Abstract
Ethanol exposure during developmental synaptogenesis can lead to brain defects referred to as fetal alcohol syndrome (FAS), which can include mental health problems such as cognitive deficits and mental retardation. In FAS, widespread neuronal death and brain mass loss precedes behavioral and cognitive impairments in adulthood. Because tissue plasminogen activator (tPA) has been implicated in neurodegeneration, we examined whether it mediates FAS. Neonatal WT and tPA−/− mice were injected with ethanol to mimic FAS in humans. In WT mice, ethanol elicited caspase-3 activation, significant forebrain neurodegeneration, and decreased contextual fear conditioning in adults. However, tPA-deficient mice were protected from these neurotoxicities, and this protection could be abrogated by exogenous tPA. Selective pharmacological modulators of NMDA and GABAA receptor pathways revealed that the effects of tPA were mediated by the NR2B subunit of the NMDA receptor. This study identifies tPA as a critical signaling component in FAS.
Ethanol exposure during developmental synaptogenesis can result in fetal alcohol syndrome (FAS), which includes cognitive deficits, mental retardation, and hyperactivity (1). Despite its prevalence as the leading preventable cause of mental retardation, little is known about the molecular basis of FAS.
There is widespread neurodegeneration (ND) of the forebrain in FAS (2). The developing brain is more vulnerable to ethanol-induced ND during synaptogenesis because ethanol reduces neuronal excitability through both antagonism of the NMDA receptor (NR) and potentiation of the GABAA receptor (GR) (3, 4). The NR is a central target for the neurobehavioral effects of ethanol because it inhibits ion flux through the receptor. NRs containing NR2A and NR2B subunits are the most sensitive to ethanol (5). Drugs that mimic ethanol via inhibition of the NR or hyperactivation of the GR lead to ND that resembles the pattern observed after ethanol exposure (2). In rodents, ethanol administered during synaptogenesis at postnatal day 7 (P7), equivalent to the third trimester of human gestation, leads to ND and cognitive impairments that mimic those in humans with FAS (6, 7). Administration of the same dose of ethanol at P21, when synaptogenesis has ended, does not result in ND (2).
Tissue plasminogen activator (tPA) is an extracellular protease that cleaves inactive plasminogen to plasmin. tPA activity is regulated by plasminogen activator inhibitor type 1 (PAI-1). tPA is expressed in the brain and is involved in learning and memory processes (8–10), mediating some of these functions via modulation of the NR (9). Besides regulating normal CNS physiology and plasticity events, tPA activity also plays a role in CNS pathology. tPA, via plasmin activation, mediates kainate-induced excitotoxic neuronal death in the hippocampus (11, 12). tPA also regulates ethanol-induced ND and withdrawal seizures in adult animals through direct interactions with NR2B in the hippocampus (13).
With the exception of mice deficient in core apoptotic factors (14), mice resistant to ethanol-induced ND have not been described. Because tPA can induce apoptosis and mediate ethanol-induced modulation of the NR (13), a main target for ethanol in the brain, we hypothesized tPA could be involved in FAS pathogenesis. Here we show that tPA is a critical factor in FAS-associated ND and cognitive deficits, acting through NR2B of the NR.
Results
Ethanol Treatment Increased Forebrain-Associated tPA Activity in P7 Mice.
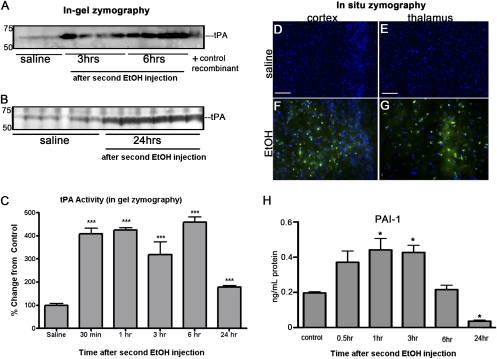
Although synaptogenesis begins in utero in humans, this process occurs postnatally in mice during the first 2 wk after birth. Therefore, pups were used at P7 to model human FAS. tPA activity and PAI-1 expression were determined following the administration of ethanol (two injections of ethanol, 2 h apart) to mouse pups at P7. In-gel zymography of forebrain homogenates showed tPA activity increased significantly over control levels at every time point examined after the second ethanol injection (Fig. 1 A–C). In situ zymography also showed tPA activity increased after ethanol exposure in the cingulate cortex (Fig. 1F) and thalamus (Fig. 1G) of WT mice compared with saline-treated animals (Fig. 1 D and E).
