Abstract
Pain is a major health concern even though numerous analgesic agents are available. Side effects and lack of wide-spectrum efficacy of current drugs justify efforts to better understand pain mechanisms. Stabilization of natural epoxy-fatty acids (EFAs) through inhibition of the soluble epoxide hydrolase (sEH) reduces pain. However, in the absence of an underlying painful state, inhibition of sEH is ineffective. Surprisingly, a pain-mediating second messenger, cAMP, interacts with natural EFAs and regulates the analgesic activity of sEH inhibitors. Concurrent inhibition of sEH and phosphodiesterase (PDE) dramatically reduced acute pain in rodents. Our findings demonstrate a mechanism of action of cAMP and EFAs in the pathophysiology of pain. Furthermore, we demonstrate that inhibition of various PDE isozymes, including PDE4, lead to significant increases in EFA levels through a mechanism independent of sEH, suggesting that the efficacy of commercial PDE inhibitors could result in part from increasing EFAs. The cross-talk between the two major pathways—one mediated by cAMP and the other by EFAs—paves the way to new approaches to understand and control pain.
Keywords: nociception, antinociceptive, epoxyeicosatrienoic acid
Persistent pain is a serious health problem associated with numerous disease states (1). The interaction of many of complex biological pathways is essential for the development of persistent pain, whether inflammatory or neuropathic (2). Thus, numerous available analgesic agents that target a single pathway lack wide-spectrum efficacy and display side effects, which justifies efforts to better understand pain mechanisms (3). Interfering with one of these pathways, the COX branch of the arachidonic acid (ARA) cascade, is a well accepted strategy for reducing inflammatory pain, although COX inhibitors are ineffective in reducing neuropathic pain (4). Another branch of the ARA cascade yields natural epoxy-fatty acids (EFAs) when ARA is oxygenated by several cytochrome P450 isozymes (5). The ARA metabolites [epoxyeicosatrienoic acids (EETs)] display anti-inflammatory and antinociceptive effects (6, 7). Linoleic, eicosapentaenoic, and docosahexaenoic acids can also be converted to EFAs by cytochrome P450 isozymes, and these metabolites, like the EETs, display similar rapid antinociceptive effects (8, 9). Stabilization and elevation of these EFAs by inhibition of the soluble epoxide hydrolase (sEH), the major enzyme that degrades EFAs, reduce inflammatory and neuropathic pain (10, 11). Consistent with the diversity of the EFAs, in vivo inhibition of sEH results in a variety of beneficial effects, including antihypertensive, anti-inflammatory, and antinociceptive (i.e., pain-blocking) activities (7, 12–14). However, in the absence of a persistent painful condition, inhibition of sEH does not alter withdrawal reflexes in response to intense thermal or mechanical stimuli (i.e., nociceptive pain) despite elevating EFAs. Here we investigated the interaction between the pain-mediating second messenger cAMP and EFAs that leads to decreased pain-related behavior in rodents.
Results
Elevation of EFAs Blocks Noninflammatory Pain.
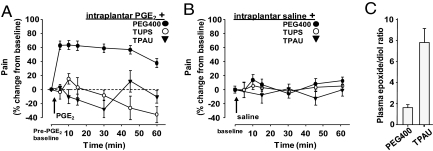
Inhibitors of sEH reduce inflammatory pain, consistent with other reports suggesting that EFAs are anti-inflammatory molecules (6, 9, 10). However, sEH inhibitors (sEHIs) also block neuropathic pain in diabetic animals (10). To test whether sEHIs are antinociceptive independent from reducing inflammation, here we induced pain by using prostaglandin E2 (PGE2). This model involving direct application of PGE2 is devoid of a major inflammatory component and therefore pain elicited by this COX product is impervious to reversal by most drugs targeting the ARA cascade, including nonsteroidal anti-inflammatory drug (NSAIDs) (15), selective cyclooxygenase inhibitors, and steroids (Fig. S1). In contrast to these agents, the sEHIs effectively blocked pain elicited by PGE2 (Fig. 1A), supporting the hypothesis that sEHIs reduce pain independent from their anti-inflammatory activity.
