Abstract
In mammals, many aspects of metabolism are under circadian control. At least in part, this regulation is achieved by core-clock or clock-controlled transcription factors whose abundance and/or activity oscillate during the day. The clock-controlled proline- and acidic amino acid-rich domain basic leucine zipper proteins D-site-binding protein, thyrotroph embryonic factor, and hepatic leukemia factor have previously been shown to participate in the circadian control of xenobiotic detoxification in liver and other peripheral organs. Here we present genetic and biochemical evidence that the three proline- and acidic amino acid-rich basic leucine zipper proteins also play a key role in circadian lipid metabolism by influencing the rhythmic expression and activity of the nuclear receptor peroxisome proliferator-activated receptor α (PPARα). Our results suggest that, in liver, D-site-binding protein, hepatic leukemia factor, and thyrotroph embryonic factor contribute to the circadian transcription of genes specifying acyl-CoA thioesterases, leading to a cyclic release of fatty acids from thioesters. In turn, the fatty acids act as ligands for PPARα, and the activated PPARα receptor then stimulates the transcription of genes encoding proteins involved in the uptake and/or metabolism of lipids, cholesterol, and glucose metabolism.
Keywords: circadian clock, liver lipid metabolism, nuclear receptors
In mammals, energy homeostasis demands that anabolic and catabolic processes are coordinated with alternating periods of feeding and fasting. There is increasing evidence that inputs from the circadian clock are required in addition to acute regulatory mechanisms to adapt metabolic functions to an animal’s daily needs. For example, mice with disrupted hepatocyte clocks display a hypoglycemia during the postabsorptive phase, supposedly because hepatic gluconeogenesis and glucose delivery into the bloodstream are dysregulated in these animals (1).
The regulation of lipid metabolism is also governed by an interaction between acute and circadian regulatory mechanisms, and the three peroxisome proliferator-activated receptors (PPARα, PPARβ/δ, and PPARγ) play particularly important roles in these processes (2). Among them, PPARα acts as a molecular sensor of endogenous fatty acids (FAs) and regulates the transcription of genes involved in lipid uptake and catabolism. Moreover, it accumulates according to a daily rhythm and reaches maximal levels around the beginning of feeding time (3, 4). For liver and many other peripheral tissues, feeding–fasting rhythms are the most dominant zeitgebers (timing cues) (5, 6). This observation underscores the importance of the cross-talk between metabolic and circadian cycles.
Circadian oscillators in peripheral tissues can participate in the control of rhythmic metabolism through circadian transcription factors, which in turn regulate the cyclic transcription of metabolically relevant downstream genes. The three PAR-domain basic leucine zipper (PAR bZip) proteins, D-site-binding protein (DBP), thyrotroph embryonic factor (TEF), and hepatic leukemia factor (HLF), are examples of such output mediators (for review, see ref. 7). Mice deficient of only one or two members of the PAR bZip gene family display rather mild phenotypes, suggesting that the three members execute partially redundant functions. However, mice deficient of all three PAR bZip genes (henceforth called PAR bZip 3KO mice) have a dramatically reduced life span due to epileptic seizures (8) and impaired xenobiotic detoxification (9).
Genome-wide transcriptome profiling of wild-type and PAR bZip 3KO mice has revealed differentially expressed genes involved in lipid metabolism, many of which are targets of the nuclear receptor PPARα. Here we present evidence for a pathway in which PAR bZip transcription factors connect the accumulation and activity of PPARα to circadian oscillators in liver.
Results
Pparα Expression in PAR bZip 3KO Mice.
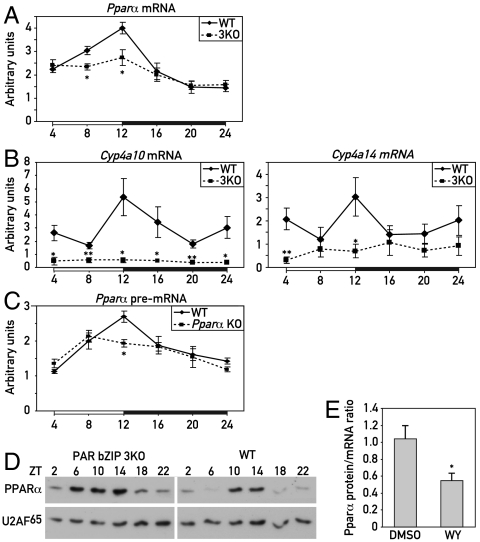
Genome-wide microarray transcriptome profiling studies with liver RNA from wild-type and PAR bZip 3KO mice revealed differentially expressed genes involved in xenobiotic detoxification (9) and lipid metabolism (this paper). The latter included Pparα, a gene specifying a nuclear receptor that is well known as a regulator of lipid metabolism, and many PPARα target genes (10) (Fig. S1A). We validated the reduced accumulation of Pparα mRNA and transcripts issued by PPARα target genes by using quantitative RT-PCR analysis (Fig. 1 A and B and Fig. S1B). The examined PPARα target genes include Cyp4a10 and Cyp4a14, encoding enzymes involved in FA ω-oxidation (whose expression is strongly reduced in Pparα KO mice, see Fig. S2A), and genes specifying enzymes involved in FA β-oxidation (Fig. S1B). PPARα has also been shown to activate transcription from its own promoter, when activated by PPARα agonists (11). To evaluate the relevance of this feed-forward loop in circadian Pparα transcription, we compared the temporal expression of Pparα pre-mRNA in the liver of wild-type mice with that of nonproductive pre-mRNA transcripts issued by the disrupted Pparα alleles in Pparα KO mice (12). As depicted in Fig. 1C, the circadian expression was indeed dampened in these animals, suggesting that PPARα contributed to the rhythmic transcription of its own gene. Therefore, PAR bZip transcription factors may have activated Pparα transcription through an indirect mechanism, for example, by promoting the cyclic generation of PPARα ligands.
