Abstract
To fuse with oocytes, spermatozoa of eutherian mammals must pass through extracellular coats, the cumulus cell layer, and the zona pellucida (ZP). It is generally believed that the acrosome reaction (AR) of spermatozoa, essential for zona penetration and fusion with oocytes, is triggered by sperm contact with the zona pellucida. Therefore, in most previous studies of sperm–oocyte interactions in the mouse, the cumulus has been removed before insemination to facilitate the examination of sperm–zona interactions. We used transgenic mouse spermatozoa, which enabled us to detect the onset of the acrosome reaction using fluorescence microscopy. We found that the spermatozoa that began the acrosome reaction before reaching the zona were able to penetrate the zona and fused with the oocyte's plasma membrane. In fact, most fertilizing spermatozoa underwent the acrosome reaction before reaching the zona pellucida of cumulus-enclosed oocytes, at least under the experimental conditions we used. The incidence of in vitro fertilization of cumulus-free oocytes was increased by coincubating oocytes with cumulus cells, suggesting an important role for cumulus cells and their matrix in natural fertilization.
Keywords: sperm–egg interaction, acrosomal exocytosis, real-time imaging, video microscopy
Fertilization is a critical moment in animal sexual reproduction and comprises sequential steps in the interaction of sperm with an egg. In eutherian mammals, natural fertilization is accomplished by sperm ascent to the oviduct ampulla, passage through the cumulus oophorus surrounding oocytes, loose binding to the oocyte's outer envelope, the zona pellucida (ZP), followed by tight sperm binding to the ZP and penetration through the ZP, culminating in sperm–oocyte membrane fusion (1). Before penetrating into the ZP, the fertilizing spermatozoon must undergo a morphological change involving disruption of the acrosome called the acrosome reaction (AR). In most studies of mouse sperm–oocyte interactions in vitro, the cumulus oophorus is removed by hyaluronidase treatment before insemination, allowing direct sperm contact with the ZP upon insemination. However, under natural in vivo conditions, spermatozoa first encounter the cumulus before reaching the ZP surface. The question this paper addresses is: Where does the fertilizing mouse spermatozoon begin the AR—in the cumulus or at the ZP?
In the mouse, it has been reported that a ZP glycoprotein, ZP3, has both sperm-binding (sperm receptor) and AR-inducing activities and is essential for formation of the zona pellucida and therefore fertility (2, 3). The specificity of sperm binding to homologous zona might be conferred by the supramolecular structure (reviewed in ref. 4) or cleavage status of ZP glycoproteins (5). It is generally assumed that—at least in the mouse—the AR occurs after sperm binding to the ZP (6, 7). Even though spermatozoa of several species other than the mouse are known to be able to undergo the AR on the ZP surface (8–10), the presence of motile spermatozoa within the cumulus at various stages of the AR in various species has also been noted by several investigators (11–14). This prompted us to reexamine the site of the AR in those mouse spermatozoa that actually participate in fertilization. We used a video microscopic in vitro fertilization (VMIVF) system, which allowed us to determine the site of the AR in fertilizing mouse spermatozoa, retrospectively (Fig. 1). We used double-transgenic male mice whose spermatozoa express enhanced green fluorescent protein (EGFP) in their acrosomes and red fluorescent protein (Ds-Red2) in their midpiece (mitochondria) (15). This allowed us to follow the changes in sperm acrosomes with ease before and after sperm contact with the cumulus and ZP. Because we were able to switch illumination from ordinary light to fluorescence-inducing wavelengths very quickly (10 ms), we could determine the status of the acrosome of individual spermatozoa at any moment during sperm–oocyte interactions. We emphasize that we used oocytes with an intact cumulus oophorus.
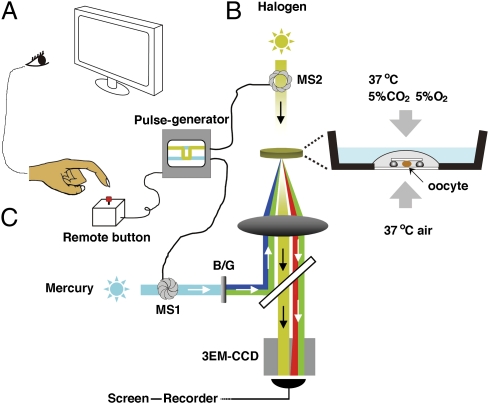
Fig. 1.
