Abstract
Neurons, astrocytes, and blood vessels are organized in functional “neurovascular units” in which the vasculature can impact neuronal activity and, in turn, dynamically adjust to its change. Here we explored different mechanisms by which VEGF, a pleiotropic factor known to possess multiple activities vis-à-vis blood vessels and neurons, may affect adult neurogenesis and cognition. Conditional transgenic systems were used to reversibly overexpress VEGF or block endogenous VEGF in the hippocampus of adult mice. Importantly, this was done in settings that allowed the uncoupling of VEGF-promoted angiogenesis, neurogenesis, and memory. VEGF overexpression was found to augment all three processes, whereas VEGF blockade impaired memory without reducing hippocampal perfusion or neurogenesis. Pertinent to the general debate regarding the relative contribution of adult neurogenesis to memory, we found that memory gain by VEGF overexpression and memory impairment by VEGF blockade were already evident at early time points at which newly added neurons could not yet have become functional. Surprisingly, VEGF induction markedly increased in vivo long-term potentiation (LTP) responses in the dentate gyrus, and VEGF blockade completely abrogated LTP. Switching off ectopic VEGF production resulted in a return to a normal memory and LTP, indicating that ongoing VEGF is required to maintain increased plasticity. In summary, the study not only uncovered a surprising role for VEGF in neuronal plasticity, but also suggests that improved memory by VEGF is primarily a result of increasing plasticity of mature neurons rather than the contribution of newly added hippocampal neurons.
Keywords: vascular biology, neural stem cells, learning
VEGF is the key factor in promoting and coordinating most if not all processes of blood vessel formation in the embryo and adult. VEGF is also required for the maintenance of vascular homeostasis, including an indispensible role in adjusting the vasculature to meet dynamic changes in oxygen supply and demand and in controlling vascular barrier functions (reviewed in ref. (1). An increasing body of evidence implicates VEGF in neuronal processes in the adult mammalian brain, notably in adult neurogenesis. Canonically, neurogenesis in the adult brain takes place in two niches: the subventricular zone, which continuously supplies new interneurons to the olfactory bulb, and the subgranular zone of the dentate gyrus (DG), which gives rise to granule neurons as well as glial cells throughout adult life. In both niches, this process takes place in close proximity to blood vessels (2, 3), which has prompted the notion of a “vascular niche” of adult neurogenesis. Exogenous VEGF was shown to increase the basal level of adult neurogenesis in the hippocampus (4–6). Conversely, inhibition of VEGF negates the increased rate of neurogenesis usually observed in mice reared in an enriched environment or under increased exercise (5, 7). Notably, enriched housing, exercise, and training in the Morris water maze induced an increase in endogenous VEGF expression (5).
VEGF was also shown to be capable of enhancing hippocampus-dependent memory (5), but it is unclear whether the effects of VEGF on neurogenesis and memory are causally related. This issue should be considered in the broader context of the ongoing debate regarding the contribution of neurogenesis to neuronal plasticity and hippocampus-dependent memory in comparison with other contributing processes, such as increased synaptic density and enhanced synaptic strength. Findings that favor a role for newly added neurons demonstrate that newly born neuroblasts not only differentiate and integrate into the existing network as functional hippocampal granule neurons, but are more responsive than older neurons to a spatial memory task and display an increased synaptic plasticity (8–10). On the contrary, experimentally damaging the proliferative capacity of DG cells yielded contradicting results concerning an impairment in hippocampus-dependent memory (11–15). (Ref. 16 reviews arguments that support and refute the notion that hippocampal neurogenesis has a major contribution to formation of new memories.)
VEGF is ideally poised to mediate a vascular–neuronal cross-talk, considering that (i) increased neuronal activity induces changes in blood flow and microvascular density and (ii) VEGF production is induced whenever there is increased need for oxygen and metabolites. However, delineating the multiple ways by which VEGF may affect neuronal activity has been greatly hampered by the lack of suitable experimental systems in which to conditionally manipulate endogenous hippocampal VEGF. In particular, it has been impossible to rule out that phenotypes of VEGF loss of function are not secondary to impaired perfusion. Here, we developed transgenic mice models for gain of cerebral VEGF function (GOF) and loss of cerebral VEGF function (LOF) in a conditional and reversible manner. With the aid of these unique mice, we uncovered a surprising role for VEGF in the adult brain in enhancing neuronal plasticity and memory functioning, independent of its described effects on blood perfusion and adult neurogenesis.