Fig. 1.
Inhibitors of sEH block pain mediated by PGE2. (A) Structurally different sEHIs—TPAU and TUPS—eliminate PGE2-elicited pain (100 ng per paw in 10 μL) whereas NSAIDs or a steroidal drug do not (Fig. S1). TPAU 10 mg/kg and TUPS 3 mg/kg were administered s.c. with PEG400 as vehicle in all panels (n ≥ 6 per group). Pain is measured by von Frey mechanical allodynia assay by a fully blinded experimenter and reported as percentage change from pre-PGE2 baseline mechanical withdrawal threshold. Baseline mechanical withdrawal, responses were measured and sEHIs were administered s.c. 1 h before PGE2. Administration of PGE2 decreased withdrawal threshold by 60%. (B) Lack of effect of high doses of sEHI on saline solution injection into the paw (without PGE2) in rats. Acute pain responses measured by von Frey assay (baseline vs. time points ANOVA, P > 0.1). All data are expressed as percentage of pre-PGE2 baseline and presented as mean ± SEM. (C) Elevation of fatty acid epoxide-to-diol ratio in rats by TPAU (10 mg/kg, n = 6 per group; Table S1 shows quantity and identities of analytes and Table S3 shows structures of sEHIs). The dose of sEHI that greatly increased plasma EFAs (Table S1) failed to show any change in perceived pain in these animals.
EFAs Act in a Pain-Dependent Manner.
The sEHIs stabilize and thus elevate antinociceptive and anti-inflammatory EFAs whereas the NSAIDs reduce pain by blocking the synthesis of proinflammatory molecules. Unlike narcotic agents that are analgesic even in the absence of pain, the sEHIs have minimal effects on basal acute pain thresholds (Fig. 1B and Fig. S2) even at doses more than 30 fold greater than that needed to reduce existing pain (10). Such sEHI levels elevate the EFAs and simultaneously decrease the inactive degradation products dihydroxy-fatty acids (FAs) in plasma and tissues regardless of the disease status of the animals (Fig. 1C and Table S1). Therefore, elevation of the EFA levels per se does not appear to be sufficient to modulate pain-related behavior.
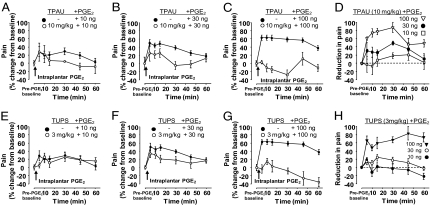
We tested if the pain-blocking effects of sEHIs require factor(s) in addition to elevated EFAs. We hypothesized that these factor(s) would be endogenously generated during the pain response. Thus, we evaluated the effect of the intensity of the pain state on the efficacy of sEHIs. Pain elicited by a series of increasing amounts of PGE2 in the presence of a constant dose of sEHI was quantified (Fig. 2 A–C and E–G). Although sEHIs effectively blocked intense pain elicited by the high dose of PGE2 (100 ng per paw), their efficacy diminished proportionally with lower doses of PGE2 (Fig. 2 D and H). A major EFA, 14,15-EpETre, was recently reported to have no interaction with D- or E-prostanoid receptors (16). Given that EFAs do not seem to be antagonists of the E-prostanoid receptors, these observations support the hypothesis that the pain-reducing effects of sEHI and EFAs are pain activity-dependent.
Fig. 2.
