypertensive rats (38), spontaneously hypertensive rats (SHR; Ref. 29), and atherosclerotic apolipoprotein E (apo E)-deficient mice (24). These data suggest that a mechanism other than reduced NOS protein expression is responsible for decreased NO/cGMP signaling in small arteries. The goal of this study was to elucidate the molecular mechanism(s) driving reduced NO/cGMP signaling in small arteries under hypertensive conditions.
Tetrahydrobiopterin (BH4) is an essential cofactor regulating NOS activity. BH4 is synthesized through two distinct pathways: the de novo biosynthetic pathway and the salvage pathway. In endothelial cells, BH4 levels are regulated by the activity of guanosine triphosphate cyclohydrolase I (GTPCH I), a rate-limiting enzyme in the de novo biosynthesis of BH4 (5). Reduction of BH4 levels occurs via decreased GTPCH I activity and/or increased BH4 oxidation to 7,8-dihydrobiopterin (BH2). BH2 may also compete for binding to NOS at the BH4 site, thus promoting NOS uncoupling and loss of NO production (22, 41). Several studies have shown that decreased BH4 levels and correspondingly increased levels of BH2 are present in aorta from diabetic rats (37) and hypertensive mice (23). Laursen et al. (24) have demonstrated that increased peroxynitrite in aorta from atherosclerotic apo E-deficient mice oxidizes vascular BH4 to BH2, thereby uncoupling NOS activity and resulting in impaired endothelium-dependent relaxation. In contrast, endothelial-specific GTPCH I overexpression attenuates the rise in blood pressure and preserves BH4 levels, NOS3 phosphorylation, and vasorelaxation in a model of salt-sensitive hypertension (9). It is well understood that small arteries have multiple endothelium-dependent vasorelaxation pathways; thus NOS activation and endothelial dysfunction under hypertensive conditions are most likely differentially regulated compared with conduit vessels (12, 18, 20, 26). Very few studies have focused on BH4-dependent changes in NO/cGMP signaling in small arteries.
Phosphorylation at multiple sites of NOS3 is another critical determinant of NOS3 activity. Phosphorylation of NOS3 at Ser1177 and Ser633 and dephosphorylation at Thr495 stimulate NOS3 activity and consequently augment NO/cGMP signaling in bovine aortic endothelial cells (28). Altered phosphorylation of NOS3, mainly Ser1177, has been observed in conduit arteries from hypertensive models such as two-kidney, one-clip hypertensive rats (16) and DOCA-salt hypertensive rats (34). However, there is a paucity of information regarding changes in phosphorylation of NOS3 in small arteries under hypertensive conditions.
We are interested in deciphering the dynamic relationship between changes in blood pressure and NOS3 regulation in small arteries. Specifically, there is a lack of knowledge whether blood pressure, per se, influences BH4/BH2 levels and phosphorylation of NOS3 in small arteries. We hypothesized that antihypertensive therapy lowers blood pressure through a BH4-dependent mechanism restoring NO/cGMP signaling and NOS3 phosphorylation in small arteries. We treated ANG rats with BH4 supplementation or triple therapy (reserperine, hydrochlorothiazide, and hydralazine) to lower blood pressure via specific or nonspecific mechanisms, respectively. Total biopterin, BH4, BH2, and GTPCH I activity and expression were evaluated in isolated small mesenteric arteries as well as measures of vascular NOS signaling, which included the following: cGMP accumulation, monomer-to-dimer ratio of NOS3 protein, and phosphorylation of NOS3.
METHODS
Animal models.
