Abstract
Our goal was to examine whether exercise training (ExT) could normalize impaired nitric oxide synthase (NOS)-dependent dilation of cerebral (pial) arterioles during type 1 diabetes (T1D). We measured the in vivo diameter of pial arterioles in sedentary and exercised nondiabetic and diabetic rats in response to an endothelial NOS (eNOS)-dependent (ADP), an neuronal NOS (nNOS)-dependent [N-methyl-d-aspartate (NMDA)], and a NOS-independent (nitroglycerin) agonist. In addition, we measured superoxide anion levels in brain tissue under basal conditions in sedentary and exercised nondiabetic and diabetic rats. Furthermore, we used Western blot analysis to determine eNOS and nNOS protein levels in cerebral vessels/brain tissue in sedentary and exercised nondiabetic and diabetic rats. We found that ADP and NMDA produced a dilation of pial arterioles that was similar in sedentary and exercised nondiabetic rats. In contrast, ADP and NMDA produced only minimal vasodilation in sedentary diabetic rats. ExT restored impaired ADP- and NMDA-induced vasodilation observed in diabetic rats to that observed in nondiabetics. Nitroglycerin produced a dilation of pial arterioles that was similar in sedentary and exercised nondiabetic and diabetic rats. Superoxide levels in cortex tissue were similar in sedentary and exercised nondiabetic rats, were increased in sedentary diabetic rats, and were normalized by ExT in diabetic rats. Finally, we found that eNOS protein was increased in diabetic rats and further increased by ExT and that nNOS protein was not influenced by T1D but was increased by ExT. We conclude that ExT can alleviate impaired eNOS- and nNOS-dependent responses of pial arterioles during T1D.
Keywords: adenosine 5′-diphosphate, N-methyl-d-aspartate, nitroglycerin, pial arterioles, Western blot, superoxide, endothelial nitric oxide synthase, neuronal nitric oxide synthase
exercise training (ExT) has been shown to play a significant role in the prevention of cardiovascular-related diseases. Although the precise cellular/molecular mechanisms accounting for the favorable effects of ExT on the cardiovascular system remain uncertain, many investigators have suggested that ExT may dramatically influence vascular endothelial function. Support for this concept can be found in studies that have examined the effects of ExT on endothelial nitric oxide synthase (eNOS)-dependent vasoreactivity in animals and human subjects (22, 23, 27, 28, 60, 69). These studies have reported that ExT can enhance eNOS-dependent responses of large and small blood vessels in skeletal muscle, heart, and skin. Mechanisms by which ExT potentiates nitric oxide synthase (NOS)-dependent relaxation/dilation of blood vessels are not entirely clear but are likely to be related to an increase in shear forces acting on endothelial cells to increase the synthesis/release of nitric oxide (29, 52, 56, 65) and/or an increase in the activity of antioxidant enzymes (superoxide dismutase, glutathione peroxidase, and/or catalase) in blood vessels/tissues surrounding blood vessels (24, 32, 35, 40, 51, 55).
In addition to examining the beneficial effects of ExT on vascular function during normal physiological states, investigators have also begun to examine the benefits of ExT in disease states. Studies have shown that ExT can improve/restore responses of peripheral blood vessels during chronic hypertension (5, 11, 26, 33), types 1 and 2 diabetes (23, 46, 69), and heart failure (30, 36). In addition, we have reported that impaired eNOS-dependent responses of the basilar artery observed in rats with type 1 diabetes (T1D) could be restored to that observed in nondiabetic rats by ExT (43). Thus it does not appear that the beneficial effects of ExT on vascular function during disease states are limited to vessels contained within skeletal muscle. Furthermore, although ExT can reverse vascular dysfunction of large blood vessels during disease states, it is not clear whether the beneficial effects of ExT include resistance arterioles and whether ExT can influence the reactivity of vessels in response to other critical vasodilator pathways, i.e., the activation of neuronal NOS (nNOS). Thus the first goal of the present study was to examine the effects of ExT on impaired eNOS- and nNOS-dependent responses of cerebral (pial) resistance arterioles during T1D. Our second goal was to examine the influence of ExT on oxidative stress in brain tissue from sedentary and exercised nondiabetic and diabetic rats. Finally, our third goal was to determine the influence of T1D and ExT on eNOS and nNOS protein levels in the brain.
METHODS
Induction of diabetes.
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Male Sprague-Dawley rats (180–200 g body wt) were randomly divided into one of four groups: sedentary nondiabetic, exercised nondiabetic, sedentary diabetic, and exercised diabetic. All rats had access to food and water ad libitum. The diabetic groups of rats were injected with streptozotocin (50 mg/kg ip) to induce diabetes, and the nondiabetic groups of rats were injected with vehicle (sodium citrate buffer). Blood samples, for the measurement of blood glucose concentration, were obtained 3–5 days after injection of streptozotocin or vehicle and on the day of the experiment. Blood glucose concentration was determined by using an Accu-Chek II Blood Glucose Monitor (Boehringer Mannheim Diagnostics, Indianapolis, IN), and an animal with a blood glucose concentration of >300 mg/dl was considered to be diabetic.