sEHIs act in a pain intensity-dependent manner. (A–C) Mean line graphs showing effect of a constant dose of TPAU (10 mg/kg s.c.) on different magnitudes of pain intensity (i.e., hyperalgesia) resulting from different doses of PGE2. TPAU did not alter baseline mechanical withdrawal thresholds (Fig. 1B). Doses of PGE2 vary as indicated (10, 30, and 100 ng per paw). Following intraplantar administration of PGE2, animals were immediately placed in acrylic chambers standing on a mesh screen. Mechanical withdrawal thresholds were measured 5, 10, 15, 30, 45, and 60 min after PGE2 by a fully blinded experimenter. For the initial time points of 5 and 10 min after PGE2 administration, one measurement per animal per time point was recorded because of the short time interval between the time points. For the rest of the time points, three measurements at 1-min intervals were recorded and averaged as the threshold (n ≥ 6 in all groups). (D) Constant dose of sEHI is less efficacious when rats have less hyperalgesia but is more effective when hyperalgesia is severe (y axis, percent difference in mechanical withdrawal threshold from mean of corresponding PGE2 group, measured by von Frey assay). (E–G) Mean line graphs showing effect of TUPS (3 mg/kg s.c.) on pain elicited by different doses of PGE2. (n = 6 in all groups) (H) A structurally different sEHI, TUPS, acts similarly to TPAU in reducing PGE2-induced pain in an intensity dependent manner (y axis, percent difference in mechanical withdrawal threshold from mean of corresponding PGE2 group measured by von Frey assay).
Phosphodiesterase 4 Inhibitor-Mediated Elevation of cAMP Instigates EFA Mediated Analgesia.
PGE2 activates E-prostanoid receptors and leads to adenylate cyclase activation, generation of cAMP, and subsequently pain (17). Thus, we hypothesized that cAMP is an important chemical mediator, which, when present, dramatically increases the ability of sEHIs to reduce pain. Given that intracellular cAMP is increased by inflammation and is itself painful (17–20), in the following experiments we used healthy rats without inflammation or neuropathy and monitored acute pain-related behavior measured as withdrawal responses to thermal and mechanical stimuli.
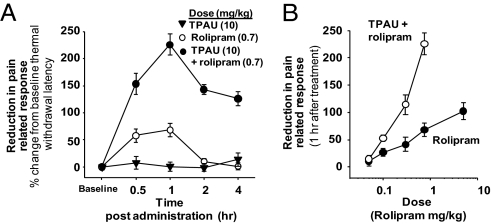
This allowed us to test the effects of a constant dose of sEHI in a paradigm that is independent of an underlying pain status but in which cAMP is artificially elevated by using rolipram, a phosphodiesterase (PDE) 4 inhibitor (PDEi). Rolipram is reported to enhance existing pain when administered locally (21). Here, systemic administration of rolipram itself was effective in elevating pain thresholds (Fig. 3). Strikingly, sEHIs that were devoid of effect in healthy animals, when coadministered with the PDEi, largely blunted pain-related behavior, displaying an opioid-like analgesic effect (Fig. 3). These findings argue that EFAs and sEHI block pain by positively interacting with a cAMP-dependent pathway.
Fig. 3.
Elevation of cAMP by PDEi substitutes for a pain-generated factor and initiates sEHI-mediated increase in nociceptive thresholds. A synergistic reduction in acute pain perception produced by simultaneous administration of PDEi and sEHI is shown. In this experiment, because pain is not elicited through inflammation or neuropathy, the increase from baseline pain thresholds (i.e., hind paw withdrawal latency from a thermal stimulus) are reported. (A) Time course of acute effects by TPAU/rolipram combination in comparison with the lack of effect of TPAU itself. Rolipram is a PDE4-selective, CNS-permeable (38) inhibitor. (B) Dose dependence of PDE4i and sEHI/PDE4i treatment 1 h after administration (TPAU 10 mg/kg constant dose in all sEHI/PDEi groups). Here pain-related behavior is measured by Hargreaves thermal withdrawal latency assay and reported as percent change from predrug baseline (Fig. S7 shows pain-related behavior measured by Randall–Selitto mechanical sensitivity assay). Notably, the combination treatment is more potent and efficacious than rolipram alone.
Although rolipram seemed to block acute nociceptive pain behavior in our experiments, it also led to decreased mobility as reported (22). In contrast, the sEHI alone did not reduce mobility (Fig. S3). At low doses of rolipram at which motor depressant effects are not maximal, a synergistic elevation in pain thresholds was evident if sEHI was coadministered (Fig. 3). Given the depressant effects of rolipram, this could be a result of a synergistic increase in motor depression when sEHI and PDEi were administered. However, we did not observe a synergy in motor depression when sEHI and PDEi were administered (Fig. S3). Strikingly, 2 and 4 h after treatments, rolipram was devoid of effect on withdrawal latency whereas sEHI plus PDEi treatment was highly effective in attenuating pain-related behavior.