Male Sprague-Dawley rats (200–250 g; Charles River Laboratories, Wilmington, MA) were divided into four groups: normotensive rats (NORM), ANG, ANG with triple therapy (ANG + TT; as follows in mg·kg−1·day−1: 30 hydralazine, 10 hydrochlorothiazide, and 0.5 reserpine), and ANG with a supplementation of BH4 (100 mg·kg−1·day−1; Schircks Laboratories, Switzerland: ANG + BH4). The dose and route of administration were previously shown to be efficacious (19, 21, 23, 40). To establish ANG II-infused hypertension, an osmotic mini-pump containing angiotension II was implanted subcutaneously into rats under isoflurane anesthesia (IsoFlo; Abbott Laboratories, North Chicago, IL). ANG II was infused chronically at the rate of 70 ng/min for 2 wk. BH4 treatment was initiated 3 days before starting ANG II infusion and was continued throughout the 2-wk ANG II treatment period. BH4 was administered daily by gavage (100 mg·kg−1·day−1) for 3 days before and throughout the 2-wk period of ANG II infusion. TT was provided in the drinking water for 3 days before and throughout the 2-wk period of ANG II infusion. The TT solution was changed every three days and modified according to the amount of water intake for each rat. Systolic blood pressure was measured after 7 and 14 days of ANG II infusion by tail-cuff plethsomyography as previously described (33). Systolic blood pressure of each rat was determined by the average of four to six independent readings. After 2 wk, rats were anesthetized with pentobarbital sodium (Nembutal; 50 mg/kg ip; Abbott) and small mesenteric arteries were isolated as previously described (38) and outlined below. All experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and approved and monitored by the Medical College of Georgia Institutional Animal Care and Use Committee.
Measurement of tissue BH4 and BH2.
The mesenteric vascular bed was cut away from the intestinal wall with the fat and veins carefully pulled away from the arteries in cold physiological saline solution [PSS (pH = 7.4); as follows in mmol/l: 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 14.9 NaHCO3, 5.5 dextrose, 0.26 EDTA, and 1.6 CaCl2]. Isolated small mesenteric arteries were homogenized in extraction buffer [as follows in mmol/l: 50 Tris (pH 7.4), 1 dithiothreitol, and 1 EDTA] at 4°C and centrifuged at 10,000 g (8 min at 4°C). Biopterin levels were determined after differential oxidation in acid (which converts both BH4 and 7,8-BH2 to biopterin) and base (which converts only 7,8-BH2 to biopterin) conditions by reverse-phase HPLC (Beckman Coulter, Fullerton, CA) with a fluorescence detector (Jasco) as described previously (7). Data were collected and analyzed by 32 Karat chromatography software (Beckman Coulter) and normalized against tissue protein levels. BH4 content was calculated from the difference in biopterin levels after acid and base oxidation.
Measurement of intracellular GTPCH I activity.
Tissue supernatant homogenates, prepared as in the biopterin assay, were filtered using a Sephadex G25M column (Amersham, Piscataway, NJ) to remove endogenous neopterin, BH4, and phenylalanine. GTPCH I enzymatic activity was assayed using reverse-phase HPLC method (Beckman Coulter) by measurements of neopterin, which was derived from dihydroneopterin triphosphate after oxidation and phosphate treatment (7).
Measurement of intracellular cGMP content.
Small mesenteric arteries were isolated, cleaned, divided into two sections, and incubated at 37°C in oxygenated PSS containing IBMX (300 μmol/l) for 15 min. Additional arterial segments were incubated with a nonselective NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 μmol/l) for 15 min. Arterial segments were snap-frozen in liquid nitrogen immediately after incubation and homogenized in ice cold 10% TCA (LabChem, Pittsburgh, PA). TCA homogenates were extracted with ether, and cGMP levels were quantitated by radioimmunoassay and normalized to milligrams of protein content as described previously (34). Protein concentrations were determined by standard Bradford assay (Bio-Rad Laboratories, Hercules, CA) using BSA as the standard.
Immunoblotting.
Arterial segments were snap-frozen in liquid nitrogen and homogenized in cold homogenization buffer (20.0 mmol/l Tris·HCl with pH 7.4, 137.0 mmol/l NaCl, 10% glycerol, and 1% NP40) containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and phosphatase inhibitor cocktail (Pierce, Pockford, IL). Protein concentrations were determined by standard Bradford assay, and Western blotting and low-temperature SDS-PAGE were performed as described previously (6, 35, 42). Briefly, after proteins were transferred to PVDF or nitrocellulose membranes, they were blocked in blocking buffer (Nacalai USA, San Diego, CA). Membranes were probed using primary antibody against GTPCH I (Ascenion, Munich, Germany), and, as a loading control, blots were rehybridized with monoclonal anti-β-actin (Sigma). For others, two-color immunoblots were performed using primary antibodies to NOS3 (BD Transduction laboratory, San Jose, CA), phosphorylated NOS3-Ser1177 (Cell Signaling, Danvers, MA), phosphorylated NOS3-Ser633 (Upstate, Lake Placid, NY), phosphorylated NOS3-Thr495 (Upstate, Lake Placid, NY), Akt (Cell Signaling), phosphorylated Akt-Ser473 (Cell Signaling), and β-actin (Sigma, St. Louis, MO). Specific bands were detected using the Odyssey Infrared Imager. IRDye800 (Rockland, Gilbertsville, PA) and AlexaFluor 680 (Molecular Probes, Eugene, OR) were used as secondary antibodies for the detection of two different primary antibodies. Densitometry was analyzed for NOS3, phosphorylated NOS3, Akt kinase, phosphorylated Akt kinase, and β-actin. Relative densitometric units for NOS3 and Akt were calculated by normalization to β-actin levels, while phosphorylated proteins were normalized to the corresponding nonphosphorylated, total protein. Low-temperature SDS-PAGE NOS3 dimer and monomer bands were detected using the Odyssey Infrared Imager.