Fig. 1.
Ethanol treatment increased forebrain-associated tPA activity in P7 mice. P7 WT mice were injected s.c. with ethanol (referred to as EtOH in the figures) in two doses of 2.5 g/kg at 0 and 2 h (n = 5–6). Control mice were injected with saline. Animals were killed at different time-points following the second ethanol injection, and the forebrains analyzed for tPA activity by in-gel zymography (A and B). Quantification of the lysis bands, represented here as percent change from control (set to 100%; C), showed tPA activity was significantly increased in the forebrains of WT mice from 30 min until 24 h after ethanol exposure (***P < 0.0001). In situ zymography experiments, using fluorescently quenched casein that fluoresces green upon plasmin cleavage, detected increased tPA activity in the cingulate cortex (F) and thalamus (G) of ethanol-treated mice 3 h after administration in comparison with saline-treated mice (D and E). PAI-1 levels were measured by ELISA (H). Ethanol administration significantly increased PAI-1 levels 1 and 3 h posttreatment. However, 24 h after ethanol, PAI-1 was significantly decreased below baseline levels (*P < 0.05). (Scale bar, 10 μm.)
Because ethanol can down-regulate the expression of PAI-1, a main inhibitor of tPA in the CNS (15), we determined whether ethanol treatment modulated PAI-1 expression. Forebrain homogenates from P7 WT mice treated with ethanol were examined for the expression of PAI-1. Ethanol exposure significantly increased PAI-1 levels initially, but PAI-1 levels decreased from baseline 24 h postethanol treatment (Fig. 1H).
tPA−/− Postnatal Mouse Pups Are Resistant to Ethanol-Induced ND.
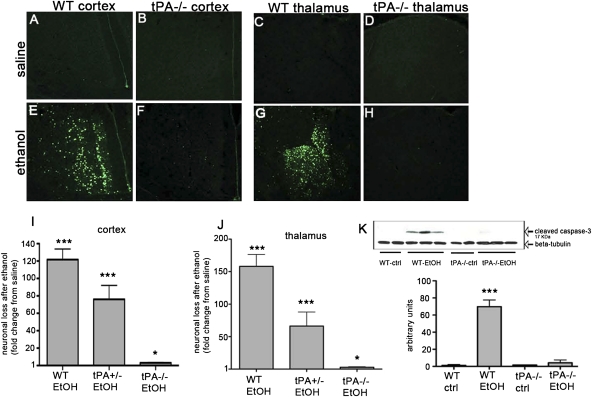
To determine whether the increased tPA activity observed after ethanol administration was associated with neuronal death, we stained brain sections with Fluoro-Jade B (FJB) to identify areas of ND in WT and tPA−/− mice 24 h after the second ethanol injection. Though saline administration did not cause neuronal death in WT, tPA+/−, or tPA−/− mice (Fig. 2 A–D), ND was widespread in the WT cortex, particularly the cingulate cortex, after ethanol treatment (Fig. 2A vs. Fig. 2E). Ethanol also induced severe ND in the thalamus (Fig. 2C vs. Fig. 2G). The hippocampus was less vulnerable to ethanol compared with thalamus and cortex, but neuronal death was significant nonetheless (16) (Fig. S1). In comparison, tPA−/− mice were significantly protected from neuronal loss throughout the forebrain (Fig. 2 B and D vs. Fig. 2 F and H). Quantification of FJB-positive staining revealed WT mice had significant ND in all brain regions in comparison with saline-treated mice and ethanol-treated tPA−/− mice (Fig. 2 I and J). The effect of tPA on ND was gene dosage-dependent, as mice heterozygous for tPA showed an intermediate phenotype in the cortex and thalamus in comparison with WT and tPA−/− mice (Fig. 2 I and J).