Fig. 1.
Expression of PPARα in PAR bZip 3KO mice. (A) Temporal expression of Pparα mRNA in the livers of WT and PAR bZip 3KO mice. RNA levels were estimated by real-time RT-PCR. Mean values ± SEM obtained from six animals are given. (B) Temporal expression of the PPARα target genes Cyp4a10 and Cyp4a14 in the liver of WT and PAR bZip 3KO mice, as determined by real-time RT-PCR. Mean values ± SEM obtained from six animals are given. (C) Temporal expression of Pparα pre-mRNA transcripts in the livers of WT or Pparα KO mice. A PCR amplicon located in the second intron was used in these quantitative RT-PCR experiments. Mean values ± SEM obtained from four animals are given. (D) Temporal expression of PPARα protein in liver nuclear extracts from PAR bZip 3KO and WT mice. Signals obtained with U2AF65 antibody were used as loading controls (U2AF65 is a constitutively expressed splicing factor). (E) Ratio of liver PPARα protein/Pparα mRNA levels after injection of the synthetic PPARα ligand WY14643 or its solvent (50% DMSO) in PAR bZip 3KO mice at ZT2. Mean values ± SEM obtained from six animals are given. The raw data used for these computations are presented in Fig. S3. The zeitgeber times (ZT) at which the animals were killed are indicated (*p ≤ 0.05, **p ≤ 0.01 KO vs. WT, Student’s t test).
Unexpectedly, hepatic PPARα protein accumulation was higher in PAR bZip 3KO mice as compared to wild-type mice, in spite of the lower mRNA levels in the former (Fig. 1D). However, nuclear receptors can be destabilized in a ligand-dependent manner (for review, see ref. 13). Hence, the higher protein to mRNA level in hepatocytes of PAR bZip 3KO mice could indicate that in these animals PPARα was less active and therefore more stable than in the liver of wild-type mice. To examine this conjecture, we measured hepatic PPARα protein and mRNA accumulation, 4 h after an intraperitoneal injection of the synthetic PPARα ligand WY14643 into PAR bZip 3KO mice. As shown in Fig. 1E and Fig. S3, the injection of the PPARα ligand led to a decrease of the protein to mRNA ratio, in keeping with the model of Kamikaze activators postulated by Thomas and Tyers (14). The lower PPARα protein to mRNA ratio in wild-type as compared to PAR bZip 3KO mice may therefore indicate that PPARα had a higher activity in the former animals than in the latter.
PAR bZip Transcription Factors May Stimulate PPARα Activity Through the Production of PPARα Ligands.
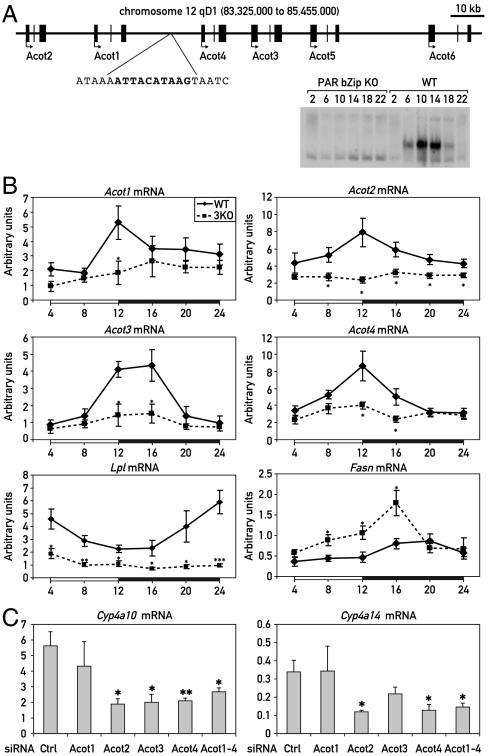
FAs generated by the metabolism of dietary lipids or de novo synthesis are the best known natural ligands for PPARα (15–17). In liver, FAs can be produced through the hydrolysis of acyl-CoA esters by acyl-CoA thioesterases (ACOTs) (18) and through the hydrolysis of lipids in lipoproteins by lipoprotein lipases (LPLs) (19). Interestingly, members of both of these two enzyme families have been reported to accumulate according to a daily rhythm in the liver (20–22), and our genome-wide transcriptome profiling experiments suggested that the mRNAs for these enzymes were expressed at reduced levels in PAR bZip 3KO mice. As shown in Fig. 2B, the accumulation of transcripts specifying ACOTs displayed temporal expression patterns expected for direct PAR bZip target genes and was indeed blunted in PAR bZip 3KO mice. The Acot genes are all located on a 120 kb cluster on mouse chromosome 12, and a perfect PAR bZip DNA binding sequence is located between Acot1 and Acot4 (Fig. 2A). At least in vitro, this sequence binds PAR bZip in a diurnal manner (Fig. 2A), which could explain the rhythmic expression of these genes. However, the phase of Lpl transcript accumulation was found to be delayed by 12 h when compared to that of Acot expression, and we suspected that PAR bZip proteins regulate Lpl transcription via an indirect mechanism. Interestingly, Acot and Lpl reached maximal concentrations at ZT12 and ZT24, respectively, suggesting a bimodal metabolism of FAs in mouse liver: hydrolysis of acyl-CoAs at the day–night transition and hydrolysis of lipids in lipoproteins at the night–day transition.