Exercise training.
Rats were exercised using standard techniques similar to that which we have previously described (43). Treadmill exercise was started 3 days following injection of streptozotocin or vehicle and was carried out 5 days/wk until the day before the experiment. Experiments were conducted 6–8 wk after starting the ExT. The length of time on the treadmill was initially 10 min/day for the first five days at 0% grade at a speed of 20 m/min. Over an 8–10-day period, the duration on the treadmill was then gradually increased to 60 min. As we have previously shown (43), this level of ExT produces an increase in citrate synthase activity in skeletal muscle of rats and is considered moderate exercise.
Preparation of animals.
Rats were prepared for in vivo studies 6–8 wk after injection of streptozotocin or vehicle. Rats were anesthetized with thiobutabarbital (Inactin; 100 mg/kg ip), and a tracheotomy was performed. The animals were ventilated mechanically with room air and supplemental oxygen. A catheter was inserted into a femoral vein for injection of supplemental anesthesia. A femoral artery was cannulated for the measurement of arterial pressure and to obtain a blood sample for the measurement of blood glucose concentration. After the placement of all catheters, the animal was placed in a head holder and a craniotomy was made over the left parietal cortex to expose the pial microcirculation (16). The cranial window was suffused with artificial cerebral spinal fluid bubbled with 95% nitrogen and 5% carbon dioxide. The temperature of the suffusate was maintained at 37 ± 1°C. The cranial window was connected via a three-way valve to an infusion pump, which allowed for the infusion of agonists into the suffusate. Arterial blood gases were monitored and maintained within normal limits.
Experimental protocol.
The cranial window was suffused for 30–45 min before examining the responses to the agonists. We then examined the dilation of pial arterioles in sedentary nondiabetic (n = 6), exercised nondiabetic (n = 13), sedentary diabetic (n = 8), and exercised diabetic (n = 6) rats in response to ADP (10 and 100 μM), N-methyl-d-aspartate (NMDA; 100 and 300 μM), and nitroglycerin (0.1 and 1.0 μM). The agonists were mixed in artificial cerebral spinal fluid and superfused over the cranial window preparation. The diameter of pial arterioles was measured immediately before the application of agonists and every minute for 5 min during the application of the agonists. Steady-state responses were reached within 2 to 3 min after the application, and the diameter of the pial arterioles returned to baseline within 5 min after the application of the agonists was stopped.
Measurement of superoxide.
Superoxide levels were measured by lucigenin-enhanced chemiluminescence as previously described (13, 14, 45). In separate groups of sedentary (n = 11) and exercised (n = 29) nondiabetic rats and sedentary (n = 34) and exercised (n = 25) diabetic rats, the brain was removed and placed in a Krebs-HEPES buffer (pH 7.4) with the following composition: (in mmol/l) 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgCl2, 1.3 CaCl2, 10 HEPES, 25 NaHCO3, and 5 (nondiabetic) or 20 (diabetic) glucose. Samples of cortex tissue, cut from brains of sedentary and exercised nondiabetic and diabetic rats, were placed in polypropylene tubes containing 5 μM lucigenin. The tubes were then read in a Sirius/FB15 luminometer (Berthold Detections Systems), which reports relative light units emitted over a 30-s interval for 5 min. The levels of superoxide reported are the value of tissue plus lucigenin-containing buffer minus background (lucigenin-containing buffer without tissue) and are normalized for tissue weight in relative light units per minute per milligram tissue.
Western blot analysis.
In separate groups of sedentary and exercised nondiabetic and diabetic rats, we measured eNOS and nNOS proteins in cerebral microvessels (eNOS) and brain tissue (nNOS), respectively. We also used Western blot analysis to measure the protein expression of GAPDH, and the values for eNOS and nNOS were normalized to GAPDH. Cerebral microvessels were isolated from brain tissue using methods previously described (61). Samples were homogenized in 20% (wt/vol) ice-cold buffer containing 10 mM Tris·HCl (pH 7.4), 1% SDS, 1 mM sodium vanadate, 10 μg/ml aprotinine, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. The homogenates were centrifuged at 12,000 g for 20 min at 4°C, and the protein concentrations in the supernatant were determined by the Bradford method (Bio-Rad; Richmond, CA) with BSA as the standard. Protein was mixed and boiled in SDS-PAGE sample buffer for 5 min and then loaded and run on standard 7.5% gels using 20 μg of protein. After SDS-PAGE, the proteins were transferred onto a polyvinylidene difluoride membrane. Immunoblotting was performed using the appropriate primary and secondary antibodies for eNOS and nNOS (Santa Cruz Biotechnology). The bound antibody was detected using an ECL kit and quantified by scanning densitometry.
Statistical analysis.
Analysis of variance with Fisher test for significance was used to compare baseline diameter of pial arterioles, responses of pial arterioles to the agonists, body weight, blood glucose concentration, mean arterial blood pressure, eNOS protein, and nNOS protein between sedentary and exercised nondiabetic and diabetic rats. A P value of 0.05 or less was considered to be significant.