Inhibitors of PDE and sEH Have Distinct Pharmacological Actions but both Modulate Bioactive Lipids in Plasma.
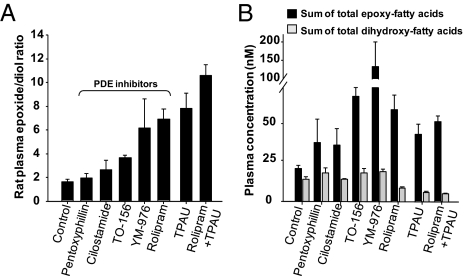
While quantifying plasma fatty acid epoxide/diol ratios in sEHI treated animals as a quantitative measure of target engagement, we included the plasma of PDEi-treated animals as negative control. It was unexpected to find that rolipram was highly effective in elevating absolute quantity of EFAs and fatty acid epoxide/diol ratios in plasma (Fig. 4). Indeed, other selective PDEis also led to elevation of EFAs (Fig. 4). Remarkably, the sEHI and PDEi modulated the EFAs distinctly, with sEHI elevating EFAs and expectedly reducing the levels of corresponding dihydroxy-FAs whereas PDEi primarily elevated EFAs and displayed minimal effects on dihydroxy-FAs (Fig. S4 demonstrates exceptions). Consistent with the structural differences in sEHI and PDEi, rolipram lacked inhibitory activity on recombinant rat or human sEH (IC50 > 100 μM). Therefore, the increase in EFAs by PDEi is a physiological response. Accordingly, the PDEis are a new class of non-sEHI pharmacological agents that selectively boost EFAs without impinging on the dihydroxy-FA metabolites (Tables S1 and S2).
Fig. 4.
sEHI and PDEi act distinctly but both modulate the levels of bioactive lipids. (A) Bar graph of ratios of plasma epoxy to dihydroxy-FAs in PDE4i- and sEHI-treated rats. Sum of epoxy- and dihydroxy-metabolites from ARA, eicosapentaenoic acid, and docosahexaenoic acid (Tables S1 and S2) were used to calculate the ratio for each animal. Because C18:2 fatty acids were much higher in concentration, they were not included in this graph, but can be found in Table S1. Rolipram, TPAU, and coadministration of rolipram and TPAU significantly elevated the EFAs over dihydroxy-FAs (ANOVA followed by Dunnett two-sided t test, P < 0.008). TPAU and pentoxifylline were administered at 10 mg/kg, all other compounds at 1 mg/kg. Samples were obtained 60 min after s.c. drug administration. (B) Different effects of sEHI and PDEi on lipid metabolites. Inhibition of sEH both increased the sum of total EFAs (all groups, ANOVA followed by Dunnett two-sided t test, P = 0.001) and reduced the sum of total dihydroxy-FAs (P = 0.04), whereas the PDEi largely increased the sum of total EFAs without impinging on the sum of total dihydroxy-FA levels (ANOVA vehicle vs. all PDEi, P > 0.55). Tables S1 and S2 display detailed information on the identity and quantity of each analyte.
Despite this unanticipated overlap in the abilities of both classes of compounds to elevate the epoxide/diol ratio, the effects of the sEHI and coadministration of the sEHI with PDEi were clearly distinguishable from PDEi alone (SI Discussion and Fig. S5). Specifically, the sEHI treatment in healthy animals elevated the epoxide/diol ratios but did not change pain-related behavior or mobility, whereas PDEi alone seemed to decrease pain-related behavior and depressed mobility. In contrast, coadministration of sEHI and PDEi produced an additive increase in the epoxy/diol fatty acid ratio in plasma while synergistically elevating the nociceptive pain thresholds.