Results
VEGF Is Constitutively Expressed in Astrocytes of the Adult Hippocampus Whereas VEGF TK Receptors Are Predominantly Expressed in Nearby Endothelial Cells.
Endogenous expression of VEGF in the adult hippocampus was demonstrated by using a LacZ knock-in reporter inserted into the 3′UTR of the endogenous VEGF gene. As shown in Fig. S1A (Left), within the hippocampus, VEGF is expressed in CA1 and DG regions. GFAP staining revealed that VEGF is produced primarily by astrocytes (Fig. S1A, Right). Nevertheless, because VEGF is a secreted protein, it is likely accessible for all CA1 and DG cells in the adult hippocampus.
As a first step to examining a putative role for VEGF in neuronal cells, we wished to determine which cells within the DG may potentially respond to the constitutively expressed VEGF by virtue of expressing VEGF receptors. The pattern of expression of VEGF-R2 (Flk1) was examined by mRNA in situ hybridization (ISH) and through the use of an Flk1 promoter-LacZ reporter mouse (17). Both methods revealed that Flk1 expression was readily detected on endothelial cells of the DG but not on neuronal cells or astrocytes (Fig. S2A). Coimmunostaining for astrocytes and Flk1 demonstrated a close proximity of the cells naturally producing (and secreting) VEGF and endothelial cells expressing cognate receptors, respectively (Fig. S2A′′). A similar analysis was extended to VEGF-R1 (Flt1), and results showed that, similarly to Flk1, Flt1 ISH signal could be detected only in endothelial cells of the DG (Fig. S2B). Next, we determined patterns of hippocampal expression of neuropilin-1 (Nrp1) and neuropilin-2 (Nrp2). Corroborating previous results (18), DG granule cells were found to be positive for both neuropilins (Fig. S2 C and D).
The fact that VEGF and its receptors are constitutively expressed in the DG prompted us to explore a role for VEGF in the adult hippocampus via its conditional manipulations.
Genetic System for Conditional and Reversible Gain or Loss of VEGF Function in the Adult Brain.
A tetracycline-regulated system was used to manipulate VEGF in the adult brain. Briefly, brain-specific expression was achieved by using a driver transgenic line composed of tet-regulated transactivator protein (13) driven by a CamKIIα promoter (19). For VEGF GOF, a tet-responsive VEGFA164 responder line was generated (20). For VEGF LOF, a transgene responder encoding a chimeric tet-regulated protein composed of the five Ig-like loops of the extracellular domain of Flt1 fused to an IgG1-Fc tail was used. The induced secreted receptor (I-sVEGF-R1) efficiently binds and sequesters VEGF, thereby precluding its signaling (21). Thus, this transgenic mouse system allows reversible induction and repression of VEGF signaling at will (SI Materials and Methods and Fig. S1B provide further details on the experimental system).
The choice of a CamKIIα-based driver mouse was based on previous studies showing its widespread expression in the hippocampus (19). Accordingly, the system was proven suitable for switching “on” expression of VEGF or, conversely, of the VEGF-trapping protein in the hippocampus (Figs. S1 C and D and S3). Note that the system is not leaky and fully reversible as evident from full rerepression of the respective transgene expression following readdition of tetracycline (i.e., “on > off”).
It is noteworthy that the induced soluble receptor I-sVEGF-R1 used in the VEGF LOF experiments sequesters not only VEGFA (abbreviated as VEGF) but also its family members VEGFB and PLGF, the inhibition of which may confound the phenotypes described here and is attributed to VEGF.
VEGF Increases Hippocampal Angiogenesis and Neurogenesis and Improves Hippocampal-Dependent Memory.