RESULTS
Baseline conditions.
Baseline diameter of pial arterioles, blood glucose concentration, mean arterial pressure, and body weight of sedentary and exercised nondiabetic and diabetic rats are shown in Table 1. We found that the baseline diameter of pial arterioles was similar in all groups of rats. In addition, ExT did not influence the blood glucose concentration in nondiabetic or diabetic rats. Blood glucose concentration remained significantly higher in diabetic compared with nondiabetic rats regardless of ExT. Body weight was similar in sedentary and exercised nondiabetic rats and in sedentary and exercised diabetic rats. Body weight in sedentary and exercised diabetic rats was significantly lower than that found in sedentary and exercised nondiabetic rats.
Table 1.
Mean arterial pressure, baseline diameter of cerebral arterioles, blood glucose concentration, and body weight in sedentary and exercised nondiabetic and diabetic rats
| Sedentary |
Exercised |
|||
|---|---|---|---|---|
| Nondiabetic | Diabetic | Nondiabetic | Diabetic | |
| Mean arterial pressure, mmHg | 136 ± 5 | 126 ± 5 | 135 ± 3 | 134 ± 6 |
| Baseline diameter, μm | 43 ± 2 | 46 ± 3 | 43 ± 2 | 45 ± 3 |
| Blood glucose, mg/dl | 107 ± 11 | 433 ± 30*† | 117 ± 6 | 418 ± 26*† |
| Body weight, g | 366 ± 15 | 264 ± 12*† | 400 ± 9 | 300 ± 8*† |
Values are means ± SE.
P < 0.05 vs. sedentary nondiabetic rats;
P < 0.05 vs. exercised nondiabetic rats.
Responses of pial arterioles.
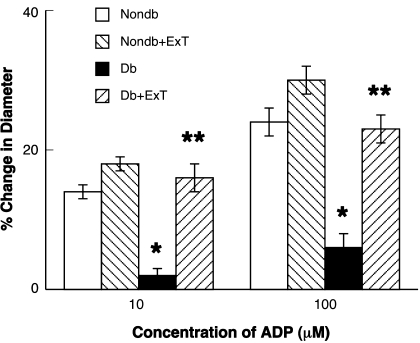
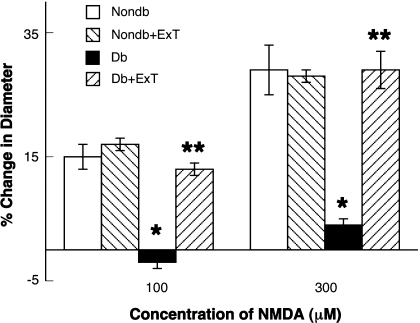
We examined the functional responses of pial arterioles to eNOS- and nNOS-dependent agonists (ADP and NMDA, respectively) and a NOS-independent agonist (nitroglycerin) in sedentary and exercised nondiabetic and diabetic rats. We found that ADP- (Fig. 1) and NMDA (Fig. 2)-induced dilation of pial arterioles was similar in sedentary and exercised nondiabetic rats. In addition, the dilation of pial arterioles in response to ADP and NMDA was significantly less in sedentary diabetic rats compared with sedentary and exercised nondiabetic rats (P < 0.05). Furthermore, we found that ExT reversed the impaired vasodilation to ADP and NMDA in diabetic rats (P < 0.05 vs. response in sedentary diabetic rats). Thus eNOS- and nNOS-induced dilation of pial arterioles is impaired in sedentary diabetic rats, and this impairment can be reversed by ExT.
Fig. 1.
Response of cerebral arterioles to ADP in sedentary nondiabetic (Nondb; white bars) and diabetic (Db; black bars) rats and in exercised nondiabetic (Nondb+ExT; right-hatched bars) and diabetic (Db+ExT; left-hatched bars) rats. Values are means ± SE. *P < 0.05 vs. nondiabetic rats; **P < 0.05 vs. diabetic rats.
Fig. 2.
Response of cerebral arterioles to N-methyl-d-aspartate (NMDA) in sedentary nondiabetic (white bars) and diabetic (black bars) rats and in exercised nondiabetic (right-hatched bars) and diabetic (left-hatched bars) rats. Values are means ± SE. *P < 0.05 vs. nondiabetic rats; **P < 0.05 vs. diabetic rats.
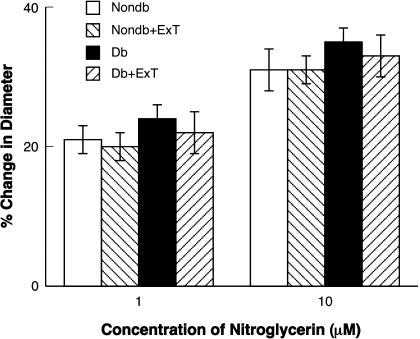
In contrast to that observed with ADP and NMDA, nitroglycerin produced a similar dose-related dilation of pial arterioles in sedentary and exercised nondiabetic and diabetic rats (Fig. 3). Thus it does not appear that the effect of ExT on ADP- and NMDA-induced vasodilation is related to a nonspecific effect of ExT on vascular function.