Fig. 2.
Regulation of the Acot genes cluster and lipid metabolizing enzymes in PAR bZip 3KO. (A) Organization of the mouse Acot gene cluster on chromosome 12. A sequence perfectly matching the PAR bZip consensus binding site is located between Acot1 and Acot4. An EMSA experiment with liver nuclear extracts from WT and PAR bZip 3KO mice shows that PAR bZip transcription factors bind this sequence in a diurnal fashion. (B) Temporal expression of acetyl-CoA thioesterase (Acot) 1–4, lipoprotein lipase (Lpl), and FA synthase (Fasn) mRNA in PAR bZip 3KO mice. Real-time RT-PCR experiments were conducted with whole-cell liver RNAs from six animals for each time point. The zeitgeber times (ZT) at which the animals were killed are indicated. Mean values ± SEM are given. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 KO vs. WT, Student’s t test. (C) Expression of Cyp4a10 and Cyp4a14 mRNA in mouse liver after treatment with siRNAs directed against Acot genes. Real-time RT-PCR experiments were conducted with whole-cell liver RNAs from four (control and individual Acot siRNA) or six animals (pool of the four precedent Acot siRNA). Mean values ± SEM are given (*p ≤ 0.05, **p ≤ 0.005, control siRNA vs. Acot siRNA, Student’s t test).
The transcription of Acots and Lpl has previously been reported to be regulated by PPARα (21–23), and the expression of these genes, in addition to that of Cyp4a10 and Cyp4a14, is activated by injection of WY14643 (Fig. S4). We thus decided to examine the role of PPARα on their diurnal expression by comparing liver RNAs harvested around the clock from Pparα KO and wild-type mice. As shown in Fig. S2B, the overall expression levels of Acots were only slightly decreased in Pparα KO animals for Acot3 and Acot4, not changed for Acot2, but 2.5-fold increased for Acot1. However, zenith levels were reached about 4–12 h later in Pparα KO as compared to wild-type mice. All in all, the changes of Acot and Lpl expression in PPARα deficient mice were complex and reflected perhaps a synergistic regulation by PAR bZip transcription factors and PPARα or other transcription factors.
In the absence of food-derived lipids, PPARα ligands can also be generated de novo by synthesis of FAs by fatty acid synthase (FASN) (24, 25). Interestingly, Fasn expression was enhanced in PAR bZip 3KO animals, supposedly to compensate for the deficient import and/or metabolism of lipids absorbed with the food. Perhaps for the same reasons, the expression of Fabp1 and Cd36, genes encoding proteins involved in FA transport and uptake, was also increased in these mice (Fig. S1B). As described previously (26), Fasn expression was decreased in the liver of Pparα KO mice, probably reflecting a perturbed activation of the sterol-response element binding protein in these animals (27).
Down-regulation of ACOT expression reduces the activity of PPARα target genes.
Our results insinuated that PAR bZip proteins may stimulate the activity of PPARα indirectly. According to this scenario, PAR bZip proteins govern the expression of the ACOT isoforms 1 to 4, which in turn liberate FAs from acyl-CoA esters that may serve as PPARα ligands. In order to examine this possibility, the hepatic expression of ACOT 1 to 4 was down-regulated by the injection of siRNAs into the tail vein (for experimental details, see SI Text, Table S1, and Fig. S5). As shown in Fig. 2C and Fig. S5, a decrease in ACOT2, ACOT3, and ACOT4 expression was sufficient to specifically inhibit the expression of the PPARα target genes Cyp4a10 and Cyp4a14, confirming the role of ACOTs in the activation of PPARα. Likewise, the intravenous application of an equimolar mixture of ACOT1-4 siRNAs specifically reduced the accumulation of Cyp4a10 and Cyp4a14 mRNAs (Fig. 2C and Fig. S5).
Impaired Activity of PPARα in the Liver of PAR bZip 3KO Mice May Be Due to a Deficiency of FAs.