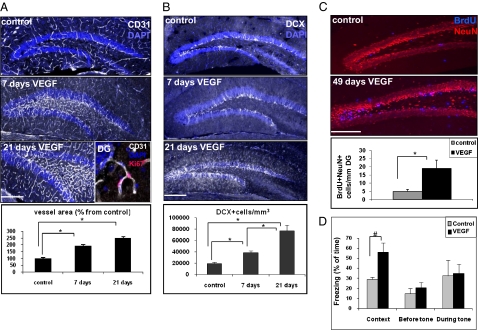
As expected, transgenic overexpression of VEGF in the hippocampus resulted in rapid, robust, and progressive addition of new blood vessels, as evidenced by staining with the endothelial marker CD31 (Fig. 1A). Many of these endothelial cells were also positive for Ki67, which is indicative of active endothelial proliferation (Fig. 1A, Inset). i.v. injection of FITC-dextran was used to show that VEGF-induced hippocampal vessels are functional and well perfused (Fig. S4).
Fig. 1.
VEGF GOF increases hippocampal angiogenesis, augments neurogenesis, and improves memory. (A and B). VEGF was switched-on at postnatal day 40 and brains analyzed 7 or 21 d later. (A) Sections were immunostained for endothelial cells (CD31+) and nuclei were highlighted with DAPI. Vascular density was quantified as the area occupied by capillaries relative to total DG area (including the neuropil; n = 3–5 per group and n = 4–6 sections per animal, representing all DG areas at the rostrocaudal axis; *P < 0.0005). (B) Immunostaining for neuroblasts (DCX+). Images are representatives of a 40-μm-thick Z-projection. Quantification was done by calculating the number of DCX+ cell bodies per a volume unit of DG cell bodies (n = 3–5 per group, n = 4–6 sections per animal; *P < 0.0005). (C) BrdU pulse was given 4 wk after the onset of VEGF induction (three injections per day of 50 mg/kg for 2 d), and brains were retrieved for analysis 3 wk thereafter. BrdU+/NeuN+ cells labeling newly added neurons that have matured meanwhile and survived were counted and normalized to the length of the DG in the image (n = 5 per group, n = 4–6 sections per animal; *P < 0.0005). (D) Control and VEGF-induced animals were subjected to a fear conditioning learning paradigm (Materials and Methods). Note a significantly enhanced contextual memory in VEGF-induced mice, reflected by a twofold increase in freezing time but no change in auditory-cued fear conditioning (during tone; n = 8 animals in the control group and n = 7 in the VEGF group; #P < 0.005). (Scale bars, 200 μm.)
To detect neurogenic activity of VEGF, proliferating neuroblasts were visualized by immunostaining with the early neuronal marker doublecortin (DCX; Fig. 1B). The number of DCX-positive cells in the subgranular zone was doubled within 1 wk from VEGF induction, and was further increased to approximately fourfold after an additional 2 wk. To determine whether the fourfold increase in the number of neuroblasts is also reflected in a parallel increase in the number of added mature neurons, a short BrdU pulse was given at 1 wk from the onset of VEGF induction, and brains were analyzed 3 wk thereafter for cells double-positive for BrdU and the mature neuronal marker NeuN. Results showed that VEGF overexpression increased the number of BrdU+/NeuN+ cells similarly by a factor of four (Fig. 1C).
To determine whether VEGF-induced neurogenesis is accompanied by improved cognitive performance, we subjected the mice to the fear-conditioning memory task. It is noteworthy that the experimental design used is capable of distinguishing hippocampus-dependent and -independent memory. As shown in Fig. 1D, mice analyzed 4 wk after switching-on of transgenic VEGF expression showed a markedly improved hippocampal-dependent contextual memory whereas their hippocampal independent auditory-cued memory remained unchanged.
VEGF LOF Impairs Memory Without Impairing Vascular Density or Decreasing Neurogenesis.
To follow the functional consequences of inhibiting signaling of the naturally produced hippocampal VEGF (Fig. S1A), I-sVEGF-R1 was induced in the adult brain and maintained in the on mode for various times and for as long as 7 wk after induction. The three processes shown here to be augmented by VEGF overexpression, namely angiogenesis, neurogenesis, and memory, were then similarly analyzed.
We have previously shown that premature withdrawal of VEGF may result in regression of newly formed vessels but that mature vessels are refractory to VEGF inhibition (22). We assumed, therefore, that withdrawing VEGF in the adult brain, i.e., after vessels have already matured, will not result in vascular loss. This was indeed confirmed in the experiment shown in Fig. 2A. Further, VEGF inhibition did not reduce vessel patency as judged by FITC-dextran perfusion (Fig. S4), did not cause hippocampal hypoxia, and did not inflict any detectable cell death (cleaved caspase-3–positive cells) within the hippocampus (Fig. S5 A and B).