Fig. 3.
Response of cerebral arterioles to nitroglycerin in sedentary nondiabetic (white bars) and diabetic (black bars) rats and in exercised nondiabetic (right-hatched bars) and diabetic (left-hatched bars) rats. Values are means ± SE.
Superoxide production.
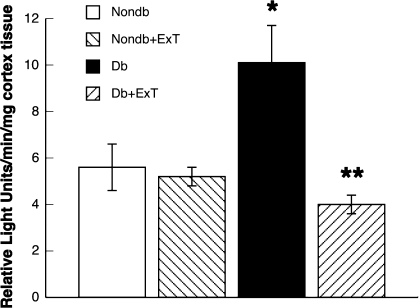
We measured superoxide levels in cortex tissue in sedentary rats and exercised nondiabetic and diabetic rats under basal conditions. We found that the levels of superoxide in cortex tissue were similar in sedentary and exercised nondiabetic rats (Fig. 4). In addition, we found that basal levels of superoxide in cortex tissue were increased in sedentary diabetic rats. Furthermore, the increase in basal levels of superoxide in cortex tissue in the sedentary diabetic rats was reversed to that observed in nondiabetic rats by ExT.
Fig. 4.
Levels of superoxide in cortex tissue from nondiabetic (white bars) and diabetic (black bars) rats and in exercised nondiabetic (right-hatched bars) and diabetic (left-hatched bars) rats. Values are means ± SE. *P < 0.05 vs. nondiabetic rats; **P < 0.05 vs. diabetic rats.
Western blot analysis.
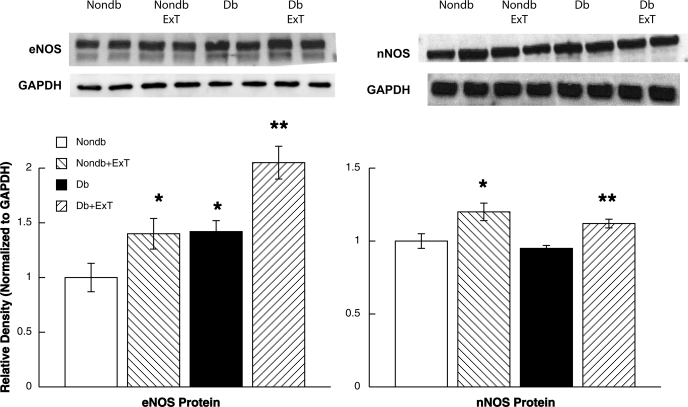
We measured eNOS and nNOS protein expression in cerebral microvessels/brain tissue, respectively, in sedentary and exercised nondiabetic and diabetic rats. First, we found that eNOS protein was significantly elevated in cerebral microvessels from sedentary diabetic rats (Fig. 5). In addition, eNOS protein was increased in microvessels from nondiabetic and diabetic rats by ExT. Thus it appears that there is a compensatory increase in eNOS protein expression in diabetic rats and that ExT can increase eNOS protein expression in nondiabetic and diabetic rats. Second, we found that nNOS protein expression was not altered in sedentary diabetic rats compared with sedentary nondiabetic rats (Fig. 5). However, we found that ExT increased nNOS protein expression in nondiabetic and diabetic rats (Fig. 5).
Fig. 5.
Top: Western blot of endothelial (eNOS) and neuronal (nNOS) nitric oxide synthase proteins from cerebral microvessels and brain tissue, respectively, in nondiabetic (white bars) and diabetic (black bars) rats and in exercised nondiabetic (right-hatched bars) and diabetic (left-hatched bars) rats. Protein levels for eNOS and nNOS are normalized to GAPDH. Values are means ± SE. *P < 0.05 vs. sedentary nondiabetic rats; **P < 0.05 vs. sedentary diabetic rats.
DISCUSSION
There are three major new findings of the present study. First, we found that ExT can restore impaired eNOS- and nNOS-dependent responses of pial arterioles in diabetic rats. This effect of ExT is specific since the responses of pial arterioles to nitroglycerin were not altered by ExT. Second, although ExT did not have a significant effect on basal levels of superoxide in the cortex tissue in nondiabetic rats, ExT reversed the increase in basal levels of superoxide in brain tissue in diabetic rats. Third, we found a compensatory increase in eNOS protein in diabetic rats that was accentuated by ExT. In addition, nNOS protein, which was not influenced by T1D, was increased by ExT in nondiabetic and diabetic rats. Based on these findings, we suggest that ExT has beneficial effects on cerebral arterioles via an influence on the balance between nitric oxide bioavailability and oxidative stress. We speculate that ExT may have important therapeutic potential for the treatment of diabetes-induced dysfunction of cerebral microvessels and may be beneficial in reducing the increased risk for stroke in diabetic subjects.
Consideration of methods.