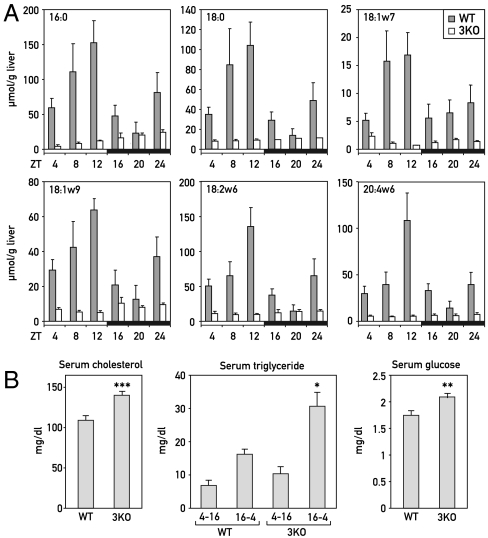
The results presented in the previous section suggested that the down-regulation of ACOTs and LPL in PAR bZip 3KO mice may have caused a decrease in the levels of hepatic FAs that can serve as PPARα ligands. We thus measured the levels of various FAs in the livers of wild-type and PAR bZip 3KO mice. In the former, the concentrations of all examined FAs displayed a robust circadian fluctuation with a maximum at ZT12 (Fig. 3A, gray columns). In addition, a second, smaller peak was observed for most of the FAs. This bimodal distribution was consistent with the hypothesis that the temporal expression of ACOTs and LPL (see Fig. 2) were responsible for the hepatic accumulation of FAs. In PAR bZip 3KO mice, the FA levels were low throughout the day (Fig. 3A, white columns). Again, these results were compatible with a down-regulation of ACOTs and LPL in PAR bZip 3KO mice (Fig. 2B). Importantly, several of the examined FAs had previously been identified as PPARα ligands. For example, C18∶1, C18∶2, and C18∶3 appear to be particularly potent PPARα ligands (15–17, 28), and the decrease in these FAs probably accounted for the down-regulation of PPARα target genes in PAR bZip 3KO animals. The blunted activation of the PPARα pathway in PAR bZip 3KO mice would be expected to manifest itself in a broad dysregulation of hepatic metabolism and associated changes in blood chemistry (26, 29). As depicted in Fig. 3B, PAR bZip 3KO mice showed indeed an increase in the serum concentrations of cholesterol, triglyceride, and glucose, similar to the observations made with Pparα KO mice.
Fig. 3.
Lipid metabolism in PAR bZip 3KO mice. (A) Temporal accumulation of FAs (C16∶0, C18∶0, C18∶1w7, C18∶1w9, C18∶2w6, and C20∶4w6) in the livers of WT and PAR bZip 3KO mice. Mean values ± SEM obtained from four animals are given. The zeitgeber times (ZT) at which the animals were killed are indicated. Note that the profiles of accumulation are daytime dependent for all analyzed FAs in WT animals (ANOVA F[5,18] = 3.29, 3.72, 9.00, 4.50, 3.86, and 4.01, and p ≤ 0.05, 0.025, 0.02, 0.015, 0.025, and 0.025, respectively), whereas they are low and virtually invariable in KO animals. In all the cases, values where statistically different between WT and KO animals (ANOVA F[1,46] = 15.85, 13.11, 10.95, 18.00, 13.96, and 11.62, and p ≤ 0.0005, 0.001, 0.0025, 0.0001, 0.001, and 0.002, respectively). (B) Serum concentrations of triglycerides, cholesterol, and glucose in WT and PAR bZip 3KO animals. Mean values ± SEM obtained from 12 WT and 17 KO animals are given. For triglycerides, values obtained between ZT4 and ZT14 were separated from the values obtained between ZT16 and ZT2, due to their strong circadian variations (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 KO vs. WT, Student’s t test).
PAR bZip 3KO Mice Have an Impaired Capacity to Adapt to Caloric Restriction.
A large number of genes induced by fasting are direct or indirect target genes of PPARα (30, 31), and Pparα KO mice have indeed difficulties in adapting to caloric restriction (29, 32–36). If the activation of the PPARα signaling was inhibited in PAR bZip 3KO mice, one would expect that these animals would also have an impaired capacity to adjust their metabolism to reduced food availability. In order to test this hypothesis, we exposed PAR bZip 3KO mice to a feeding regimen in which the quantity of food was reduced to 60% of what these mice absorbed when food was offered ad libitum. As shown in Fig. S6, PAR bZip 3KO mice subjected to this regimen suffered from a rapid and dramatic weight loss, as compared to wild-type mice. However this difference could not be attributed to a difference in energy expenditure, as O2 consumption and CO2 production were nearly identical in wild-type and PAR bZip 3KO animals (Fig. S7). We also compared the food anticipatory activity (FAA) of wild-type and PAR bZip 3KO mice (Fig. S8 A and B). FAA manifests itself in the onset of enhanced locomotor activity (wheel-running) a few hours before the time when food becomes available. When food availability was limited to a 6-h time span between ZT03 to ZT09, PAR bZip 3KO mice displayed exacerbated FAA and actually shifted a large fraction of their wheel-running activity to this time window during the light phase. As expected, wild-type mice did show FAA but kept running the wheel mainly during the dark phase. These results suggested that the activity associated with food searching equaled or even dominated suprachiasmatic nucleus-driven locomotor activity in PAR bZip 3KO animals when food availability became limiting. Because PPARα KO mice did not show enhanced FAA (Fig. S8C), the exacerbated FAA cannot have been caused solely by the impaired PPARα activity in PAR bZip 3KO mice.