Discussion
PDEis are used therapeutically to treat inflammatory diseases, but in rodent pain models, elevation of cAMP produces pain (20, 21, 23–25). The anti-inflammatory versus pain-producing effects of cAMP and PDEi are contradictory. Although rolipram is a cognition-enhancing antidepressant agent, it has strong anti-inflammatory properties, but it will prolong inflammatory pain if given locally (21, 26–29). It was suggested earlier that rolipram may have analgesic-like effects (26). Our observation that a considerable fraction of rolipram's effect is regulated by EFAs in the CNS may explain the lack of similar effects of locally administered rolipram (Fig. S5) (21). We speculate that the broad effects produced by a range of isozyme selective PDEis, including anti-inflammatory, antidepressant, and memory-enhancing activities are partially modulated by the dramatic increase in EFA. This hypothesis is supported by the finding that inhibiting isozymes of cytochrome P450 is noncompetitively antagonistic to rolipram's ability to elevate nociceptive thresholds. This suggests that a portion of the analgesia-like effects produced by rolipram is dependent on EFAs (Fig. S5E).
The increase in EFAs produced by various PDE inhibitors was unexpected. It is possible that rolipram and other PDEis induced the expression of cytochrome P450 isozymes, in particular epoxygenases, in which case the levels of EFAs would be elevated. However, the short time scale of our bioassays and blood sampling argue against a cytochrome P450 induction-dependent increase in EFAs. Given that cAMP, rolipram, and other agents that elevate cAMP levels are known to lead to lipolysis, release of free fatty acids into the plasma is a more probable explanation for the observed increase in EFAs from intracellular stores (30–32). Rolipram and other PDEis (10–1,000 nM) in vitro elevate free fatty acid concentrations by approximately twofold, similar to the in vivo increase in EFAs we observed in this study (Fig. 4B). Therefore, a likely mechanism of the increase in EFAs seems to be lipolysis. A mechanism for cAMP-induced lipolysis is described whereby cAMP activated PKA phosphorylation of hormone-sensitive lipase and perilipin residing on intracellular lipid droplets destabilizes the lipid droplets. This allows hormone-sensitive lipase to access and break down triglycerides, releasing free fatty acids from this organelle (30–33). However, in vivo for the PDEis we tested, a number of factors including tissue type (adipose vs. liver), identity and expression profiles of the PDEs, free fatty acid uptake, and membrane reincorporation may influence the selectivity in increasing certain EFAs and particular EFA regioisomers.
In this study we demonstrate two aspects of the physiological roles of EFAs. First, it appears that a biological switch (i.e., pain state) is required for the EFAs or sEHIs to display biological activity. This is important from a therapeutical and safety standpoint if EFAs or their mimics are to be used to treat pain. We identified that this switch may be the increase in cAMP that is known to occur in inflammatory pain states or a downstream event that is initiated by cAMP-mediated signaling. Given the ubiquitous nature of cAMP-mediated signaling, the selective interaction of EFAs with cAMP opens unexplored venues to attain therapeutic effect in disease states such as opioid withdrawal-induced pain, wherein cAMP is elevated. Concurrent inhibition of the sEH and PDEs provide a number of advantages. In particular, these combinations can be used as postoperative analgesic agents or during recovery from general anesthesia, when the pain-relieving effects of sEHI coupled with transient somatosensory and motor-depressant effects of PDEi are desirable. Second, we demonstrate an approach to modulate the levels of EFAs without inhibiting the major enzyme that degrades the EFAs. The ability of PDE4-selective inhibitors to elevate EFAs as efficiently as a potent sEHI argues that a portion of the effects of the PDEi may be mediated by EFAs. This is an interesting hypothesis to test because, besides elevating cAMP, most of the mechanisms of effects mediated by PDE inhibition are not well understood. From a practical standpoint, elevating EFAs by coinhibiting sEH with PDE may be more advantageous than PDE alone because coinhibition of sEH would stabilize the EFAs and sustain the higher EFA levels while also suppressing the levels of dihydroxy-FAs that may have adverse biological effects.