The video microscopic in vitro fertilization system consists of three components. (A) Oocyte-holding Petri dish (Materials and Methods). An oocyte surrounded by cumulus cell mass was slightly compressed under a 3 × 3 mm coverslip supported by four silicon grease spots. The dish was placed into an incubation chamber supplied with a mixed gas (5% CO2, 5% O2, and 90% N2). (B) Mercury–halogen illumination system: two sets of mechanical shutters (VS25; Uniblitz), one near the mercury lamp (MS1) and the other next to the halogen lamp (MS2), were controlled via a pulse generator. (C) Image-capturing system: a supersensitive video camera (NC-R550b; NEC) and a blue/green dual bandpass filter set enabled simultaneous visualization of EGFP and Ds-Red2 fluorescence in swimming spermatozoa. All spermatozoa entering the cumulus were viewed first at low magnification (10× objective lens). When a spermatozoon was seen approaching the oocyte, it was then focused under higher magnification (20× objective lens; Fig. S6). The spermatozoon under observation was exposed to blue/green light intermittently (125 ms) by pressing a remote shutter control button. After about 90 min of recording, all of the spermatozoa were individually analyzed for the status of their acrosomes. Motile (live) spermatozoa with EGFP fluorescence in their acrosomes were considered “acrosome intact” and those without EGFP fluorescence were considered “acrosome reacted.”
Results
Acrosome Reaction Detected by EGFP Fluorescence of the Sperm Head.
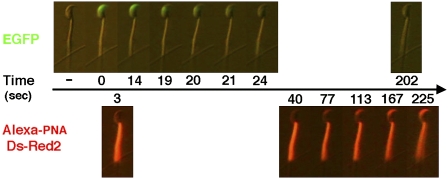
The AR of mammalian spermatozoa involves multiple fusions between the outer acrosomal membrane and the overlying plasma membrane, followed by the exposure and release of intraacrosomal materials (1, 16). According to Nakanishi et al. (17), EGFP that is expressed by the spermatid stage of spermatogenesis and is destined to accumulate in the acrosome disappears from the sperm head at about 3 s after the onset of the AR in vitro. We found previously that the disappearance of EGFP was immediately followed by the appearance of intraacrosomal materials that had strong affinity to Alexa 594-conjugated peanut agglutinin (PNA) (Fig. 2) (18, 19). We considered that the disappearance of EGFP fluorescence from the acrosomal cap region represented the earliest stage of membrane vesiculation. Therefore, the appearance of PNA lectin-affinity materials on the sperm head must represent more advanced stages of the AR.
Fig. 2.
The progression of acrosomal exocytosis. Capacitated spermatozoa immobilized on a coverslip were incubated with BSA–HTF containing Alexa-594 PNA and thereafter observed under an epifluorescence microscope. Representative time-lapse photographs show a spermatozoon undergoing the acrosomal exocytosis induced by 10 μM ionomycin. Acrosomal EGFP disappearance took place within about 10 s followed by acrosomal staining with PNA lectin.
Behavior of in Vitro Capacitated Spermatozoa Before and During Fertilization, with Particular Reference to the Status of Acrosomes in Fertilizing Spermatozoa.
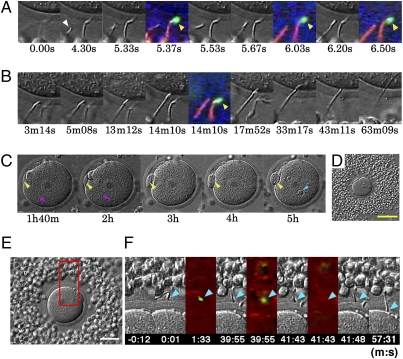
The video microscopic apparatus allowed us to observe spermatozoa continuously, from their first approach to the oocytes until pronuclear development in the penetrated oocytes. In each experiment, a single cumulus-enclosed oocyte was inseminated under a miniature coverslip (Materials and Methods). In 56 experiments, 30 (54%) of 56 oocytes that were illuminated intermittently with blue/green light were fertilized and reached the two-cell stage in 24 h. In the control cohort of 161 oocytes that were not illuminated with blue/green light, 104 (64.6%) cleaved, indicating that the intermittent illumination for fluorescence excitation did not have any detrimental effects on oocytes. In 13 of 30 fertilized oocytes, we could identify the status of acrosomes before sperm binding to the ZP. In 12 of 13 spermatozoa that eventually fertilized successfully, EGFP fluorescence was absent from sperm heads that we considered to have undergone the AR before they bound to the ZP (Fig. 3 A–D and Figs. S1–S4 and Movies S1–S5). Fertilizing spermatozoa began to enter the ZP soon after contact with it (in less than 1 min). Fig. 3 E and F and Movie S6 show a rare case in which an EGFP+ (acrosome intact) spermatozoon attached to the ZP; EGFP fluorescence disappeared from the sperm head (i.e., the AR commenced) about 40 min later. This spermatozoon passed through the ZP within the next 16 min. Approximately half (56.7%) of 120 acrosome-intact spermatozoa remained on the ZP for less than 20 min (the mode of sperm's residence time on the ZP was 1–2 min) before they swam away (n = 21). Others stayed on the ZP for more than 20 min before examinations were terminated. As shown in Table 1, 57 (49.6%) of 115 spermatozoa that had begun the AR before reaching the ZP penetrated it successfully, whereas only 4 (1.9%) of 214 spermatozoa did so when they had intact acrosomes at the time of their first contact with the ZP.