Fig. 2.
Ethanol induced ND in WT, but not tPA−/−, mice. P7 WT, tPA+/−, and tPA−/− mice were injected with ethanol or saline (n = 6–7/group). Brains were collected 24 h after treatment and stained with FJB to visualize ND. Saline treatment did not cause ND in any genotype (A–D). Ethanol induced significant ND in the cortex and thalamus of WT mice compared with saline-treated mice (A vs. E; C vs. G). tPA−/− mice were significantly protected from ND after ethanol exposure in comparison with saline group (B vs. F; D vs. H). Quantification of FJB-positive staining density revealed WT mice had significant ND in all brain regions in comparison with their saline-treated counterparts and ethanol-treated tPA−/− mice (I and J; *P < 0.05, ***P < 0.0001). The effect of tPA on ND was gene dosage dependent, as mice heterozygous for tPA showed an intermediate phenotype in the cortex and thalamus in comparison with WT and tPA−/− mice (I and J). We examined forebrain samples for cleaved caspase-3 by Western blot. WT mice showed activation of caspase-3 8 h after ethanol treatment, whereas tPA−/− mice did not (K; ***P < 0.0001). (Scale bar, 10 μm.)
Apoptotic ND induced by ethanol at P7 is mediated by Bax, requires the release of cytochrome c, and culminates with the activation of caspase-3 (7, 14, 17, 18). To determine if activation of ethanol-induced apoptosis after ethanol exposure is tPA dependent, we measured cleaved caspase-3 by Western blot. WT forebrains showed an increase in activation of caspase-3 8 h after the second ethanol treatment, whereas tPA−/− mice did not show these effects (Fig. 2K).
Another molecule of interest is GAP43, which plays a critical role in synaptogenesis because its phosphorylation promotes cytoskeletal remodeling (19). Phospho-GAP43 was significantly increased in tPA−/− mice, but not WT, 24 h following ethanol administration. However, total GAP43 levels were unchanged in both genotypes and treatment groups (Fig. S2). These findings suggest that ethanol alters synaptic activity in tPA−/− but not WT animals.
The disparities in severity of ethanol-induced ND between tPA−/− and WT mice could have resulted from differing blood ethanol concentrations due to metabolic differences. Therefore, we determined plasma ethanol concentrations in these mice at several time-points following administration. We found no differences in blood ethanol concentrations between tPA−/− and WT mice (Fig. S3). These findings indicate that the neuroprotection in tPA−/− mice did not stem from differences in the rate of ethanol metabolism.
To examine if the effects of tPA were plasminogen dependent, P7 WT and plasminogen-deficient mouse pups were exposed to ethanol and examined for ND. There was no difference in the extent of neuronal loss in the two mouse lines (Fig. S4), indicating that tPA acts independently of plasminogen in this system.
tPA Catalytic Activity Is Not Required to Promote Ethanol-Induced ND.
To determine if tPA−/− mice are resistant to neuronal death as a result of developmental abnormalities and not as a direct effect of tPA up-regulation following ethanol exposure, we injected P7 tPA−/− mice with catalytically inactive S481A tPA 30 min before ethanol treatment. Active tPA restores ND in tPA−/− mice in another excitotoxicity model (20), and therefore administration of inactive tPA allowed us to determine if the proteolytic activity of tPA was necessary to mediate ND. Ethanol treatment alone did not induce significant ND in the cortex (Fig. S5A vs. Fig. S5 B and D) or thalamus (Fig. S5E vs. Fig. S5 F and H) of tPA−/− mice. However, S481A tPA restored ethanol-induced ND in tPA−/− mice (Fig. S5 C, D, G, and H).