We used ADP and NMDA to examine eNOS- and nNOS-dependent responses of cerebral arterioles, respectively. Others and we have suggested that ADP dilates cerebral arterioles via the activation of NOS, presumably eNOS (6, 17, 44). Others (42, 70) have suggested that the relaxation of the rat middle cerebral artery to purines is related, in part, to the synthesis/release of nitric oxide and to the synthesis/release of an endothelium-derived hyperpolarizing factor (EDHF). We did not examine a role for EDHF in response to ADP in the present study. However, studies by other investigators (10, 12, 18, 21, 64) have suggested that the activation of potassium channels, presumably by EDHF, does not play a significant role in the dilatation of cerebral arterioles to the agonists used in the present study. Regarding responses to NMDA, we and others have shown that NMDA dilates cerebral arterioles via the activation of nNOS and the subsequent synthesis/release of nitric oxide (18–20, 63). Based on these previous findings, we suggest that ADP and NMDA are appropriate agonists to evaluate NOS-dependent dilatation of cerebral arterioles.
We measured superoxide levels in the parietal cortex tissue using lucigenin-derived chemiluminescence, as we (2, 3) have previously described. We found that our measurement of superoxide from the parietal cortex tissue was elevated in diabetic rats and was attenuated by ExT. Superoxide production can occur from several cell types, including endothelium, vascular smooth muscle, neurons, and glia. In the present study, we cannot determine the precise cellular source of superoxide. It is likely that T1D stimulates an increase in the production of superoxide from more than one cellular source. Thus it would be very difficult to determine, even with the use of cell culture methodologies, the overall importance of individual sources of superoxide by the various cell types in relation to altered cerebrovascular function. However, we suggest that our inability to determine the precise source of superoxide does not diminish the importance of the finding that T1D can lead to an increase in superoxide, that this increase can contribute to cerebrovascular dysfunction, and that ExT can reduce superoxide in T1D.
Role of eNOS and nNOS.
In the present study, we found that eNOS protein was greater in sedentary diabetic compared with sedentary nondiabetic rats. This finding, which is similar to that reported in previous studies (34, 43, 68), suggests a compensatory response to altered vascular reactivity in diabetic animals. In addition, we found that eNOS protein was significantly elevated in cerebral vessels from exercised diabetic compared with sedentary diabetic rats. This finding would seem to suggest that the effects of ExT on eNOS-dependent reactivity of cerebral arterioles during T1D may be related, in part, to an increase in the synthesis/release of nitric oxide in response to ADP. However, since we also observed a significant decrease in levels of superoxide by ExT in diabetic rats, we suggest that the restoration of vascular function by ExT in diabetic rats may be related to a combination of a decrease in oxidative stress and an increase in nitric oxide production by eNOS.
We also found that ExT increased eNOS protein in cerebral microvessels in nondiabetic rats. However, we did not find a difference in eNOS-dependent reactivity of cerebral arterioles between sedentary and exercised nondiabetic rats. This finding was surprising given the fact that others have shown that ExT increases the reactivity of peripheral blood vessels (5, 11, 22, 37). However, in a previous study (43), we found that ExT did not influence eNOS-dependent responses of the basilar artery. Thus it is conceivable that levels of eNOS observed in sedentary nondiabetic rats are sufficient to produce the degree of vasodilation observed in the present study, and thus an increase in eNOS protein and/or activity may not contribute to agonist-induced changes in vascular reactivity in nondiabetic animals.
Unlike that reported for eNOS, we did not find a difference in nNOS protein between nondiabetic and diabetic rats. Some investigators have reported that T1D produces a decrease in the expression of nNOS protein in peripheral tissue and specific regions of the brain (54, 71, 72). However, others have reported that nNOS protein expression is actually increased by T1D (25). The discrepancy between the present study and previous studies is not clear but may be related to the area of the brain examined and/or the duration/severity of T1D. In any event, although we did not examine nNOS activity, our finding of a similar level of nNOS protein between nondiabetic and diabetic rats seems to suggest that impaired responses of cerebral arterioles during T1D are not related to a decrease in the synthesis/release of nitric oxide via activation of nNOS.
In addition to examining the influence of T1D on nNOS, studies have examined the influence of ExT on the expression of nNOS protein. These investigators have reported that ExT produces an increase in the expression of nNOS protein in peripheral tissues and in the brain (8, 31, 48, 66, 73). The mechanism that accounts for the increase in expression of nNOS protein during ExT is not entirely clear. In the present study, we also found a small increase in the expression of nNOS protein in exercised nondiabetic and diabetic rats. Surprisingly, the increase in the expression of nNOS protein by ExT did not influence the reactivity of cerebral arterioles in nondiabetic rats, a finding similar to that reported for eNOS-dependent agonists. However, we did find that ExT restored the impaired nNOS-dependent responses of the pial arterioles in diabetic rats. Given that this increase in nNOS by ExT was similar in nondiabetic and diabetic rats, we suggest that the overall mechanism responsible for the restoration of responses to NMDA in exercised diabetic rats is probably not solely related to changes in nNOS protein and/or activity but is probably related to a change in the balance between oxidative stress, which was reduced by ExT, and nitric oxide production. In fact, others (1, 50) have shown that oxidative stress can inhibit responses of cerebral arteries to NMDA.