Materials.
ANG II was purchased from Phoenix Pharmaceuticals (Belmont, CA). Mini-osmotic pumps were purchased from Alzet (Cupertino, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Data analysis.
Values are expressed as means ± SE and analyzed by Student's t-test for paired comparison or ANOVA followed by a Bonferroni's correction for multiple comparisons (Prism, La Jolla, CA). A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Systolic blood pressure.
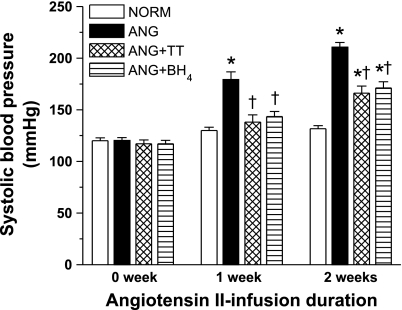
Systolic blood pressure was significantly greater in ANG compared with NORM following 1 wk of infusion, and this difference in pressure was maintained through the following weeks (n = 19–21; Fig. 1). Treatment with either TT or BH4 in the ANG group significantly blocked the increase in blood pressure at week 1 and attenuated the increase in pressure at week 2.
Fig. 1.
Systolic blood pressure of normotensive rats (NORM; n = 19), ANG rats (n = 19), ANG rats with triple therapy (ANG + TT; n = 21), and ANG rats with tetrahydrobiopterin (ANG + BH4; n = 21) before and after 1 and 2 wk of ANG II infusion. *Significant difference vs. NORM at each period (P ≤ 0.05). †Significant difference vs. ANG at each period (P ≤ 0.05).
BH4, BH2 content, and BH4-to-BH2 ratio in small mesenteric arteries.
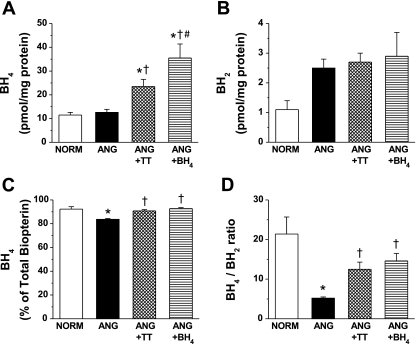
As shown in Fig. 2 (n = 10), BH4 levels in small arteries from the ANG group was not different from NORM. However, concomitant treatment with either TT or BH4 significantly increased BH4 levels (Fig. 2A). Treatment with BH4 further increased BH4 levels (Fig. 2A). BH2 levels showed a tendency to increase in the ANG group (P = 0.07 compared with NORM group; Fig. 2B). This in turn significantly decreased the percentage BH4 of total biopterin (Fig. 2C) and BH4-to-BH2 ratio (Fig. 2D) in small arteries from ANG compared with NORM. TT or BH4 supplementation did not affect BH2 levels in ANG (Fig. 2B). However, the percentage BH4 of total biopterin and the BH4-to-BH2 ratio was restored with either TT or BH4 treatment (Fig. 2, C and D, respectively; P < 0.05, compared with the untreated ANG group).
Fig. 2.
BH4 level (A), BH2 level (B), BH4 percentage of total biopterin (C), and BH4-to-BH2 ratio (D) in small mesenteric arteries (n = 10) from NORM, ANG, ANG + TT, and ANG + BH4. *Significant difference vs. NORM (P ≤ 0.05). †Significant difference vs. ANG (P ≤ 0.05). #Significant difference vs. ANG + TT (P ≤ 0.05).