To verify that S481A reached the brain parenchyma, we examined S481A tPA-treated P7 tPA−/− brain sections. We did not detect tPA immunoreactivity in the brains of tPA−/− mice treated with saline (Fig. S5I), but we did observe tPA immunoreactivity in the brains treated with S481A tPA (Fig. S5J). The blood brain barrier (BBB) is not developed in P7 mice (21, 22), thus facilitating passive transport of S481A tPA. To confirm BBB permeability, tPA−/− mice at ages P7 and P90 were administered an i.v. injection of Evans Blue, a dye that does not cross the mature intact BBB and is used to determine BBB integrity (23). Evans Blue dye was observed in the brain parenchyma of P7 tPA−/− mice injected with the dye (Fig. S6 A, B, D, and E), but not P7 mice injected with saline (Fig. S6C) or adult tPA−/− mice injected with Evans Blue (Fig. S6F), indicating that the BBB was incomplete at P7. Therefore, tPA could be passively transported through the immature BBB into the CNS parenchyma.
tPA-Deficient Mice Are Resistant to ND Induced by MK801 but Not Diazepam.
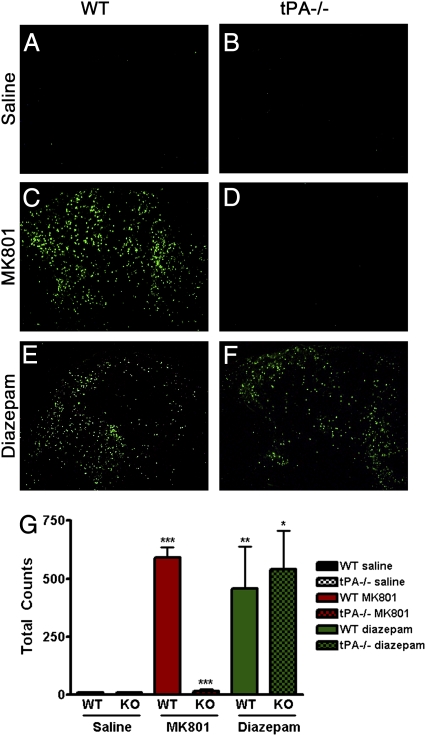
Ethanol reduces synaptic activity through inhibition of the NR and potentiation of the GR. To determine which neurotransmitter system mediates tPA's effects after ethanol administration, we injected P7 WT and tPA−/− mice with saline, diazepam (GR agonist), or MK801 (nonselective antagonist of NR) and analyzed neuronal death (24, 25). Saline did not induce ND in the cortex or thalamus of either mouse line (Fig. 3 A, B, and G). As reported (2), both diazepam and MK801 treatment induced significant neuronal death in the developing WT cortex and thalamus (Fig. 3 C, E, and G). tPA−/− mice demonstrated almost complete neuroprotection after MK801 administration (Fig. 3 D and G), but there was significant ND in the thalamus (Fig. 3 F and G; P < 0.0001) and cortex (P < 0.005) of tPA−/− mice after diazepam administration compared with saline. Furthermore, the level of diazepam-induced cell death observed in the tPA−/− thalamus (P = 0.75) and cortex (P = 0.06) was consistent with that of WT. These findings suggest that tPA mediates ethanol-induced ND through the NR in WT mice.
Fig. 3.
tPA−/− mice are resistant to MK801-induced ND. P7 WT and tPA−/− mice were injected with saline, the nonselective NR antagonist MK801, or the GR agonist diazepam. Both diazepam and MK801 treatment induced extensive ND in the developing WT thalamus (A vs. C and E; ***P < 0.0001, **P = 0.007). tPA−/− mice demonstrated almost complete neuroprotection after MK801 administration (C vs. D; ***P < 0.001). However, there was ND in tPA−/− mice after diazepam administration (B vs. F; *P = 0.019). Although there was less cell death in tPA−/− mice than in WT after diazepam administration, this difference was not significant (P = 0.75). Bar graph in G represents raw cell death counts in images from thalamus. Results were similar in cortex.
tPA−/− Mice Are Resistant to ND in the Presence of NR2B-Specific Blockade.