Fig. 3.
Viewing a fertilizing spermatozoon. (A–D) Photographs showing sperm penetration into the ZP and the cytoplasm of a single oocyte. Whether it was the actual fertilizing spermatozoon was determined retrospectively after video recording. Times are shown in seconds (s), minutes (m), and hours (h) after the fertilizing spermatozoon entered the field of view (0 s). (A) Successive stages of sperm attachment to the ZP. The fertilizing spermatozoon (arrowhead at 4.30 s) had no EGPF fluorescence (i.e., was acrosome reacted) before reaching the ZP surface at 6.20 s. (B) The head of this fertilizing spermatozoon passed through the ZP and reached the egg surface at 13 m 12 s. Note that an acrosome-intact spermatozoon (arrowhead at 14 m 10 s) with EGFP fluorescence remained on the ZP surface. (C) The oocyte under low magnification, showing formation of a second polar body (yellow arrowheads), sperm tail (red arrowheads), and sperm pronucleus (blue arrowhead). (D) The cumulus-enclosed oocyte before insemination under low magnification. (Scale bar, 100 μm.) (E and F) Fertilization by a spermatozoon that had undergone the AR after binding to the ZP. (E) A low-magnification view of a cumulus-enclosed oocyte used in this experiment. (Scale bar, 50 μm.) (F) The time of initial sperm binding to the ZP was set at 0. This spermatozoon remained on the ZP surface with an intact acrosome until 39 m 55 s and thereafter lost acrosomal EGFP by the time of the next fluorescence exposure at 41 m 43 s. After the AR, this spermatozoon initiated ZP penetration and fused with the plasma membrane of the oocyte at about 57 m 31 s. Arrowheads point to the sperm head.
Table 1.
The fate of spermatozoa that reached ZP surface
| No. of spermatozoa (%) |
||
| Spermatozoa | Acrosome-reacted before contact with ZP | Acrosome-intact when first contacted with ZP |
| Entered the cumulus and reached ZP surface | 115 | 214 |
| Initiated the AR on ZP | — | 4 (1.9) |
| Penetrated through ZP | 57 (49.6) | 4 (1.9) |
| Developed to sperm pronucleus | 12 (10.4) | 1 (0.5) |
Behavior of in Vivo Capacitated Spermatozoa Before and During Fertilization.
Under natural conditions, spermatozoa undergo capacitation in the female reproductive tract before they become capable of interacting with oocytes. Because the spermatozoa used in the above experiments were collected from the cauda epididymidis and then capacitated in vitro, we suspected that the behavior of these spermatozoa toward the cumulus and ZP could be different from those of spermatozoa capacitated in vivo. Therefore, in the next series of experiments, we examined the behavior of spermatozoa released from the oviducts of mated females (Fig. S5). Because these females were mated soon after hCG injection and killed 12 h later, most if not all of the spermatozoa released from the oviduct's isthmus were believed to be capacitated. Because only very few spermatozoa were released from dissected oviducts, the number that entered the cumulus was small, resembling similar conditions of the physiological sperm–egg ratio in vivo (20). The frequency of sperm entry into the cumulus was 2.0 sperm/h (n = 20, Table S1). Eleven (23%) of 48 spermatozoa we saw in the cumulus had intact acrosomes (EGFP+), whereas the remaining 37 (77%) were acrosome reacted (EGFP−). No acrosome-intact spermatozoa were observed to bind to the ZP. Nine acrosome-reacted spermatozoa stayed bound to the ZP. Only one passed partially through the ZP during our observations (Fig. S5 D and E and Movie S7). Together, these results suggest that under conditions where capacitation is induced naturally, acrosome-reacted spermatozoa reach the ZP through the cumulus more successfully than do intact spermatozoa.