PPARα Ligands Can Be Generated from Food-Derived and de Novo Synthesized Lipids.
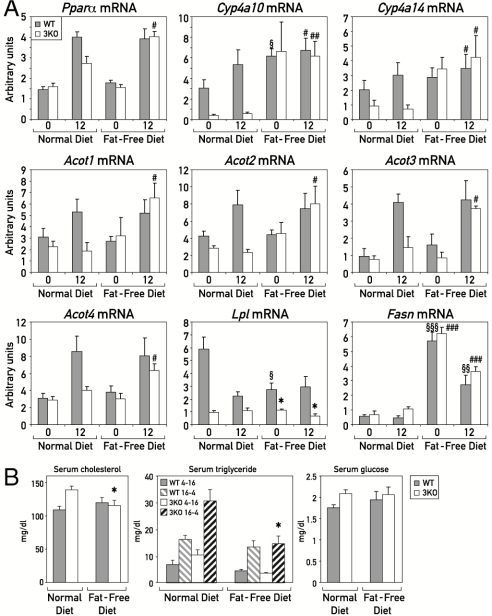
As discussed above, PPARα ligands can be generated from diet-derived lipids or de novo synthesis by FASN, and the first pathway appeared to be deficient in PAR bZip 3KO mice. We wished to determine the expression of putative PPARα target genes and genes with key functions in the production of PPARα ligands in wild-type and PAR bZip 3KO mice that were fed with a fat-free diet during an extended time span (5 wk). Under these conditions, FAs can be produced exclusively through de novo synthesis. As shown in Fig. 4A, the mRNAs of PPARα target genes Cyp4a10 and Cyp4a14 accumulated to similar levels in wild-type and PAR bZip 3KO mice receiving a fat-free diet, unlike what had been observed in animals fed on normal chow. The similar expression of these PPARα target genes in mice receiving a fat-free diet suggested that de novo synthesis of FAs serving as PPARα agonists was not affected by the absence PAR bZip transcription factors, and Fasn mRNA was indeed expressed at similar levels in wild-type and PAR bZip 3KO mice receiving fat-free food. Hence, the fat-free diet rescued the deficiency of PPARα activity in PAR bZip 3KO mice, presumably because de novo synthesis of FAs in liver did not depend upon pathways requiring the circadian PAR bZip proteins. This interpretation was validated by our observation that the hepatic concentrations of various FAs were similar in wild-type and PAR bZip 3KO mice exposed to a fat-free diet (Fig. S9). Interestingly, the expression of Pparα and Acots was also rescued by the fat-free diet in PAR bZip 3KO mice and, in keeping with earlier observations (11, 21, 22), both of these genes were indeed activated by PPARα ligands. Lpl expression did not exhibit large differences between mice fed with normal and fat-free chow. Similarly, blood glucose, cholesterol, and triglyceride levels were not significantly different between wild-type and PAR bZip 3KO mice kept on a fat-free diet (Fig. 4B), unlike what we have observed for animals fed with normal chow.
Fig. 4.
Effect of fat-free diet on PPARα target genes expression and serum biochemistry (A) Mice were fed ad libitum during 5 wk with a fat-free diet. For each condition, four mice were killed at ZT0 and ZT12. Total liver RNAs were extracted and analyzed by real-time RT-PCR for the expression of mRNAs specified by PPARα target genes and Fasn, a marker gene of lipogenesis that is induced by the fat-free diet (#p ≤ 0.05, ##p ≤ 0.005, ###p ≤ 0.005 fat-free vs. normal diet in 3KO; §p ≤ 0.05, §§p ≤ 0.01, §§§p ≤ 0.00005 fat-free vs normal diet in WT; *p ≤ 0.05 KO vs. WT, Student’s t test). (B) Serum concentrations of triglycerides, cholesterol, and glucose were measured in WT and PAR bZip 3KO animals fed with regular or fat-free chow. Mean values ± SEM obtained from eight WT and KO animals are given. For FAs, values obtained between ZT4 and ZT14 were separated from the values obtained between ZT16 and ZT2 (*p ≤ 0.05 fat-free vs. normal diet in 3KO).
Discussion
PAR bZip Transcription Factors DBP, HLF, and TEF Regulate Circadian PPARα Activity.