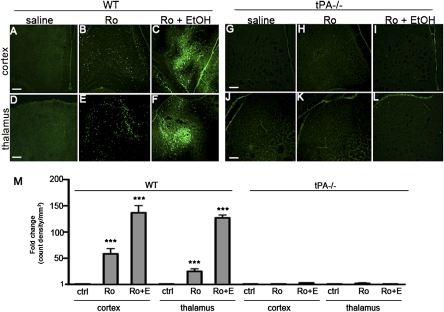
Because NR2B is the prominent NR2 subunit during synaptogenesis, and tPA interacts with NR2B in the adult brain (9, 13), we examined ND after injection of the NR2B-specific antagonist Ro25-6981 (26) into P7 pups. Saline treatment did not cause ND (Fig. 4 A, D, G, and J), but Ro25-6981 treatment induced significant ND in WT (Fig. 4 B, E, and M) mice. However, tPA−/− mice were protected from Ro25-6981-induced ND (Fig. 4 H, K, and M). We investigated if Ro25-6981 and ethanol would act synergistically in WT mice. Pups were treated with Ro25-6981 30 min before treatment with ethanol. Ethanol potentiated Ro25-6981-induced ND in the cortex and thalamus (Fig. 4 C, F, and M). We administered the same treatment combination to tPA−/− pups in an attempt to potentiate inhibition of the NR system in these mice, but tPA−/− mice remained protected (Fig. 4 I, L, and M), further suggesting that tPA promotes ND via an NR2B-dependent mechanism.
Fig. 4.
tPA−/− mice are resistant to ND in the presence of NR2B-specific blockade. We examined ND after treating P7 WT and tPA−/− pups with the NR2B-specific antagonist Ro25-6981. Saline treatment did not induce ND in WT or tPA−/− mice (A, D, G, and J). Treatment with Ro25-6981 induced significant ND in the cortex (B and M) and thalamus (E and M) of WT mice after drug treatment (***P < 0.001). tPA−/− mice were protected from Ro25-6981 ND in both cortex (H) and thalamus (K; P > 0.05). Mice were also treated with Ro25-6981 30 min before treatment with ethanol. Ethanol potentiated Ro25-6981-induced ND in the cortex (C and M) and thalamus (F and M; ***P < 0.001), whereas tPA−/− mice remained protected from ND (I, L, and M; P > 0.05).
tPA can regulate NR function (9, 13, 27), and we found that NR2B expression changed in response to ethanol. Western blots of forebrain homogenates from P7 tPA−/− and WT mice showed no difference in basal NR2B expression. However, NR2B was up-regulated 24 h after ethanol exposure in both genotypes, but even more so in tPA−/− animals (Fig. S7). Furthermore, tPA activity was examined after administration of diazepam or MK801 in WT mice, and we found that MK801 exposure increased tPA activity, whereas diazepam treatment did not (Fig. S8). These data further suggest a correlation between tPA and NR2B.
tPA-Deficient Adult Mice Are Protected from Cognitive Defects Induced by Early Postnatal Ethanol Exposure.
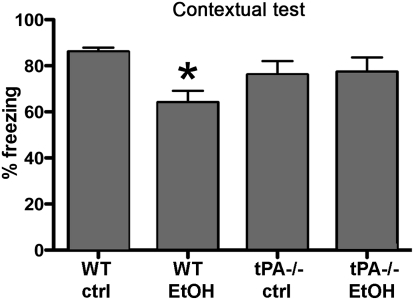
We evaluated the cognitive ability of adult mice exposed to ethanol or saline at P7 by fear conditioning. There were no differences in baseline freezing between genotypes or treatments (Fig. S9A). Consistent with previous reports (16), ethanol-exposed WT mice had ~20% reduction in freezing behavior in contextual fear conditioning compared with the WT saline group (Fig. 5). In contrast, tPA−/− mice that received ethanol at the same age did not show impairments compared with their saline-treated counterparts (Fig. 5). There were no significant differences in freezing behavior during cued fear conditioning (Fig. S9B). These data demonstrate that after exposure of the immature brain to a binge-like dose of ethanol, the absence of tPA protects mice from long-term effects on the brain and behavior. These properties are consistent with the effects of tPA on FAS-related ND and suggest a role for tPA as a regulator of ethanol-induced neurotoxicity and FAS development.