GTPCH I enzymatic activity and protein expression in small mesenteric arteries.
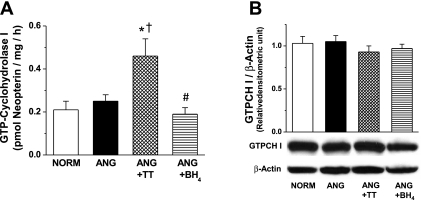
To evaluate the mechanism of increased BH4 level in ANG with TT, GTPCH I enzymatic activity and protein expression were determined. GTPCH I activity was not changed in ANG group compared with NORM group (n = 6–7; Fig. 3A). GTPCH I activity was significantly increased with TT but not with BH4 supplementation (n = 6–7; Fig. 3A). GTPCH I protein expression was similar between groups (n = 3; Fig. 3B).
Fig. 3.
A: GTP cyclohydrolase I (GTPCH I) enzymatic activity in small mesenteric arteries (n = 6–7) from NORM, ANG, ANG + TT, and ANG + BH4. *Significant difference vs. NORM (P ≤ 0.05). †Significant difference vs. ANG (P ≤ 0.05). #Significant difference vs. ANG + TT (P ≤ 0.05). B: representative Western blot and relative densitometry analysis of GTPCH I protein expression normalized to β-actin in small mesenteric arteries (n = 3) from NORM, ANG, ANG + TT, and ANG + BH4.
cGMP in small mesenteric arteries.
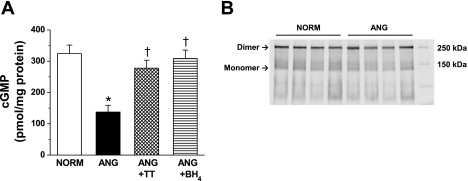
NO/cGMP signaling in small mesenteric arteries was assessed by measuring the cGMP content in the presence and absence of l-NAME (n = 6; Fig. 4). cGMP accumulation in small arteries from ANG was significantly less compared with the NORM group (Fig. 4A). cGMP levels were restored by both antihypertensive treatments (Fig. 4A). Preincubation with l-NAME abolished cGMP accumulation in NORM and ANG (data not shown), confirming that cGMP levels were dependent on NOS-mediated signaling and consistent with our previous publication (20).
Fig. 4.
A: cGMP accumulation in small mesenteric arteries (n = 6) from NORM, ANG, ANG + TT, and ANG + BH4. *Significant difference vs. NORM (P ≤ 0.05). †Significant difference vs. ANG (P ≤ 0.05). B: representative Western blot analysis of low-temperature SDS-PAGE evaluation of endothelial NOS (NOS3) dimer and monomer in small mesenteric arteries (n = 4) from NORM and ANG.
NOS3 monomer-to-dimer ratio.
The native state of NOS3 is as a dimer (42). Increased NOS3 monomers, reported as an increase in the monomer-to-dimer ratio, has been used as a marker of NOS3 uncoupling in conduit arteries (44). Low-temperature SDS-PAGE analysis revealed that NOS3 is expressed predominately as the native dimer form in small arteries from both NORM and ANG (Fig. 4B).
Total and phosphorylated NOS3 and Akt kinase.
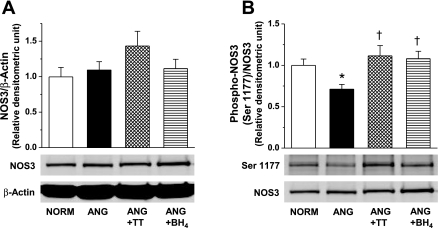
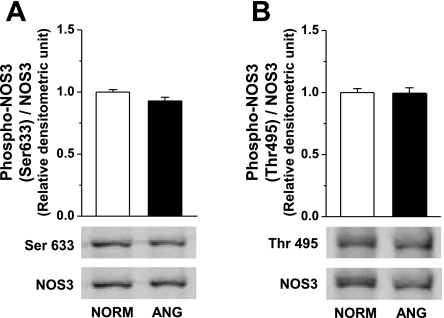
Since NOS3-mediated NO/cGMP signaling is regulated dynamically through phosphorylation and dephosphorylation (28) and phosphorylation status may modulate NOS3 activity and uncoupling (25), we analyzed phosphorylation of NOS3 at Ser1177, Ser633, and Thr495 in small mesenteric arteries (Fig. 5; n = 5–13). Total NOS3 protein expression was comparable between groups (n = 5–13; Fig. 5A). Phosphorylation of NOS3 at Ser1177 in small arteries from ANG rats was significantly less than NORM rats. Furthermore, NOS3 phosphorylation at Ser1177 site was restored when blood pressure was lowered with either treatment (Fig. 5B). Phosphorylation of NOS3 at Ser633 and Thr495 was similar in ANG and NORM rats (n = 10–11; Fig. 6A and B).