Here we present evidence for a metabolic clock output pathway operative in hepatocytes, which connects the PAR bZip transcription factors DBP, HLF, and TEF to the circadian activity of PPARα. This nuclear receptor has long been known to play a key role in the coordination of lipid metabolism and, like several other nuclear receptors, it accumulates in a circadian manner (3, 4). Our studies revealed that Pparα mRNA levels were reduced in PAR bZip 3KO mice. However, PPARα protein accumulated to higher than wild-type levels in these animals, presumably due to its reduced transactivation potential.
Our gene expression studies, combined with hepatic FAs measurements, offered a plausible biochemical pathway for the PAR bZip-dependent activation of PPARα , schematized in Fig. 5. PAR bZip proteins drive directly or indirectly the expression of Acots and Lpl, which in turn release FAs from acyl-CoA esters and lipoproteins, respectively. FAs then serve as ligands of PPARα and initiate a feed-forward loop, in which PPARα enhances transcription from its own gene. This scenario is supported by our observation that the siRNA-mediated dampening of ACOT2, ACOT3, and ACOT4 expression led to a down-regulation of the expression of Cyp4a10 and Cyp4a14, two bona fide target genes of PPARα.
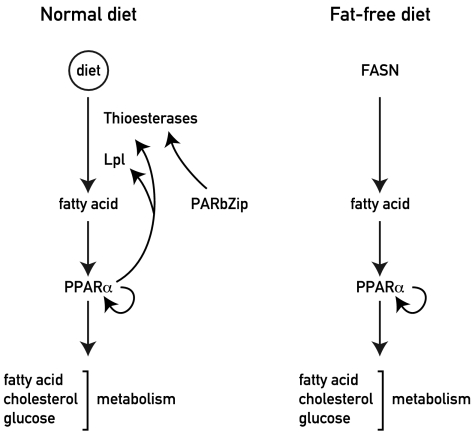
Fig. 5.
Model showing the regulation of PPARα by metabolism and PAR bZip transcription factors. (Left) Under normal diet conditions, the expression of ACOTs are under the control of circadian PAR bZip transcription factors. These transcription factors thus control the release of free FA from acyl-CoA thioesters, and the free FAs stimulate PPARα activity. The activated PPARα then stimulates transcription of Acot and Lpl, and in a feed-forward loop reinforces its own expression and activity. (Right) Under a fat-free diet, all free FAs are derived from the de novo synthesis pathway. Under these conditions, PPARα activity is not dependent on PAR bZip transcription factors.
The accumulation cycles of Acots and Lpl mRNA had widely different phases, yet both were strongly attenuated in PAR bZip 3KO mice. Whereas the phase of Acot expression was compatible with that expected for direct PAR bZip target genes, Lpl mRNA reached maximal levels at a time (ZT24) when all three PAR bZip proteins were expressed at nadir values. We thus suspect that Lpl transcription was controlled by a complex pathway, in which the precise roles of PPARα and PAR bZip proteins remain to be clarified. The temporal accumulation of most determined FAs revealed a major peak at ZT12, when Acots were maximally expressed, and a minor peak at ZT24, when Lpl was maximally expressed. The control of FAs catabolism through oxidation and lipid uptake are major functions of PPARα. On first sight, the low hepatic FAs levels in PAR bZip 3KO mice, in which PPARα activity appeared to be blunted, was perhaps surprising. However, this apparent conundrum can be rationalized as follows. Free FAs are natural ligands for PPARα, and a minimal FA threshold concentration may thus be required for the activation of PPARα (15–17, 28). Moreover, acyl-CoA esters antagonize the activation of PPARα by free FAs (37, 38). Because, due to the reduced expression of Acots in PAR bZip 3KO mice, these esters were probably less efficiently hydrolyzed, the ratio of free FAs to acyl-CoA esters is expected to be lower in these animals as compared to wild-type mice. The attenuation of PPARα activity in the PAR bZip 3KO mice is expected to be associated with an impaired uptake of FAs from the blood (39–41).
PPARα expression has first been found to follow a daily rhythm by Lemberger et al. (3). Subsequently, Oishi et al. (42) demonstrated that the core-clock transcription factor CLOCK is required for circadian Pparα transcription and that CLOCK binds to a series of E-box sequences within the first intron. This regulation by CLOCK might explain why Pparα expression is still circadian in PAR bZip 3KO mice, albeit with reduced amplitude and magnitude.
PPARα Target Gene Expression is Rescued in PAR bZip 3KO Mice Fed with a Fat-Free Diet.
In animals kept on a fat-free diet, hepatic FA synthesis is strongly induced (43). We thus suspected that the intracellular availability of FAs rescued PPARα-mediated transcription in PAR bZip 3KO mice. Indeed, the production of mRNAs encoding enzymes implicated in FAs synthesis, such as FASN, was strongly induced in wild-type and PAR bZip 3KO mice receiving a fat-free diet. Furthermore, in contrast to mice fed on a normal chow, PAR bZip 3KO and wild-type animals fed on a fat-free diet accumulated similar hepatic levels of mRNAs specified by Pparα, and the putative PPARα target genes Cyp4a10 and Cyp4a14. We did notice, however, that Acot expression, whose overall magnitude was only slightly changed in Pparα KO mice, was also rescued in PAR bZip 3KO mice kept on a fat-free diet. Hence, as previously suggested (21, 22), Acot transcription was also augmented by PPARα, but probably required high concentrations of natural ligands (i.e., FAs). It is noteworthy that 1-palmitoyl-2-oleoly-sn-glycerol-3-phosphocholine, whose FASN-dependent synthesis was activated under a fat-free diet, has recently been discovered as a highly potent PPARα ligand (24).