Fig. 5.
Neonatal ethanol exposure leads to learning and memory deficits in WT, but not tPA−/−, mice. We evaluated the cognitive ability of adult mice exposed to ethanol or saline at P7. WT mice that had undergone ethanol treatment at P7 had a significant reduction in freezing behavior in contextual fear conditioning compared with the WT saline treatment group (*P < 0.05). In contrast, tPA−/− mice that received ethanol at the same age did not show impairments compared with their saline-treated counterparts (P > 0.05).
Discussion
The effects of ethanol on the developing brain may result in lifelong behavioral and cognitive complications. The behavioral sequela—specifically, the cognitive deficits that are associated with FAS—originate from massive forebrain degeneration resulting from high doses of ethanol. Our data identify tPA as a key regulator of the neurotoxic effects of alcohol in a murine model of FAS.
Imaging studies of humans with FAS show microencephaly (28), reductions in cortical gray matter (29), decreased basal ganglia size (30), and abnormal development or absence of the corpus callosum (31). In our experiments, WT mice showed >100-fold increase in FJB staining in the thalamus and cortex after ethanol exposure. In comparison, tPA−/− mice treated with ethanol showed less than a fourfold increase in FJB staining. Concomitantly, activation of caspase-3, an effector molecule in the apoptotic cascade, was observed in WT but not tPA−/− mice. Blood ethanol levels were similar in WT and tPA−/− mice after treatment, and plasma concentrations were consistent with those required to induce ND (2), indicating both genotypes have similar ethanol metabolism rates.
The NR is critical for proper developmental synaptogenesis (32). Blocking the NR exacerbates the basal rate of apoptosis that occurs developmentally and impairs the formation of synapses and neuronal circuitry. Our data show that ethanol, the nonspecific NR antagonist MK801, and the NR2B-specific NR antagonist Ro25-6981 are unable to induce ND in tPA−/− mice, suggesting NR function is abnormal in these mice. As reported and consistent with our results, enhanced basal NR currents were detected in tPA−/− cortical cultures from adult mice (33). The underlying cause for increased excitatory signaling in these mice remains unclear.
Early exposure to ethanol interferes with synaptic contact formation, affecting development of neural circuits. The cortex, thalamus, and hippocampus are regions vulnerable to ethanol-induced ND during synaptogenesis. Consistent with previous reports, we show that neonatal exposure of ethanol to WT, but not tPA−/−, mouse brain results in decreased contextual fear conditioning, a learning task that depends on intact hippocampal function (34). The hippocampus is essential for memory storage, and the thalamus, typically referred to as the brain's relay station, allows communication between the cortex and other brain regions, including the hippocampus. Therefore, it is expected that ethanol-induced ND in these areas is followed by behavioral deficits.
It remains unclear what triggers increased tPA in the WT brain. tPA is not a constitutively secreted protease; it is stored in dense core vesicles and released into the extracellular space upon depolarization (35). In the adult brain, tPA-induced pathology is triggered by excitotoxicity, as in ethanol withdrawal-induced ND (13). The sequence of events in the adult points to a positive feedback mechanism between NR and tPA initially stimulated by chronic ethanol. However, this mechanism does not account for what we observed in our model with neonatal animals, because ethanol exposure was acute and not chronic.
Overall, our study suggests that tPA regulates the NR and the apoptotic cascade that is crucial in FAS pathogenesis. Pharmacological manipulation of tPA in the brain might be a unique therapeutic avenue for the treatment of FAS.
Materials and Methods
Animals.
C57/BL6/J WT and tPA−/− mice backcrossed for 10 generations were used. Mouse pups were used at P7. Animal procedures were approved by the Institutional Animal Care and Use Committee.
Materials.