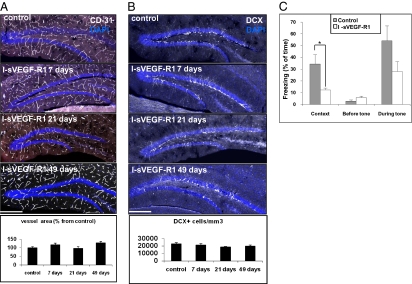
Fig. 2.
VEGF LOF in the hippocampus impairs memory without reducing vessel density or neurogenesis. (A and B) I-sVEGF-R1 transgenic mice were switched at postnatal day 40 for 7, 21, and 49 d. (A) CD31 immunostaining: quantification of the area occupied by vessels (Bottom) reveals no significant difference among all time points (P > 0.1; n = 4 per group, n = 4–6 images each). (Scale bar, 200 μm.) (B) DCX immunostaining: quantification shows no significant decrease in neurogenesis (P > 0.1; n = 4 per group, n = 4–6 images each). (C) Control and VEGF LOF animals were subjected to a fear-conditioning learning paradigm. Note a 2.5-fold reduction in contextual memory. Auditory-cued memory was also reduced but this did not reach statistical significance (n = 6–8 in all groups; *P < 0.01). (Scale bars, 200 μm.)
To determine the effect of VEGF inhibition on basal neurogenesis, hippocampal slices were stained with DCX. Results showed no decrease in the number of neuroblasts (Fig. 2B). Additionally, newly formed neurons (highlighted via DG injections of GFP-encoding retrovirus) developed to have typical dendritic trees and spines with branching points equal in number to control (Fig. S5C).
To determine whether VEGF sequestration is accompanied by impairment of cognitive performance, animals were subjected to a memory task as described earlier. Despite the fact that neurogenesis was not compromised, animals with VEGF LOF displayed impaired contextual memory compared with their control littermates (Fig. 2C). Auditory-cued memory was also moderately reduced, but this effect did not reach statistical significance. To provide additional support to the finding that VEGF LOF may result in memory impairment, animals were subjected to a radial eight-arm maze test that measured hippocampus-dependent spatial learning (SI Materials and Methods). As shown in Fig. S6, VEGF LOF animals completely lost spatial learning skills. VEGF GOF animals, however, failed to further improve spatial learning in this particular test.
Taken together, GOF and LOF experiments clearly showed that VEGF is required for proper memory. Intriguingly, however, the LOF experiments also suggest that the role of VEGF in the memory process is unrelated to its neurogenic activity. Therefore, we wished to further establish that VEGF-induced neurogenesis and VEGF-improved memory are causally unrelated.
Uncoupling the Effect of VEGF on Memory from Its Effects on Neurogenesis.
To evaluate the relative contribution of newly added neurons to memory, we subjected mice to a memory task as early as 5 d after VEGF induction (or, conversely, after VEGF blockade). This experiment is based on the premise that, at this time point, newly added neurons may not yet have become functional considering that newly added hippocampal neurons are known to mature within 3 to 4 wk and project their first axons and dendrites no earlier than 10 d from their birth (23). As shown in Fig. 3 A and B, at 5 d after VEGF induction or blockade, there was already a significant improvement or reduction, respectively, in memory functioning and at a magnitude comparable to changes observed after 1 mo from onset. These results suggest that a mechanism other than neurogenesis is likely to account for VEGF-enhanced memory.
Fig. 3.
Effects of VEGF GOF or LOF on fear conditioning are observed already after 5 d. Animals were analyzed for fear conditioning after 5 d of VEGF GOF (A) or 5 d of VEGF LOF (B). A significant (*P < 0.05) increase or decrease, respectively, in hippocampal-dependent contextual memory was observed in both groups, whereas auditory-cued memory was reduced in the LOF group only (n = 6–7 in the different groups).
VEGF-Mediated Cognitive Gain Is Reversible Whereas Cognitive Loss Induced by VEGF Inhibition Is Irreversible.