Fig. 5.
Representative Western blot and relative densitometry analysis of total NOS3 normalized to β-actin (A) and NOS3 phosphorylation at Ser1177 normalized to total NOS3 (B) in small mesenteric arteries from NORM (n = 12), ANG (n = 13), ANG + TT (n = 5), and ANG + BH4 (n = 5). *Significant difference vs. NORM (P ≤ 0.05). †Significant difference vs. ANG (P ≤ 0.05).
Fig. 6.
Representative Western blot and relative densitometry analysis of NOS phosphorylation at Ser633 (A; n = 11) or Thr495 (B; n = 10–11) in small mesenteric arteries from NORM and ANG. Relative densitometric units were normalized to total NOS3.
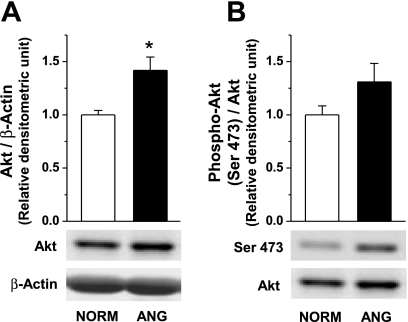
Phosphorylation at Ser1177 of NOS3 is regulated by Akt kinase activation (13, 28, 36), while ANG II increases Akt phosphorylation at Ser473 in renal thick ascending limb (15). Thus we analyzed total and phosphorylated Akt at Ser473 in small mesenteric arteries from NORM and ANG rats (n = 7; Fig. 7). We found that total Akt is greater in small arteries from ANG rats compared with NORM (Fig. 7A). However, the ratio of phosphorylated Akt to total Akt was not significantly different (Fig. 7B).
Fig. 7.
Representative Western blot and relative densitometry analysis of total Akt normalized to β-actin (A) and Akt phosphorylation at Ser473 normalized to total Akt (B) in small mesenteric arteries (n = 7) from NORM and ANG. *Significant difference vs. NORM (P ≤ 0.05).
DISCUSSION
The present study focused on the blood pressure-dependent regulation of BH4-to-BH2 levels, GTPCH I activity, NO/cGMP signaling, and NOS3 phosphorylation in small mesenteric arteries. We found that the antihypertensive treatments, triple therapy, or oral BH4 increased vascular BH4 levels and the BH4-to-BH2 ratio, restored NO/cGMP signaling, and restored NOS3 phosphorylation at Ser1177. Furthermore, triple therapy (but not oral BH4 therapy) significantly increased GTPCH I activity in small arteries without a change in GTPCH I expression. In addition, we found that NOS3 monomerization, NOS3 phosphorylation at Ser633 and Thr495, and Akt phosphorylation at Ser473 in small mesenteric arteries were not altered despite changes in blood pressure. These data indicate that the increase in BH4 levels and consequently the increase in NO/cGMP signaling lowered blood pressure.
Vascular BH4-to-BH2 ratio and blood pressure regulation.