Human recombinant tPA was from Genentech, and plasminogen was isolated from human plasma provided by the New York Blood Center (36). S481A tPA was from Molecular Innovations; MK801 from Calbiochem; Ro25-6981 from Tocris; and diazepam from Sigma.
Acute Ethanol Treatment.
P7 pups were injected s.c. with two injections of 2.5 g/kg ethanol (20% solution) at 0 and 2 h. Controls were injected with saline. Groups of mice were killed at the desired time point after the second ethanol injection, and brains were analyzed histologically for ND with FJB.
S481A tPA, MK801, Ro25-6981, and Diazepam Treatments.
S481A tPA (1 μg/g of body weight) was administered 30 min before the first ethanol injection. Diazepam was administered at 30 mg/kg, MK801 at 1.5 mg/kg, and Ro25-6981 at 6 mg/kg. All reagents were administered i.p. Mice were killed 24 h following treatments, and brains were analyzed for ND.
tPA In-Gel Zymography.
Saline- and ethanol-treated WT mice were perfused with saline. Forebrains were collected at 0.5, 1, 3, 6, or 24 h after the second ethanol injection. Tissue was homogenized in 100 mM Tris containing 0.2% Triton X-100 and phosphatase inhibitors, and homogenates were centrifuged at 14,000 × g for 20 min. Zymographies were carried out as described (9). Recombinant tPA was used as a positive control. Bands representing tPA activity were quantified with National Institutes of Health Scion software. Fold change is the ratio of the measured value for an experimental sample to the control sample.
tPA Fluorescent in Situ Zymography.
To determine extracellular tPA activity in neonatal mice, 20-μm sections were incubated with an overlay containing plasminogen and fluorescently quenched casein (Molecular Probes), which fluoresces green when cleaved by plasmin. Coverslipped slides were incubated at 37 °C for 2 h. Images were acquired using the fluorescein filter to visualize lysis area.
PAI-1 ELISA.
Forebrain homogenates from saline- and ethanol-treated WT mice were collected at 0.5, 1, 3, 6, or 24 h after the second ethanol treatment. ELISAs were performed according to the manufacturer (Molecular Innovations).
Western Blotting.
For information, see SI Materials and Methods.
Cleaved Caspase-3 Homogenization Buffer.
Forebrains were lysed in boiling SDS buffer. Homogenates were centrifuged at 14,000 × g for 20 min, and the supernatant collected for Western blotting.
tPA Immunostaining.
P7 tPA−/− mice treated with S481A tPA and ethanol (or saline) were anesthetized and perfused with saline and 4% PFA. Brains were removed, postfixed, and immersed in 30% sucrose. After sectioning, slices were blocked (1% BSA, 5% goat serum, and 0.5% Triton X-100 in PBS) for 4 h at room temperature followed by incubation in primary antibody, which had been preabsorbed on tPA−/− tissue for 4 h at room temperature (37). Slides were washed and incubated with FITC-tagged secondary antibody.
Antibodies.
Rabbit anti-cleaved caspase-3 was from Cell Signaling, and polyclonal anti-tPA was from Molecular Innovations or American Diagnostica. All antibodies were used at 1:500 in 5% milk.
Fluoro-Jade B Staining.
Twenty-four hours after treatment, mice were anesthetized and perfused with saline and 4% PFA. Brains were removed and postfixed for 48 h, followed by immersion in 30% sucrose. Brains were frozen and sectioned at 40 μm. Sections were collected in PBS, mounted on slides, and air-dried for 24 h, followed by FJB staining as described (38). Slides containing sections obtained from the same experiment were processed concurrently.
FJB Quantitation and Analysis.
After FJB staining, the slides were observed on a Zeiss Axiovert 200 microscope. Cells were counted on one tissue section of six (240 μm apart). National Institutes of Health Image J software was used to trace and examine the cingulate cortex (Bregma from 1.34 to −0.82), thalamus (−1.06 to −1.46), and CA1 of the hippocampus (−1.22 to −1.84). Images were converted to 1 bit, the background subtracted from the image, and the relative area densities (counts/mm2) of FJB-positive cell profiles was determined. The values (counts/mm2) obtained from ethanol-treated samples were analyzed with factorial ANOVA (GraphPad Prism software) and represented either as fold-change compared with the values from saline-treated samples or raw values.