Evidence Suggesting That the Cumulus Oophorus Assists Fertilization Through Interaction with Spermatozoa.
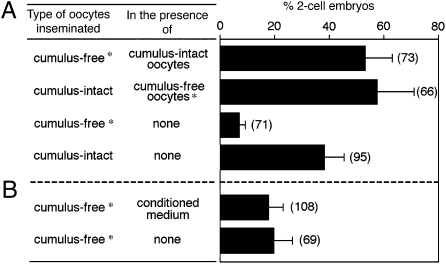
To examine whether the cumulus oophorus affects the sperm's fertilizing ability, we performed experiments in which cumulus-free oocytes were inseminated with or without the copresence of cumulus-enclosed oocytes or cumulus-conditioned medium. Cumulus-free oocytes from transgenic animals were distinguished by Ds-Red2+ mitochondria that exhibited red fluorescence. Sperm concentration in insemination medium was adjusted such that ~10–20% of cumulus-free oocytes were fertilized without the copresence of the cumulus. Cumulus-free oocytes were fertilized at higher rates when cumulus-enclosed oocytes were present in the same insemination droplet (Fig. 4A). However, cumulus-conditioned medium failed to increase the fertilization rate of cumulus-free oocytes (Fig. 4B).
Fig. 4.
The presence of an intact cumulus layer on oocytes increases the fertilizing ability of capacitated spermatozoa. Asterisks denote cumulus-free oocytes from transgenic females whose oocytes express red fluorescence. (A and B) Approximately 10–20 cumulus-free oocytes incubated with or without cumulus-enclosed oocytes (A) or with cumulus–oocyte complex conditioned medium (B) were inseminated at a final concentration of 2 × 104 sperm/mL. The numbers of two-cell embryos per total oocytes examined was scored after 24 h. Shown are the percentages obtained from seven independent experiments. The total number of oocytes scored is indicated in parentheses.
Discussion
Under natural conditions, mammalian spermatozoa undergo the AR and lose/discard the reacted acrosomal carapace before penetrating through the ZP and fusing with the oocyte (1). In the mouse—the most commonly used model for studying the molecular aspects of fertilization in eutherian mammals—analyses using cumulus-free eggs have fostered the belief that the AR occurs on the ZP surface. The key points in support of this belief were the observations that: (i) acrosome-intact spermatozoa bind to the zona surface; (ii) one zona glycoprotein component of the zona, ZP3, can trigger the AR; and (iii) the population of acrosome-reacted spermatozoa on the ZP surface increased steadily with time (7, 21, 22). However, recent investigations using EGFP-expressing spermatozoa from transgenic mice failed to detect the AR on the ZP of cumulus-free oocytes (17, 23). According to Gahlay et al. (5), ZP of transgenic mice (ZP2Mut and ZP3Mut) were unable to induce the AR, yet the oocytes of these mice were still fertilized, indicating that either the AR occurred during sperm passage through the ZP or before reaching it. These findings suggest that the dogma that the physiologically relevant AR occurs at the ZP (24) requires serious reconsideration.
Fertilization in vitro and in vivo is certainly possible without the cumulus oophorus. However, the cumulus seems to be beneficial for fertilization (25–27). According to Toyoda et al. (28), fertilization rates of cumulus-free oocytes in vitro are rather erratic, whereas those of cumulus-enclosed oocytes are high and consistent (figure 30 of ref. 1). Although the ZP—in particular its component ZP3—is certainly capable of inducing the AR under experimental conditions (9, 22, 29), cumulus components also have the ability to prime the ZP-induced AR or even to initiate the AR (30–34). The cumulus matrix might also be important for selecting spermatozoa competent to fertilize oocytes (35). For these reasons, we used cumulus-enclosed oocytes to determine the site of the physiologically relevant AR in those spermatozoa that actually participate in fertilization.
The strength of the present study was threefold. First, we used spermatozoa of transgenic mice and a newly developed fluorescence/differential interference contrast microscopy system, which allowed us to visualize changes in acrosomes continuously without damaging spermatozoa. Second, we could distinguish fertilizing from nonfertilizing spermatozoa by retrospective review of video-recorded images of individual spermatozoa. Third, we used cumulus-enclosed and not “denuded” oocytes. In most eutherian mammals, including the mouse, fertilizing spermatozoa in vivo must encounter the cumulus oophorus first before contacting the ZP.