BH4 is an essential cofactor for NO biosynthesis, and BH4 deficiency has been shown to be a primary mechanism for NOS uncoupling (1, 2, 22). In addition to the reduction of absolute BH4 levels, a decrease in the ratio between the fully reduced BH4 and oxidized BH2 may also contribute to NOS3 uncoupling (5, 41). A reduced vascular BH4-to-BH2 ratio was observed in studies from apo E-deficient mice (8) and diabetic (db/db) mice (32), whereas absolute BH4 levels have been shown to be either increased in apo E-deficient mice (7), unchanged in diabetic (db/db) mice (32) and ANG II-infused hypertensive rats (21), or decreased in insulin-resistant rats (37), spontaneously hypertensive rats (17), apo E-deficient mice (24), and DOCA-salt mice (9). BH2 is not a NOS cofactor but may effectively reduce NO bioavailability by competitively binding to NOS3 at the BH4 binding site resulting in loss of NO production and enhancement of NOS uncoupling (5, 41). Vasquez-Vivar et al. (41) demonstrated that both coupled and uncoupled NOS3 activity may exist when both BH4 and BH2 are present utilizing an in vitro system. The current study furthers this observation by demonstrating that triple therapy and oral BH4 increased the BH4-to-BH2 ratio in small arteries. Previously, we reported that NOS generates both NO and H2O2 in small arteries from ANG II-infused hypertensive rats (20). The data in the current study support the hypothesis that the ratio of BH4 to BH2 is a critical determinant for NOS uncoupling in small arteries in ANG II-induced hypertension.
Oral BH4 therapy dramatically increased tissue levels of BH4, yet BH2 levels did not follow resulting in an increased BH4-to-BH2 ratio in small arteries. BH4 is known to have antioxidant effects independent of its role as a cofactor for NOS (22). However, our data suggest that the antioxidant activity of oral BH4 therapy does not influence BH4 oxidation to BH2 in small arteries. Yet, substantial reduction of tissue O2− production was previously demonstrated in aortic tissue from DOCA-salt hypertensive mice with oral BH4 therapy (23). Interestingly, triple therapy significantly increased BH4 levels and in turn the BH4-to-BH2 ratio in small arteries similar to oral BH4 therapy. Blood pressure, per se, does not appear to play a role in the regulation of GTPCH I activity in small arteries, since this is similar in normotensive and hypertensive rats. Triple therapy increased GTPCH I enzymatic activity without a concomitant change in protein expression, suggesting that the treatment paradigm activates GTPCH I. At this point, there is a lack of knowledge regarding whether reserpine, hydrochlorothiazide, and/or hydralazine directly influence GTPCH I activity. Further experimentation is necessary to determine the mechanism of triple therapy mediated activation of GTPCH I.
NO/cGMP signaling in small arteries and blood pressure regulation.
Our laboratory and others (20, 34) have demonstrated reduced NO/cGMP signaling in arteries from various hypertensive animal models. The present study confirmed our previous observation that small mesenteric arteries from hypertensive rats have reduced cGMP accumulation. Triple therapy and oral BH4 both reduced blood pressure to similar levels in the ANG II-dependent model of hypertension after 1 and 2 wk of treatment. Expectedly, BH4 supplementation increased vascular NO/cGMP signaling. Interestingly, lowering blood pressure via triple therapy also increased vascular NO/cGMP signaling. Several reports (40) have shown that triple therapy does not influence the renin-ANG-aldosterone system, most likely excluding this possibility in the mechanism. Blood pressure dependent changes in NO/cGMP signaling have previously been reported in the two-kidney, one-clip model of hypertension (43) in agreement with our study. Furthermore, Du et al. (9) showed that NOS3 uncoupling was attenuated in DOCA-salt hypertensive mice with an endothelial-specific overexpression of GTPCH I increasing BH4 synthesis and the vascular BH4-to-BH2 ratio along with a reduction in blood pressure. These data support the hypothesis that vascular NO/cGMP signaling via changes in the BH4-to-BH2 ratio regulates blood pressure.
In vitro studies have demonstrated that the native dimer state of NOS3 generates NO, while monomer expression has been associated with NOS3 uncoupling (11) and increases in the monomer-to-dimer ratio has been used in many studies with conduit arteries as a marker of NOS3 uncoupling (44). We found that native NOS3 was expressed as dimers in small arteries from both normotensive and ANG II-induced hypertensive rats. Therefore, since we have previously published that NOS generates H2O2 in small mesenteric arteries from hypertensive rats, the monomer-to-dimer ratio of NOS3 does not appear to be related to enzymatic uncoupling of NOS3, at least, in small arteries. Similarly, Du et al. (9) found increased NOS-mediated O2− production with no increase in monomerization of NOS3 in small mesenteric arteries from DOCA-salt hypertensive mice. These data indicate that NOS3 monomer in small arteries is unrelated to blood pressure or NOS3 uncoupling.