See SI Materials and Methods for blood ethanol levels, Evans Blue extravasation, contextual fear conditioning, and statistical analyses.
Supplementary Material
Acknowledgments
We thank Dr. Bruce McEwen's laboratory at The Rockefeller University for the use of its behavioral room and equipment. This work was supported by National Institutes of Health Grant AA014630.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017608108/-/DCSupplemental.
References
- 1.Famy C, Streissguth AP, Unis AS. Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects. Am J Psychiatry. 1998;155:552–554. doi: 10.1176/ajp.155.4.552. [DOI] [PubMed] [Google Scholar]
- 2.Ikonomidou C, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 3.Ikonomidou C, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 4.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 5.Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: Effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- 6.Dikranian K, et al. Apoptosis in the in vivo mammalian forebrain. Neurobiol Dis. 2001;8:359–379. doi: 10.1006/nbdi.2001.0411. [DOI] [PubMed] [Google Scholar]
- 7.Olney JW, et al. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12:488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baranes D, et al. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 9.Norris EH, Strickland S. Modulation of NR2B-regulated contextual fear in the hippocampus by the tissue plasminogen activator system. Proc Natl Acad Sci USA. 2007;104:13473–13478. doi: 10.1073/pnas.0705848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 12.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 13.Pawlak R, Melchor JP, Matys T, Skrzypiec AE, Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc Natl Acad Sci USA. 2005;102:443–448. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young C, et al. Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell Death Differ. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- 15.Grenett HE, et al. Identification of a 251-bp fragment of the PAI-1 gene promoter that mediates the ethanol-induced suppression of PAI-1 expression. Alcohol Clin Exp Res. 2001;25:629–636. [PubMed] [Google Scholar]
- 16.Wozniak DF, et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AP, et al. The proapoptotic BH3-only, Bcl-2 family member, Puma is critical for acute ethanol-induced neuronal apoptosis. J Neuropathol Exp Neurol. 2009;68:747–756. doi: 10.1097/NEN.0b013e3181a9d524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 19.Widmer F, Caroni P. Phosphorylation-site mutagenesis of the growth-associated protein GAP-43 modulates its effects on cell spreading and morphology. J Cell Biol. 1993;120:503–512. doi: 10.1083/jcb.120.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996;384:123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Ling EA. Studies of the ultrastructure and permeability of the blood-brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J Anat. 1994;184:227–237. [PMC free article] [PubMed] [Google Scholar]
- 22.Liebner S, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coan EJ, Saywood W, Collingridge GL. MK-801 blocks NMDA receptor-mediated synaptic transmission and long term potentiation in rat hippocampal slices. Neurosci Lett. 1987;80:111–114. doi: 10.1016/0304-3940(87)90505-2. [DOI] [PubMed] [Google Scholar]
- 25.Study RE, Barker JL. Diazepam and (—)-pentobarbital: Fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci USA. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer G, et al. Ro 25-6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 27.Park L, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swayze VW, 2nd, et al. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- 29.Rivkin MJ, et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattson SN, et al. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 31.Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- 32.Benn SC, Woolf CJ. Adult neuron survival strategies—slamming on the brakes. Nat Rev Neurosci. 2004;5:686–700. doi: 10.1038/nrn1477. [DOI] [PubMed] [Google Scholar]
- 33.Park L, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Kim JJ, Thompson RF, Tonegawa S. Hippocampal lesions impair contextual fear conditioning in two strains of mice. Behav Neurosci. 1996;110:1177–1180. doi: 10.1037//0735-7044.110.5.1177. [DOI] [PubMed] [Google Scholar]
- 35.Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deutsch DG, Mertz ET. Plasminogen: Purification from human plasma by affinity chromatography. Science. 1970;170:1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 37.Salles FJ, Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmued LC, Hopkins KJ. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.