The results of our observations were rather surprising. The spermatozoa that had intact acrosomes when they first contacted a ZP seldom passed through it, whereas those that began the AR (loss of acrosomal EGFP) before contacting the ZP could readily do so (Table 1). This implies that the physiologically relevant AR starts before spermatozoa contact the ZP. The AR can indeed occur at the ZP surface, but this was the exception rather than the rule. We further found that EGFP− fertilizing spermatozoa did not spend much time on the ZP surface (less than 1 min) before entering it. Oocytes collected from oviducts during fertilization seldom have spermatozoa on or in the ZP (12, 36), indicating that sperm contact and penetration through the ZP must be a rapid process. Because we did not examine spermatozoa using electron microscopy, we are not certain whether EGFP− fertilizing spermatozoa have undergone vesiculation of the acrosomal membranes or have lost the shroud completely (37, 38). In the macaque, the acrosomal shroud remains on sperm bound to the zona surface before zona penetration (39). However, we can assert that EGFP− live spermatozoa have initiated the AR. A possible role of ZP3 in facilitating the complete AR rather than initiating it cannot be ruled out. Nonetheless, it would be interesting to determine how long spermatozoa undergoing the AR maintain their fertilizing ability (37). Notably, it was reported that acrosome-reacted rabbit spermatozoa collected from the perivitelline space of one-cell embryos were still capable of penetrating and fertilizing fresh cumulus-intact oocytes (40). Although this result has yet to be confirmed, it suggests considerably greater persistence of fertilizing ability than is actually needed by the spermatozoon in vivo.
Progesterone secreted by cumulus cells is known to induce or promote the AR of spermatozoa of various species (41–45). Cumulus extracts and hyaluronic acid are able to induce or enhance the AR (31, 46). However, what really triggers the AR of mouse spermatozoa in the cumulus remains to be determined.
Materials and Methods
Media.
Human tubal fluid (HTF) medium and Hepes-buffered HTF designed for human in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) were purchased from InVitroCare. BSA was from Sigma-Aldrich.
Animals.
Spermatozoa of double-gene knockout males [BDF1-Tg (CAG-mtDsRed2, Acr-EGFP) RBGS0020sb] have acrosomal vesicles expressing green EGFP fluorescence and midpieces (mitochondria) expressing red Ds-Red2 fluorescence (15). Those male mice were crossed with imprinting control region (ICR) females and their gametes were used for our observations. In all experiments except for those depicted in Fig. 4, ICR females (at least 7 wk old) were used without induction of superovulation. They were each injected with 5 units of human chorionic gonadotropin (hCG) on the day of proestrus; 14.5 h later, oocytes surrounded by intact cumulus oophorus masses were collected from oviducts. In the experiments shown in Fig. 4, ICR or transgenic females (at least 7 wk old) were induced to superovulate by injection of 5 units of pregnant mare serum gonadotropin (PMSG) followed by an injection of hCG 48 h later. All experiments were performed with the approval of the Animal Care and Use Committee of Ochanomizu University.
Preparation of Cumulus–Oocyte Complexes (COCs).
When first retrieved from the ovary, oocyte–cumulus complexes are separate. However, in the oviduct, they adhere to form a single large cumulus mass. Because this mass was too large to detect fertilizing spermatozoa within it, we separated the mass into smaller individual cumulus–oocyte complexes using a brief (0.5–2.0 min) treatment with bovine testicular hyaluronidase (Sigma-Aldrich; 80 units/mL at 37 °C) with gentle pipetting. In some cases, a large cumulus mass was left in hyaluronidase-free HTF–BSA medium for 1 h (5% CO2, 37 °C), allowing the mass to dissociate spontaneously into several individual cumulus masses. These were washed four times with Hepes-buffered HTF (pH 7.35 ± 0.1) containing 0.3% BSA followed by a final wash with HTF–BSA.
Preparation of Observation Dish.
A round hole (16 mm in diameter) was made at the center of a plastic dish (35 × 13 mm; Iwaki). The outer edge of the hole was covered with molten dental wax before a coverslip was pressed against the bottom of the dish. Similar plastic dishes with glass “windows” are available commercially (Iwaki; 3911-035). Four small spots of silicon grease (ShinEtsu; KS-62M) were placed on the inner surface of the glass window, 3 mm apart. After a single or several cumulus masses in HTF–BSA medium was placed at the center of the grease spots, a small coverslip (3 × 3 mm) was gently placed over the cumulus mass, which was then slightly compressed under the coverslip. The final volume of HTF–BSA medium covering the cumulus mass under the coverslip was 100 μL. The medium was then covered with 700 μL of prewarmed (37 °C) mineral oil (Sage In-Vitro Fertilization). All procedures for handling oocytes were performed under a stereomicroscope (Leica) equipped with a thermal plate warmed to 37 °C. The quality of the glass-bottomed plastic dish, silicon grease, and mineral oil needed to be checked before experiments because some such materials are toxic to gametes.