NOS3 phosphorylation in small arteries and blood pressure regulation.
In the present study, we demonstrated that NOS3 phosphorylation at Ser1177 was reduced in small arteries from ANG. These data indicate that reduced NO/cGMP signaling in small arteries from hypertensive rats may be due, in part, to dysfunctional phosphorylation at Ser1177. Numerous studies (16, 34) have demonstrated reduced NOS3 phosphorylation at Ser1177 in the vasculature from hypertensive animal models. However, the dynamic change of NOS3 phosphorylation in small arteries by reduction of blood pressure has rarely been studied. We demonstrate that NOS3 phosphorylation at Ser1177 is restored with triple therapy and oral BH4, suggesting that the Ser1177 phosphorylation site is blood pressure dependent. The Ser1177 phosphorylation site is linked to shear stress-mediated activation of NOS3 in cultured endothelial cells (4); thus we hypothesize that lowering blood pressure leads to a functional recoupling of the Ser1177 phosphorylation pathway in vivo. In studies with endothelial cells, the serine/threonine kinase Akt has been identified as a major kinase contributing to the phosphorylation at the Ser1177 site (13). Akt activity is also regulated by posttranslational phosphorylation at Ser473 in endothelial cells (14). In our results, Akt phosphorylated at Ser473 in small mesenteric arteries from hypertensive rats shows a tendency to increase compared with the normotensive control (P = 0.07), which may be due to the ANG II and not blood pressure (15). Reports of the regulation of Akt phosphorylation in the vasculature under hypertensive conditions show inconsistent results: decreased in DOCA (31) and obese Zucker rats (30), unchanged in NG-nitro-l-arginine-treated rat (31) and DOCA (34), or increased in two-kidney, one-clip (16) and ANG II infusion (3). This discrepancy may be due to the level of hypertension as well as the type of vascular tissue examined. It should also be noted that Akt measurements in endothelium-intact vascular tissue may not be reflective of endothelial-dependent changes and may be a result of changes in smooth muscle-derived Akt. Our results suggest that Akt activation under ANG II infusion does not associate with the dysfunctional NOS3 phosphorylation at Ser1177 in small arteries. NOS3-bound Akt may regulate NOS3 activity more precisely than total Akt (10, 39). Thus further investigation is needed to determine the phosphorylation status of Akt and NOS3 from vascular tissues in hypertensive animal models.
We previously reported that NOS activity generates both NO and H2O2 in small arteries from hypertensive rats, indicating that both coupled and uncoupled NOS are present. NOS3 phosphorylation at Ser633 and Thr495 was similar in small arteries from hypertensive and normotensive rats. Therefore, these data led us to hypothesize that phosphorylation at Ser633 and Thr495 is responsible for maintenance of basal NO production and NO/cGMP signaling in small arteries. Further investigation is necessary to fully elucidate this possible mechanism.
In conclusion, the present study demonstrates that de novo biosynthesis of BH4 via GTPCH I or supplementation of BH4 leading to increased NO/cGMP signaling and NOS3 phosphorylation at Ser1177 results in lowering blood pressure. Further evaluation of the molecular mechanisms underlying antihypertensive therapy and activation of GTPCH I may lead to novel therapies to activate and restore NO/cGMP signaling.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-60653 and HL-69999 (to J.S. Pollock) and HL-53524 (to Z. Katusic) and the American Heart Association (to J. S. Pollock: Established Investigator 0440073N; L. d'Uscio: Scientist Development Award 0730133N).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance of Heather Walker Smith. We would also thank Dr. David Pollock for expert editorial suggestions.
Present address for K. Kang: Vascular Biology Program, Karp Family Research Building (#12.004A), Children's Hospital Boston, 300 Longwood Avenue, Boston, MA 02115.