PAR bZip 3KO Mice Are Unable to Adapt to Restricted Feeding.
Wild-type mice exposed to caloric restriction lost about 13% of their body mass during the first 3 wk and then kept their mass within narrow boundaries over several months. In contrast, PAR bZip 3KO animals rapidly lost more than 20% of their weight and had to be killed after about a week, because they probably would have succumbed to wasting after this time period. At least in part, the failure of PAR bZip deficient mice may be due to a decreased PPARα activity, as Pparα KO mice have been reported to adapt poorly to calorie restriction (29, 32–36). However, not all phenotypes of PAR bZip 3KO mice related to feeding could be assigned to an impaired PPARα activity. Thus, in contrast to PAR bZip 3KO mice, Pparα KO mice did not exhibit an exacerbated FAA.
The capacity to adapt activity and metabolism to feeding–fasting cycles is primary to an animal’s health and survival, and the disruption of the circadian timing system has indeed been linked to obesity and other metabolic disorders (44–46).
Experimental Procedures
Animal Housing Conditions.
All animal studies were conducted in accordance with the regulations of the veterinary office of the State of Geneva and of the State of Vaud. PAR bZip 3KO mice with disrupted Dbp, Tef, and Hlf genes (8) and mice with Pparα null alleles (12) have been described previously. Mice were maintained under standard animal housing conditions, with free access to food and water, and a 12-h-light–12-h-dark cycle. Specific treatments and feeding regimens are described in SI Text.
Blood Chemistry.
Blood samples were harvested after decapitation of the animals, and sera were obtained by centrifugation of coagulated samples for 10 min at 2,000 × g at room temperature. The sera were stored at -20 °C until analyzed. Triglycerides and total cholesterol were measured using commercially available enzymatic kits according to the manufacturer’s instructions (Triglyceride; Cholesterol; Roche/Hitachi Mannheim GmbH). Glucose was measured using the glucose oxidase method adapted to rodent (GO assay kit Sigma-Aldrich, Handels GmbH).
Liver FA Measurement:
Mouse livers were homogenized in 0.5 mL of phosphate buffered saline and 0.5 mL of methanol. This procedure inhibits triglycerides lipases and allows their elimination. Each sample was immediately spiked with 50 nmol of 15∶0 FAs as an internal standard. Subsequently, lipids were extracted according to Bligh and Dyer (47) and FAs were then measured by GC-MS as described in SI Text.
RNA Isolation and Analysis.
Livers were removed within 4 min after decapitation, frozen in liquid nitrogen, and stored at -70 °C until use. The extraction of whole-cell RNA and its analysis by real-time RT-PCR were conducted as described previously (8). The values were normalized to those obtained for Gapdh mRNA. Sequences of the oligonucleotides used are given in Table S2.
Preparation of Nuclear Protein Extracts and Western Blotting.
Liver nuclear proteins were prepared by using the NaCl-Urea-NP40 procedure (48). Western blotting was carried out as described (9). The rabbit anti-PPARα and murine anti-U2AF65 antibodies were purchased from Cayman Chemical and Sigma, respectively.
Supplementary Material
Acknowledgments.
We thank Nicolas Roggli for the artwork, and Joel Gyger and Bernard Thorens from the Mouse Metabolic Facility of the University of Lausanne for indirect calorimetry experiments. This research was supported by the Swiss National Science Foundation through individual research grants (U.S., W.W., and F.G.) and the National Center of Competence in Research Program Frontiers in Genetics (U.S. and W.W.), the Cantons of Geneva and Vaud, The Louis Jeantet Foundation of Medicine, the Bonizzi-Theler-Stiftung (U.S. and W.W.), and the Sixth European Framework Project EUCLOCK (U.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002862108/-/DCSupplemental.