Effects of ExT on vascular function.
Whereas no studies to our knowledge have examined the influence of ExT on nNOS-dependent responses of blood vessels, many studies have examined the effects of ExT on eNOS-dependent reactivity. Several investigators (28, 37–39, 60, 62) have shown that ExT enhances eNOS-dependent responses of peripheral arteries from animal models and human subjects. However, not all studies are in agreement. Franke et al. (22) found that ExT enhanced eNOS-dependent increases in forearm vascular conductance but did not alter vascular responses to eNOS-dependent agonists. In addition, Oltman et al. (47) found that ExT did not influence NOS-dependent responses of porcine coronary arteries, and Rogers et al. (53) report that ExT actually decreased responsiveness of isolated canine coronary arteries to β-adrenergic agonists. Furthermore, we have reported that ExT does not alter eNOS-dependent dilation of the basilar artery in nondiabetic rats (43). In the present study, we did not find a difference in eNOS-dependent reactivity of cerebral arterioles between nondiabetic sedentary and exercised rats. Thus this finding is similar to that reported for the basilar artery (43). The discrepancy between studies that have reported an increase in eNOS-dependent reactivity and those that do not find a change in eNOS-dependent reactivity during physiological states is difficult to determine but may be related, in part, to differences in the intensity of ExT, vascular beds examined, and/or species differences. In the present study, we also found that nNOS-dependent responses of cerebral arterioles were not altered by ExT. To our knowledge, this is the first report regarding the influence of ExT on nNOS-dependent vasoreactivity.
Several studies have examined the influence of ExT on eNOS-dependent responses of peripheral blood vessels during a variety of disease states, including chronic hypertension (5, 33), heart failure (30, 36, 67), and T1D (43) or type 2 diabetes (46, 58). Arvola et al. (5) reported that ExT enhanced the relaxation of mesenteric arterioles and the carotid artery to eNOS-dependent and -independent agonists in hypertensive obese rats compared with normal rats via a mechanism that appeared to be related to an increase in the synthesis/release of nitric oxide. Higashi et al. (33) reported an enhancement in eNOS-dependent responses in human subjects with chronic hypertension following ExT. In addition, studies have shown that ExT improved eNOS-dependent relaxation of the aorta in type 2 diabetic rats (58) and forearm blood flow in type 2 diabetic humans (46). Furthermore, we have reported that ExT restored an impaired eNOS-dependent dilation of the basilar artery in diabetic rats (43). Thus the effects of ExT are not limited to large peripheral blood vessels and/or blood vessels contained within skeletal muscle but also involve the cerebral circulation.
What are the potential implications for altered vascular function to eNOS- and nNOS-dependent agonists in T1D? Diabetes is characterized by hyperglycemia induced by a lack of insulin and/or a resistance to the actions of insulin. Despite the ability to maintain blood glucose within an acceptable range, complications still occur in humans with T1D. Diabetic subjects and animals have cognitive deficits, as well as other neurophysiological/neurochemical changes in the brain (4, 7, 9, 41, 57). In addition, these deficits can only partially be reversed by insulin treatment (9). The pathogenesis of cerebral dysfunction in T1D may be related to both functional (e.g., altered vascular reactivity) and structural (e.g., changes in the basement membrane) alterations. How these functional and structural changes in cerebral blood vessels contribute to the regulation of cerebral blood flow in T1D remains controversial, with studies showing increases (59), decreases (15), or no change (49) in cerebral blood flow in T1D. Understanding the potential role for vascular disorders and a disruption in the neurovascular unit (as we demonstrate by examining responses to NMDA) in cerebral dysfunction during T1D may provide a new therapeutic approach whereby treatment aimed at correcting vascular disorders may lead to a decrease in other T1D-induced complications. Support for this concept can be found in a study by Manschot et al. (41). These investigators reported that treatment to improve vascular function could improve cognitive function in diabetic rats. Thus changes in vascular function and/or function of the neurovascular unit may play a critical role in cerebral dysfunction in T1D.
In summary, to our knowledge, this is the first study that examined the effects of ExT on eNOS- and nNOS-dependent reactivity of cerebral resistance arterioles. We found that ExT restored an impaired eNOS- and nNOS-dependent dilation of cerebral arterioles in diabetic rats but did not alter NOS-independent vasodilation. In addition, we found that levels of superoxide were elevated in diabetic rats and that ExT decreased these basal levels of superoxide in diabetic rats. Furthermore, we found that eNOS protein was increased by ExT and T1D and that nNOS protein was increased by ExT but not altered by T1D. Taken together, these findings suggest that ExT can restore the impaired eNOS- and nNOS-dependent dilation of cerebral arterioles via mechanisms that may regulate the balance between oxidative stress and nitric oxide synthesis/release.