Overall, two lines of evidence support the hypothesis that natural EFAs act cooperatively with cAMP: the dependence of sEHI activity on an existing pain state and the profound analgesia produced by coadministration of sEHI with PDEi. Consequently, modulating the levels of EFAs and cAMP by sEHI and sEHI/PDEi combinations should prove useful in the clinic for alleviating inflammatory and noninflammatory pain.
Materials and Methods
Details of the experimental protocols are given in SI Materials and Methods.
Animals and Chemicals.
This study was approved by the institutional University of California, Davis, Animal Care and Use Committee. Male Sprague–Dawley rats weighing 250 to 300 g were obtained from Charles River Laboratories. A subset of rats was a donation from Charles River Laboratories. The sEHIs 1-trifluoromethoxyphenyl-3-(1-acetylpiperidin-4-yl) urea (TPAU) and 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS) were synthesized as previously reported (34, 35). Rolipram was purchased from Biomol. All other chemicals were obtained from Fisher Scientific.
Pain Models and Nociceptive Testing.
For the PGE2-elicited pain model, the procedure of Khasar et al. was followed with modifications (15). Pain-related behavior was assessed by quantifying hindlimb withdrawal responses to thermal and mechanical stimuli by using the Hargreaves, von Frey, and Randall–Selitto tests as described earlier (7, 10). All drug administrations were done s.c. on the backs of the animals away from limbs.
Inhibitor and Eicosanoid Analyses.
For quantification of brain inhibitor levels, animals were killed while under deep isoflurane anesthesia and perfused with cold saline solution to remove traces of blood from brain tissue. The plasma and brain levels of TPAU were determined as described previously (10). Blood samples for eicosanoid analysis were collected by using a 24-gauge i.v. catheter (Insyte Autoguard; BD) from the tail vein. Plasma samples were stored at −80 °C until analyses. Oxylipin analyses were carried out as described by Yang et al. with minor modifications (36). Inhibitory potencies of the sEHIs were determined by using a modified procedure as described previously (10, 37). Data were analyzed by ANOVA followed by Dunnett two-sided t test for between-group comparisons with the SPSS analysis package. Results are depicted as mean ± SEM. Regression equations were used for the calculation of IC50 values.
Supplementary Material
Acknowledgments
We thank Dr. Leslie Morrow for detailed discussions. This work was supported by National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (to B.D.H.), NIEHS Superfund Basic Research Program P42 ES004699, National Institutes of Health Grant R01 GM 078167 (to S.L.J.), and NIEHS Grant T32ES007059 (to K.W.). B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation. N.H.S. was supported by a fellowship from the Schormueller Foundation (Berlin, Germany) of the German Society of Food Chemists.
Footnotes
Conflict of interest statement: Several of the authors are authors of intellectual property in the areas of treating inflammation, pain, metabolic disease, hypertension and other disorders by the manipulation of oxylipins, and the use of inhibitors of the soluble epoxide hydrolase (B.I., K.W., C.M., S.L.J., A.U., T.R., B.D.H.).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101073108/-/DCSupplemental.