References
- 1.Lamia KA, Storch K-F, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 3.Lemberger T, et al. Expression of the peroxisome proliferator-activated receptor α gene is stimulated by stress and follows a diurnal rhythm. J Biol Chem. 1996;271:1764–1769. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokkan K-A, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 7.Gachon F. Physiological function of PARbZip circadian clock-controlled transcription factors. Ann Med. 2007;39:562–571. doi: 10.1080/07853890701491034. [DOI] [PubMed] [Google Scholar]
- 8.Gachon F, et al. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004;18:1397–1412. doi: 10.1101/gad.301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gachon F, Fleury Olela F, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Leuenberger N, Pradervand S, Wahli W. Sumoylated PPARα mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J Clin Invest. 2009;119:3138–3148. doi: 10.1172/JCI39019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart J-C, Staels B. Characterization of the human PPARα promoter: Identification of a functional nuclear receptor response element. Mol Endocrinol. 2002;16:1013–1028. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- 12.Lee SS, et al. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochette-Egly C. Dynamic combinatorial networks in nuclear receptor-mediated transcription. J Biol Chem. 2005;280:32565–32568. doi: 10.1074/jbc.R500008200. [DOI] [PubMed] [Google Scholar]
- 14.Thomas D, Tyers M. Transcriptional regulation: Kamikaze activators. Curr Biol. 2000;10:R341–R343. doi: 10.1016/s0960-9822(00)00462-0. [DOI] [PubMed] [Google Scholar]
- 15.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krey G, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 18.Hunt MC, Alexson SEH. The role Acyl-CoA thioesterases play in mediating intracellular lipid metabolism. Prog Lipid Res. 2002;41:99–130. doi: 10.1016/s0163-7827(01)00017-0. [DOI] [PubMed] [Google Scholar]
- 19.Ziouzenkova O, et al. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: Evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benavides A, Siches M, Llobera M. Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Am J Physiol. 1998;275:R811–817. doi: 10.1152/ajpregu.1998.275.3.R811. [DOI] [PubMed] [Google Scholar]
- 21.Hunt MC, et al. Involvement of the peroxisome proliferator-activated receptor α in regulating long-chain acyl-CoA thioesterases. J Lipid Res. 2000;41:814–823. [PubMed] [Google Scholar]
- 22.Hunt MC, Solaas K, Kase BF, Alexson SEH. Characterization of an Acyl-CoA thioesterase that functions as a major regulator of peroxisomal lipid metabolism. J Biol Chem. 2002;277:1128–1138. doi: 10.1074/jbc.M106458200. [DOI] [PubMed] [Google Scholar]
- 23.Schoonjans K, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarthy MV, et al. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarthy MV, et al. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Patel DD, Knight BL, Wiggins D, Humphreys SM, Gibbons GF. Disturbances in the normal regulation of SREBP-sensitive genes in PPARα-deficient mice. J Lipid Res. 2001;42:328–337. [PubMed] [Google Scholar]
- 27.Knight BL, et al. A role for PPARα in the control of SREBP activity and lipid synthesis in the liver. Biochem J. 2005;389:413–421. doi: 10.1042/BJ20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor α. Biochemistry. 1999;38:185–190. doi: 10.1021/bi9816094. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T, et al. Defect in peroxisome proliferator-activated receptor α-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 30.Bauer M, et al. Starvation response in mouse liver shows strong correlation with life-span-prolonging processes. Physiol Genomics. 2004;17:230–244. doi: 10.1152/physiolgenomics.00203.2003. [DOI] [PubMed] [Google Scholar]
- 31.Corton JC, et al. Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. J Biol Chem. 2004;279:46204–46212. doi: 10.1074/jbc.M406739200. [DOI] [PubMed] [Google Scholar]
- 32.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki T, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Kersten S, et al. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SST, et al. Requirement of PPARα in maintaining phospholipid and triacylglycerol homeostasis during energy deprivation. J Lipid Res. 2004;45:2025–2037. doi: 10.1194/jlr.M400078-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: The PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elholm M, et al. Acyl-CoA esters antagonize the effects of ligands on peroxisome proliferator-activated receptor α conformation DNA binding, and interaction with co-factors. J Biol Chem. 2001;276:21410–21416. doi: 10.1074/jbc.M101073200. [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen C, et al. Opposing effects of fatty acids and Acyl-CoA esters on conformation and cofactor recruitment of peroxisome proliferator-activated receptors. Ann N Y Acad Sci. 2002;967:431–439. doi: 10.1111/j.1749-6632.2002.tb04299.x. [DOI] [PubMed] [Google Scholar]
- 39.Bremer J. The biochemistry of hypo- and hyperlipidemic fatty acid derivatives: Metabolism and metabolic effects. Prog Lipid Res. 2001;40:231–268. doi: 10.1016/s0163-7827(01)00004-2. [DOI] [PubMed] [Google Scholar]
- 40.Fruchart J-C, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today. 2006;42:39–64. doi: 10.1358/dot.2006.42.1.963528. [DOI] [PubMed] [Google Scholar]
- 41.Martin G, Schoonjans K, Lefebvre A-M, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and Acyl-CoA synthetase genes by PPARα and PPARγ activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- 42.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor α (PPARα) in mice. Biochem J. 2005;386:575–581. doi: 10.1042/BJ20041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Towle HC, Kaytor EN, Shih H-M. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu Rev Nutr. 1997;17:405–433. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 44.Buijs RM, Kreier F. The metabolic syndrome: A brain disease? J Neuroendocrinol. 2006;18:715–716. doi: 10.1111/j.1365-2826.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 45.Staels B. When the clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–55. doi: 10.1038/nm0106-54. [DOI] [PubMed] [Google Scholar]
- 46.Turek FW, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 48.Lavery D, Schibler U. Circadian transcription of the cholesterol 7 α hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993;7:1871–1884. doi: 10.1101/gad.7.10.1871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.