GRANTS
This study was supported by National Institutes of Health Grants HL-090657 and AA-11288 and by funds from the University of Nebraska Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Armstead WM. NOC/oFQ PKC-dependent superoxide generation contributes to hypoxic-ischemic impairment of NMDA cerebrovasodilation. Am J Physiol Heart Circ Physiol 279: H2678–H2684, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Arrick DM, Mayhan WG. Acute infusion of nicotine impairs nNOS-dependent reactivity of cerebral arterioles via an increase in oxidative stress. J Appl Physiol 103: 2062–2067, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res 1184: 365–371, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61: 661–666, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Arvola P, Wu X, Kahonen M, Makynen H, Riutta A, Mucha I, Solakivi T, Kainulainen H, Porsti I. Exercise enhances vasorelaxation in experimental obesity associated hypertension. Cardiovasc Res 43: 992–1002, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Ayajiki K, Okamura T, Toda N. Involvement of nitric oxide in endothelium-dependent, phasic relaxation caused by histamine in monkey cerebral arteries. Jpn J Pharmacol 60: 357–362, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH. Cerebral function in diabetes mellitus. Diabetologia 37: 643–650, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med 44: 224–229, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Brands AM, Kessels RP, de Haan EH, Kappelle LJ, Biessels GJ. Cerebral dysfunction in type 1 diabetes: effects of insulin, vascular risk factors and blood-glucose levels. Eur J Pharmacol 490: 159–168, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Brayden JE. Hyperpolarization and relaxation of resistance arteries in response to adenosine diphosphate. Circ Res 69: 1415–1420, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Collins HL, DiCarlo SE. Daily exercise enhances acetylcholine-induced dilation in mesenteric and hindlimb vasculature of hypertensive rats. Clin Exp Hypertens 21: 353–376, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Chrissobolis S, Ziogas J, Chu Y, Faraci FM, Sobey CG. Role of inwardly rectifying K+ channels in K+-induced cerebral vasodilatation in vivo. Am J Physiol Heart Circ Physiol 279: H2704–H2712, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Didion SP, Faraci FM. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am J Physiol Heart Circ Physiol 282: H688–H695, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol 281: H1697–H1703, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during chronic and acute hyperglycemia. Stroke 18: 52–58, 1987 [DOI] [PubMed] [Google Scholar]
- 16. Fang Q, Sun H, Mayhan WG. Impairment of nitric oxide synthase-dependent dilatation of cerebral arterioles during infusion of nicotine. Am J Physiol Heart Circ Physiol 284: H528–H534, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Faraci FM. Role of endothelium-derived relaxing factor in cerebral circulation: large arteries vs. microcirculation. Am J Physiol Heart Circ Physiol 261: H1038–H1042, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-d-aspartate receptors in brain. Circ Res 72: 476–480, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Faraci FM, Breese KR, Heistad DD. Responses of cerebral arterioles to kainate. Stroke 25: 2080–2084, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Faraci FM, Brian JE. 7-Nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation in response to N-methyl-d-aspartate. Stroke 26: 2172–2176, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Faraci FM, Heistad DD. Role of ATP-sensitive potassium channels in the basilar artery. Am J Physiol Heart Circ Physiol 264: H8–H13, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Franke WD, Stephens GM, Schmid PG. Effects of intense exercise training on endothelium-dependent exercise-induced vasodilatation. Clin Physiol 18: 521–528, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Fuchsjager-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L, Wolzt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care 25: 1795–1801, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105: 1631–1639, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giove TJ, Deshpande MM, Gagen CS, Eldred WD. Increased neuronal nitric oxide synthase activity in retinal neurons in early diabetic retinopathy. Mol Vis 15: 2249–2258, 2009 [PMC free article] [PubMed] [Google Scholar]
- 26. Graham DA, Rush JW. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol 96: 2088–2096, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation 104: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Harris MB, Mitchell BM, Sood SG, Webb RC, Venema RC. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol 104: 795–802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther 102: 87–96, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Role of endothelium-derived nitric oxide. Circulation 100: 1194–1202, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Hink U, Li H, Mollnau H, Oelse M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: e14–e22, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Johnson P. Antioxidant enzyme expression in health and disease: effects of exercise and hypertension. Comp Biochem Physiol C Toxicol Pharmacol 133: 493–505, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Katz SD, Yuen J, Bijou R, LeJemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol 82: 1488–1492, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Circ Res 76: 544–550, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Kvernmo HD, Stefanovska A, Kirkeboen KA, Osterud B, Kvernebo K. Enhanced endothelium-dependent vasodilatation in human skin vasculature induced by physical conditioning. Eur J Appl Physiol 79: 30–36, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Laughlin MH, Rubin LJ, Rush JW, Price EM, Schrage WG, Woodman CR. Short-term training enhances endothelium-dependent dilation of coronary arteries, not arterioles. J Appl Physiol 94: 234–244, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Lawson DL, Chen L, Mehta JL. Effects of exercise-induced oxidative stress on nitric oxide release and antioxidant activity. Am J Cardiol 80: 1640–1642, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Manschot SM, Biessels GJ, Cameron NE, Cotter MA, Kamal A, Kappelle LJ, Gispen WH. Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin-diabetic rats. Brain Res 966: 274–282, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Marrelli SP, Khorovets A, Johnson TD, Childres WF, Bryan RM., Jr P2 purinoceptor-mediated dilations in the rat middle cerebral artery after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 276: H33–H41, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Mayhan WG, Sun H, Mayhan JF, Patel KP. Influence of exercise on dilatation of the basilar artery during diabetes mellitus. J Appl Physiol 96: 1730–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Mayhan WG. Endothelium-dependent responses of cerebral arterioles to adenosine 5′-diphosphate. J Vasc Res 29: 353–358, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Miller FJ, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Miorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetics. J Am Coll Cardiol 38: 860–866, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol 79: 33–40, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Ozbek E, Tasci AI, Ilbey YO, Simsek A, Somay A, Metin G. The effect of regular exercise on penile nitric oxide synthase expression in rats. Int J Androl 33: 623–628, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Pelligrino DA, Albrecht RF. Chronic hyperglycemic diabetes in the rat is associated with a selective impairment of cerebral vasodilatory responses. J Cereb Blood Flow Metab 11: 667–677, 1991 [DOI] [PubMed] [Google Scholar]
- 50. Philip S, Armstead WM. Differential role of PTK, ERK and p38 MAPK in superoxide impairment of NMDA cerebrovasodilation. Brain Res 979: 98–103, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 107: 987–997, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol Endocrinol Metab 277: E390–E394, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Rogers PJ, Miller TD, Bauer BA, Brum JM, Bove AA, Vanhoutte PM. Exercise training and responsiveness of isolated coronary arteries. J Appl Physiol 71: 2346–2351, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Roghani M, Baluchnejadmojarad T. Mechanisms underlying vascular effects of chronic resveratrol in streptozotocin-diabetic rats. Phytother Res 10: 1–7, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 46: 940–948, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Sakamoto S, Minami K, Niwa Y, Ohnaka M, Nakaya Y, Mizuno A, Kuwajima M, Shima K. Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka long-evans Tokushima fatty rat, a model of spontaneous NIDDM. Diabetes 47: 82–86, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Simpson RE, Phillis JW, Buchannan J. A comparison of cerebral blood flow during basal, hypotensive, hypoxic and hypercapnic conditions between normal and streptozotocin diabetic rats. Brain Res 531: 136–142, 1990 [DOI] [PubMed] [Google Scholar]
- 60. Sun D, Huang A, Koller A, Kaley G. Enhanced NO-mediated dilations in skeletal muscle arterioles of chronically exercised rats. Microvasc Res 64: 491–496, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG. Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke 37: 495–500, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol 76: 2241–2247, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Sun H, Patel KP, Mayhan WG. Impairment of neuronal nitric oxide synthase-dependent dilatation of cerebral arterioles during chronic alcohol consumption. Alcohol Clin Exp Res 26: 663–670, 2002 [PubMed] [Google Scholar]
- 64. Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca2+-dependent K+ channels. Circ Res 76: 1057–1062, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Tanabe T, Maeda S, Miyauchi T, Iemitsu M, Takanashi M, Irukayama-Tomobe Y, Yokota T, Ohmori H, Matsuda M. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand 178: 3–10, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Ueda H, Urano Y, Sakurai T, Kizaki T, Hitomi Y, Ohno H, Izawa T. Enhanced expression of neuronal nitric oxide synthase in islets of exercise-trained rats. Biochem Biophys Res Commun 312: 794–800, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Varin R, Mulder P, Richard V, Tamion F, Devaux C, Henry JP, Lallemand F, Lerebours G, Thuillez C. Exercise improves flow-mediated vasodilatation of skeletal muscle arteries in rats with chronic heart failure. Role of nitric oxide, prostanoids and oxidant stress. Circulation 99: 2951–2957, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Wolka AM, Huber JD, Davis TP. Pain and the blood-brain barrier: obstacles to drug delivery. Adv Drug Deliv Rev 55: 987–1006, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R987–R991, 2005 [DOI] [PubMed] [Google Scholar]
- 70. You J, Johnson TD, Marrelli SP, Mombouli JV, Bryan RM. P2u receptor-mediated release of endothelium-derived relaxing factor/nitric oxide and endothelium-derived hyperpolarizing factor from cerebrovascular endothelium in rats. Stroke 30: 1125–1133, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Yu WJ, Juang SW, Chin WT, Chi TC, Chang CJ, Cheng JT. Insulin restores neuronal nitric oxide synthase expression in streptozotocin-induced diabetic rats. Life Sci 68: 625–634, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Yu WJ, Juang SW, Chin WT, Chi TC, Wu TJ, Cheng JT. Decrease of nitric oxide synthase in the cerebrocortex of streptozotocin-induced diabetic rats. Neurosci Lett 272: 99–102, 1999 [DOI] [PubMed] [Google Scholar]
- 73. Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005 [DOI] [PubMed] [Google Scholar]