References
- 1.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 2.Hucho T, Levine JD. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Mao J. Translational pain research: Achievements and challenges. J Pain. 2009;10:1001–1011. doi: 10.1016/j.jpain.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeilhofer HU, Brune K. Analgesic strategies beyond the inhibition of cyclooxygenases. Trends Pharmacol Sci. 2006;27:467–474. doi: 10.1016/j.tips.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 6.Node K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inceoglu B, et al. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fer M, et al. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471:116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Morisseau C, et al. Naturally occurring mono epoxides of EPA and DHA are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inceoglu B, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostag Oth Lipid M. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 13.Schmelzer KR, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terashvili M, et al. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther. 2008;326:614–622. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khasar SG, Ho T, Green PG, Levine JD. Comparison of prostaglandin E1- and prostaglandin E2-induced hyperalgesia in the rat. Neuroscience. 1994;62:345–350. doi: 10.1016/0306-4522(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 16.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: Identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther. 2009;328:231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 17.Vonvoigtlander PF, Losey EG. Prostaglandin E2, cyclic adenosine monophosphate and morphine analgesia. Brain Res. 1977;128:275–283. doi: 10.1016/0006-8993(77)90994-5. [DOI] [PubMed] [Google Scholar]
- 18.Burstein S, Gagnon G, Hunter SA, Maudsley DV. Elevation of prostaglandin and cyclic AMP levels by arachidonic acid in primary epithelial cell cultures of C3H mouse mammary tumors. Prostaglandins. 1977;13:41–53. doi: 10.1016/0090-6980(77)90041-7. [DOI] [PubMed] [Google Scholar]
- 19.Zor U, Kaneko T, Schneider HPG, et al. Stimulation of anterior pituitary adenyl cyclase activity and adenosine 3′ 5′ cyclic phosphate by hypothalamic extract and prostaglandin E1. Proc Natl Acad Sci USA. 1969;63:918–925. doi: 10.1073/pnas.63.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song X-J, Wang Z-B, Gan Q, Walters ET. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J Neurophysiol. 2006;95:479–492. doi: 10.1152/jn.00503.2005. [DOI] [PubMed] [Google Scholar]
- 21.Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- 22.Wachtel H. Characteristic behavioural alterations in rats induced by rolipram and other selective adenosine cyclic 3′, 5′-monophosphate phosphodiesterase inhibitors. Psychopharmacology (Berl) 1982;77:309–316. doi: 10.1007/BF00432761. [DOI] [PubMed] [Google Scholar]
- 23.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 24.Field SK. Roflumilast: An oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin Investig Drugs. 2008;17:811–818. doi: 10.1517/13543784.17.5.811. [DOI] [PubMed] [Google Scholar]
- 25.Dolan S, Nolan AM. Biphasic modulation of nociceptive processing by the cyclic AMP-protein kinase A signalling pathway in sheep spinal cord. Neurosci Lett. 2001;309:157–160. doi: 10.1016/s0304-3940(01)02063-8. [DOI] [PubMed] [Google Scholar]
- 26.Siuciak JA, Chapin DS, McCarthy SA, Martin AN. Antipsychotic profile of rolipram: Efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2007;192:415–424. doi: 10.1007/s00213-007-0727-x. [DOI] [PubMed] [Google Scholar]
- 27.Francischi JN, et al. Anti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritis. Eur J Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- 28.Aoki M, et al. Studies on mechanisms of low emetogenicity of YM976, a novel phosphodiesterase type 4 inhibitor. J Pharmacol Exp Ther. 2001;298:1142–1149. [PubMed] [Google Scholar]
- 29.Cooper DMF, Crossthwaite AJ. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci. 2006;27:426–431. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Egan JJ, et al. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA. 1992;89:8537–8541. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schimmel RJ, Buhlinger CA, Serio R. Activation of adenosine 3′,5′-monophosphate-dependent protein kinase and its relationship to cyclic AMP and lipolysis in hamster adipose tissue. J Lipid Res. 1980;21:250–256. [PubMed] [Google Scholar]
- 32.Nilsson NÖ, Strålfors P, Fredrikson G, Belfrage P. Regulation of adipose tissue lipolysis: Effects of noradrenaline and insulin on phosphorylation of hormone-sensitive lipase and on lipolysis in intact rat adipocytes. FEBS Lett. 1980;111:125–130. doi: 10.1016/0014-5793(80)80776-9. [DOI] [PubMed] [Google Scholar]
- 33.Snyder PB, Esselstyn JM, Loughney K, Wolda SL, Florio VA. The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J Lipid Res. 2005;46:494–503. doi: 10.1194/jlr.M400362-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Jones PD, Tsai H-J, Do ZN, Morisseau C, Hammock BD. Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett. 2006;16:5212–5216. doi: 10.1016/j.bmcl.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morisseau C, et al. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol. 2002;63:1599–1608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wixtrom RN, Hammock BD. Continuous spectrophotometric assays for cytosolic epoxide hydrolase. Anal Biochem. 1988;174:291–299. doi: 10.1016/0003-2697(88)90548-9. [DOI] [PubMed] [Google Scholar]
- 38.Krause W, Kühne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.