Sperm Capacitation and Insemination.
Spermatozoa from the cauda epididymidis were induced to capacitate by suspending them in a 100-μL droplet of HTF–BSA medium at ~105 cells/mL and incubating them for 1–3 h at 37 °C under an atmosphere of 5% CO2 and 95% air. Insemination was performed by placing about 1 μL of capacitated sperm suspension (about 2,000 sperm) at the edge of a coverslip overlaying a slightly compressed cumulus–oocyte complex (Fig. 1). When no spermatozoa reached the immediate vicinity of the oocyte within 20 min of insemination, another aliquot of capacitated spermatozoa was added to the same or another edge of the coverslip. Preparations were kept for up to 24 h to examine whether inseminated oocytes developed to the pronuclear or two-cell stage. In some experiments, cumulus-enclosed and cumulus-free oocytes were inseminated together or separately in the dish and cultured for 24 h to determine fertilization rates (on the basis of cleavage). Cumulus-conditioned medium was prepared by incubating 18–24 cumulus-enclosed oocytes in 100-μL aliquots of HTF–BSA medium for 2 h under 5% CO2 in air at 37 °C.
Histochemical Staining of Living Spermatozoa.
Spermatozoa from the cauda epididymidis were suspended in 4 mL of HTF–BSA and incubated under 5% CO2 in air at 37 °C for 1 h. Spermatozoa were then allowed to adhere to Cell-Tak–coated coverslips (BD Biosciences) for 20 min followed by a gentle wash with HTF–BSA. Alexa 594-conjugated PNA lectin (Invitrogen) at a final concentration of 0.2 μg/mL was added to this medium and spermatozoa immobilized on the coverslip were observed by epifluorescence microscopy. Preliminary experiments showed that Alexa-PNA at this concentration did not interfere with fertilization under standard IVF conditions.
Supplementary Material
Acknowledgments
We are grateful to Professor R. Yanagimachi for his invaluable advice during the preparation of the manuscript. We thank Drs. V. D. Vacquier and G. L. Gerton for critically reading the original manuscript. Thanks are due to Drs. T. Sato, T. Kojima, K. Ishii, T. Ebisawa, J. Otsuki, N. Kawano, F. Nakaya, and T. Nishiyama for their technical support. We are indebted to Drs. T. Baba, M. Yamashita, and M. G. Buffone for providing us with antibodies. A long-term free loan of a supersensitive 3EM-CCD camera (NC-R550b) was granted by NEC and the cell culture system for microscopy was a gift from Taiei Electric. This study was supported by the Japan Society for the Promotion of Science (N.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018202108/-/DCSupplemental.
See Commentary on page 4703.