REFERENCES
- 1. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, Yokoyama M, Kawashima S, Channon KM. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res 97: 864–871, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Benkirane K, Viel EC, Amiri F, Schiffrin EL. Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension 47: 102–108, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14: 323–327, 2004 [DOI] [PubMed] [Google Scholar]
- 6. d'Uscio LV, Katusic ZS. Erythropoietin increases endothelial biosynthesis of tetrahydrobiopterin by activation of protein kinase B alpha/Akt1. Hypertension 52: 93–99, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. d'Uscio LV, Katusic ZS. Increased vascular biosynthesis of tetrahydrobiopterin in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol 290: H2466–H2471, 2006 [DOI] [PubMed] [Google Scholar]
- 8. d'Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res 92: 88–95, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117: 1045–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 90: 866–873, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Freitas MR, Schott C, Corriu C, Sassard J, Stoclet JC, Andriantsitohaina R. Heterogeneity of endothelium-dependent vasorelaxation in conductance and resistance arteries from Lyon normotensive and hypertensive rats. J Hypertens 21: 1505–1512, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677–682, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Herrera M, Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT2 receptors and Akt1-dependent nitric oxide synthase 3 (NOS3) activation. J Biol Chem 14932–14940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiyoshi H, Yayama K, Takano M, Okamoto H. Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension 45: 967–973, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Hong HJ, Hsiao G, Cheng TH, Yen MH. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 38: 1044–1048, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Houghton JL, Davison CA, Kuhner PA, Torossov MT, Strogatz DS, Carr AA. Heterogeneous vasomotor responses of coronary conduit and resistance vessels in hypertension. J Am Coll Cardiol 31: 374–382, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Inscho EW, Cook AK, Murzynowski JB, Imig JD. Elevated arterial pressure impairs autoregulation independently of AT(1) receptor activation. J Hypertens 22: 811–818, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase-dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Kase H, Hashikabe Y, Uchida K, Nakanishi N, Hattori Y. Supplementation with tetrahydrobiopterin prevents the cardiovascular effects of angiotensin II-induced oxidative and nitrosative stress. J Hypertens 23: 1375–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol 281: H981–H986, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of l-arginine metabolism to efficient nitric oxide production. J Biol Chem 278: 44719–44726, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Luscher TF. Heterogeneity of endothelial dysfunction in hypertension. Eur Heart J 13, Suppl D: 50–55, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Nava E, Llinas MT, Gonzalez JD, Salazar FJ. Nitric oxide synthase activity in renal cortex and medulla of normotensive and spontaneously hypertensive rats. Am J Hypertens 9: 1236–1239, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Nishimatsu H, Suzuki E, Satonaka H, Takeda R, Omata M, Fujita T, Nagai R, Kitamura T, Hirata Y. Endothelial dysfunction and hypercontractility of vascular myocytes are ameliorated by fluvastatin in obese Zucker rats. Am J Physiol Heart Circ Physiol 288: H1770–H1776, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Northcott CA, Poy MN, Najjar SM, Watts SW. Phosphoinositide 3-kinase mediates enhanced spontaneous and agonist-induced contraction in aorta of deoxycorticosterone acetate-salt hypertensive rats. Circ Res 91: 360–369, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol 140: 701–706, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol Regul Integr Comp Physiol 275: R1719–R1723, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Sasser JM, Sullivan JC, Elmarakby AA, Kemp BE, Pollock DM, Pollock JS. Reduced NOS3 phosphorylation mediates reduced NO/cGMP signaling in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 43: 1080–1085, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sessa WC. eNOS at a glance. J Cell Sci 117: 2427–2429, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Shinozaki K, Nishio Y, Okamura T, Yoshida Y, Maegawa H, Kojima H, Masada M, Toda N, Kikkawa R, Kashiwagi A. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ Res 87: 566–573, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension 39: 597–602, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem 278: 30821–30827, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Vanourkova Z, Kramer HJ, Huskova Z, Vaneckova I, Opocensky M, Chabova VC, Tesar V, Skaroupkova P, Thumova M, Dohnalova M, Mullins JJ, Cervenka L. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens 24: 2465–2472, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogs controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733–739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Venema RC, Ju H, Zou R, Ryan JW, Venema VJ. Subunit interactions of endothelial nitric-oxide synthase. Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem 272: 1276–1282, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Wickman A, Andersson IJ, Jia J, Hedin L, Bergstrom G. Endothelial nitric oxide synthase protein is reduced in the renal medulla of two-kidney, one-clip hypertensive rats. J Hypertens 19: 1665–1673, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol 26: 2688–2695, 2006 [DOI] [PubMed] [Google Scholar]