References
- 1.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neil J, editors. The Physiology of Reproduction. New York: Raven; 1994. pp. 189–317. [Google Scholar]
- 2.Liu C, et al. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA. 1996;93:5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rankin T, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996;122:2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- 4.Hoodbhoy T, Dean J. Insights into the molecular basis of sperm-egg recognition in mammals. Reproduction. 2004;127:417–422. doi: 10.1530/rep.1.00181. [DOI] [PubMed] [Google Scholar]
- 5.Gahlay G, Gauthier L, Baibakov B, Epifano O, Dean J. Gamete recognition in mice depends on the cleavage status of an egg's zona pellucida protein. Science. 2010;329:216–219. doi: 10.1126/science.1188178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florman HM, Storey BT. Mouse gamete interactions: The zona pellucida is the site of the acrosome reaction leading to fertilization in vitro. Dev Biol. 1982;91:121–130. doi: 10.1016/0012-1606(82)90015-x. [DOI] [PubMed] [Google Scholar]
- 7.Storey BT, Lee MA, Muller C, Ward CR, Wirtshafter DG. Binding of mouse spermatozoa to the zonae pellucidae of mouse eggs in cumulus: Evidence that the acrosomes remain substantially intact. Biol Reprod. 1984;31:1119–1128. doi: 10.1095/biolreprod31.5.1119. [DOI] [PubMed] [Google Scholar]
- 8.Cherr GN, Lambert H, Meizel S, Katz DF. In vitro studies of the golden hamster sperm acrosome reaction: Completion on the zona pellucida and induction by homologous soluble zonae pellucidae. Dev Biol. 1986;114:119–131. doi: 10.1016/0012-1606(86)90388-x. [DOI] [PubMed] [Google Scholar]
- 9.Schroer SC, Yudin AI, Myles DG, Overstreet JW. Acrosomal status and motility of guinea pig spermatozoa during in vitro penetration of the cumulus oophorus. Zygote. 2000;8:107–117. doi: 10.1017/s0967199400000885. [DOI] [PubMed] [Google Scholar]
- 10.Tollner TL, Yudin AI, Cherr GN, Overstreet JW. Real-time observations of individual macaque sperm undergoing tight binding and the acrosome reaction on the zona pellucida. Biol Reprod. 2003;68:664–672. doi: 10.1095/biolreprod.102.009175. [DOI] [PubMed] [Google Scholar]
- 11.Austin CR, Bishop MW. Some features of the acrosome and perforatorium in mammalian spermatozoa. Proc R Soc Lond B Biol Sci. 1958;149:234–240. doi: 10.1098/rspb.1958.0065. [DOI] [PubMed] [Google Scholar]
- 12.Yanagimachi R. Time and process of sperm penetration into hamster ova in vivo and in vitro. J Reprod Fertil. 1966;11:359–370. doi: 10.1530/jrf.0.0110359. [DOI] [PubMed] [Google Scholar]
- 13.Yanagimachi R, Phillips D. The status of the acrosomal caps of hamster spermatozoa immediately before fertilization in vivo. Gamete Res. 1984;9:1–20. [Google Scholar]
- 14.Bedford JM, Mori T, Oda S. The unusual state of the cumulus oophorus and of sperm behaviour within it, in the musk shrew, Suncus murinus. J Reprod Fertil. 1997;110:127–134. doi: 10.1530/jrf.0.1100127. [DOI] [PubMed] [Google Scholar]
- 15.Hasuwa H, et al. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59:105–107. doi: 10.1538/expanim.59.105. [DOI] [PubMed] [Google Scholar]
- 16.Barros C, Bedford JM, Franklin LE, Austin CR. Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol. 1967;34:1–5. doi: 10.1083/jcb.34.3.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi T, et al. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449:277–283. doi: 10.1016/s0014-5793(99)00433-0. [DOI] [PubMed] [Google Scholar]
- 18.Kallajoki M, Virtanen I, Suominen J. The fate of acrosomal staining during the acrosome reaction of human spermatozoa as revealed by a monoclonal antibody and PNA-lectin. Int J Androl. 1986;9:181–194. doi: 10.1111/j.1365-2605.1986.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 19.Cheng FP, et al. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J Androl. 1996;17:674–682. [PubMed] [Google Scholar]
- 20.Hunter RHF. Ovarian control of very low sperm/egg ratios at the commencement of mammalian fertilisation to avoid polyspermy. Mol Reprod Dev. 1996;44:417–422. doi: 10.1002/(SICI)1098-2795(199607)44:3<417::AID-MRD15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Saling PM, Storey BT. Mouse gamete interactions during fertilization in vitro. Chlortetracycline as a fluorescent probe for the mouse sperm acrosome reaction. J Cell Biol. 1979;83:544–555. doi: 10.1083/jcb.83.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bleil JD, Wassarman PM. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 23.Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–943. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- 24.Kopf GS, Gerton GL. The mammalian sperm acrosome and the acrosome reaction. In: Wassarman PM, editor. Elements of Mammalian Fertilization. Boca Raton, FL: CRC; 1991. pp. 153–203. [Google Scholar]
- 25.Salustri A, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 26.Shimada M, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 27.Tanii I, Aradate T, Matsuda K, Komiya A, Fuse H. PACAP-mediated sperm-cumulus cell interaction promotes fertilization. Reproduction. 2011;141:163–171. doi: 10.1530/REP-10-0201. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda Y, Sato E, Naito K. Role of the cumulus oophorus in mammalian fertilization. In: Mohri H, Takahashi M, Tachi C, editors. Biology of the Germ Line in Animals and Man. Tokyo, Japan: Japan Scientific Societies Press; 1993. pp. 111–124. [Google Scholar]
- 29.Litscher ES, Williams Z, Wassarman PM. Zona pellucida glycoprotein ZP3 and fertilization in mammals. Mol Reprod Dev. 2009;76:933–941. doi: 10.1002/mrd.21046. [DOI] [PubMed] [Google Scholar]
- 30.Baltes P, et al. Evidence for the synthesis and secretion of a CBG-like serpin by human cumulus oophorus and fallopian tubes. Andrologia. 1998;30:249–253. doi: 10.1111/j.1439-0272.1998.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 31.Hong SJ, et al. Cumulus cells and their extracellular matrix affect the quality of the spermatozoa penetrating the cumulus mass. Fertil Steril. 2009;92:971–978. doi: 10.1016/j.fertnstert.2008.07.1760. [DOI] [PubMed] [Google Scholar]
- 32.Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–1581. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- 33.Tesarík J, Pilka L, Drahorád J, Cechová D, Veselský L. The role of cumulus cell-secreted proteins in the development of human sperm fertilizing ability: implication in IVF. Hum Reprod. 1988;3:129–132. doi: 10.1093/oxfordjournals.humrep.a136645. [DOI] [PubMed] [Google Scholar]
- 34.Yin L, et al. A sperm GPI-anchored protein elicits sperm-cumulus cross-talk leading to the acrosome reaction. Cell Mol Life Sci. 2009;66:900–908. doi: 10.1007/s00018-009-8482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummins JM, Yanagimachi R. Sperm-egg ratios and the site of the acrosome reaction during in vivo fertilization in the hamster. Gamete Res. 1982;5:239–256. [Google Scholar]
- 36.Austin CR. Entry of spermatozoa into the fallopian-tube mucosa. Nature. 1959;183:908–909. doi: 10.1038/183908a0. [DOI] [PubMed] [Google Scholar]
- 37.Buffone MG, Foster JA, Gerton GL. The role of the acrosomal matrix in fertilization. Int J Dev Biol. 2008;52:511–522. doi: 10.1387/ijdb.072532mb. [DOI] [PubMed] [Google Scholar]
- 38.Buffone MG, Rodriguez-Miranda E, Storey BT, Gerton GL. Acrosomal exocytosis of mouse sperm progresses in a consistent direction in response to zona pellucida. J Cell Physiol. 2009;220:611–620. doi: 10.1002/jcp.21781. [DOI] [PubMed] [Google Scholar]
- 39.VandeVoort CA, Yudin AI, Overstreet JW. Interaction of acrosome-reacted macaque sperm with the macaque zona pellucida. Biol Reprod. 1997;56:1307–1316. doi: 10.1095/biolreprod56.5.1307. [DOI] [PubMed] [Google Scholar]
- 40.Kuzan FB, Fleming AD, Seidel GE., Jr Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil Steril. 1984;41:766–770. doi: 10.1016/s0015-0282(16)47847-7. [DOI] [PubMed] [Google Scholar]
- 41.Osman RA, Andria ML, Jones AD, Meizel S. Steroid induced exocytosis: The human sperm acrosome reaction. Biochem Biophys Res Commun. 1989;160:828–833. doi: 10.1016/0006-291x(89)92508-4. [DOI] [PubMed] [Google Scholar]
- 42.Meizel S, Turner KO, Nuccitelli R. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction. Dev Biol. 1997;182:67–75. doi: 10.1006/dbio.1997.8477. [DOI] [PubMed] [Google Scholar]
- 43.Melendrez CS, Meizel S, Berger T. Comparison of the ability of progesterone and heat solubilized porcine zona pellucida to initiate the porcine sperm acrosome reaction in vitro. Mol Reprod Dev. 1994;39:433–438. doi: 10.1002/mrd.1080390412. [DOI] [PubMed] [Google Scholar]
- 44.Meyers SA, Overstreet JW, Liu IK, Drobnis EZ. Capacitation in vitro of stallion spermatozoa: Comparison of progesterone-induced acrosome reactions in fertile and subfertile males. J Androl. 1995;16:47–54. [PubMed] [Google Scholar]
- 45.Thérien I, Manjunath P. Effect of progesterone on bovine sperm capacitation and acrosome reaction. Biol Reprod. 2003;69:1408–1415. doi: 10.1095/biolreprod.103.017855. [DOI] [PubMed] [Google Scholar]
- 46.Sabeur K, Cherr GN, Yudin AI, Overstreet JW. Hyaluronic acid enhances induction of the acrosome reaction of human sperm through interaction with the PH-20 protein. Zygote. 1998;6:103–111. doi: 10.1017/s0967199498000021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.