To determine whether the cognitive gain induced by VEGF could be reversed on withdrawal of transgene expression, VEGF was switched off after 1 mo from induction (i.e., on > off), and 1 mo later, animals were subjected to the fear-conditioning test (Fig. 4). Results showed that enhanced memory induced by VEGF was completely lost after switching off transgenic VEGF and was now indistinguishable from that of control littermates (Fig. 4A). To rule out that the reversal was because the newly added neurons did not survive VEGF withdrawal, a BrdU pulse was given during the on period and the number of BrdU+ neurons was determined at the end of the off' period. Results showed that the number of BrdU+ neurons remained significantly higher in VEGF on > off animals than in controls (Fig. 4B). Moreover, the number of DCX+ neuroblasts remained significantly higher in VEGF on > off animals (Fig. S7), indicating that neuronal cell production did not decrease upon VEGF withdrawal. These results indicate that ongoing VEGF signaling is required to maintain the cognitive gain and that this cannot be attributed to the neurogenic activity of VEGF.
Fig. 4.
Reversibility of phenotypes induced by VEGF GOF and LOF. Transgenic expression of VEGF (A, B, and D) or I-sVEGF-R1 (C) was switched on for 4 wk. A 4-d long BrdU pulse (50 mg/kg i.p. twice daily) was given during the second week. Animals were then switched off and subjected to analysis 4 wk later. (A) VEGF on > off transgenic animals subjected to fear conditioning. Note a comparable performance to control animals (n = 7 animals per group). (B) Hippocampi from VEGF on > off transgenic animals immunostained for BrdU and NeuN. Note an increase in the number of double-positive neurons comparable to the increase observed in VEGF on animals shown in Fig. 2C (n = 3 animals per group, n = 6 images each; *P < 0.0001). (Scale bar, 200 μm.) (C) I-sVEGF-R1 on > off transgenic animals subjected to fear conditioning. Note that the deficit in contextual memory was not rectified (n = 9–11 animals per group; #P < 0.05). (D) VEGF on > off transgenic animals immunostained for CD31. Note that the vascular gain remained unchanged following VEGF withdrawal (n = 4–5 animals per group, n = 3–5 images each; *P < 0.0001). (Scale bar, 200 μm.)
A similar reversal experiment was performed for VEGF LOF. Here, the memory deficit induced via VEGF inhibition was not rectified after cessation of VEGF blockade (Fig. 4C). The reason for this result is currently not understood given that, under these conditions, perfusion and neurogenesis were not compromised.
Uncoupling the Effect of VEGF on Memory from Its Effects on Perfusion.
A bidirectional link between perfusion and neuronal activity is well established. Because VEGF-induced angiogenesis functions to improve tissue perfusion, we wished to determine whether improved memory could be attributed to increased microvascular density. VEGF induction indeed led to markedly increased microvascular density in the hippocampus (highlighted by CD31 staining), which persisted 1 mo after VEGF withdrawal (Fig. 4D). As enhanced memory was lost despite the fact that added vessels persisted after VEGF withdrawal, it could be ruled out that VEGF-induced hyperperfusion is the cause of improved memory.
VEGF Enhances Long-Term Potentiation (LTP) Whereas I-sVEGF-R1 Abrogates LTP.
As VEGF-induced memory changes could not be accounted for by VEGF-enhanced neurogenesis, we examined other mechanisms of neuronal plasticity such as LTP. Animals with VEGF GOF or LOF were subjected in vivo to tetanic stimulation of the perforant path (PP) while recording the postsynaptic field potential of DG granule cells. Importantly, LTP was measured 5 to 15 d after induction of the respective transgene to rule out a contribution by newly added neurons. Results showed that VEGF overexpression resulted in a highly significant reactivity to the afferent stimulation persisting for at least 60 min. Conversely, VEGF LOF resulted in complete abrogation of the normal LTP response (Fig. 5). To characterize changes in the dynamic range of DG synapses, input/output relations were determined; no significant difference between the groups was found (Fig. S8A). To determine whether there are apparent differences in short-term synaptic plasticity in these synapses, paired-pulse responses were examined (Fig. S8B). A decreased facilitation in the VEGF LOF animals at the interstimulus interval of 60 ms was indeed found, which provided a partial explanation for the LTP loss. Remarkably, augmentation of the LTP response by ectopic VEGF appeared to be hippocampus region-specific, taking place at the PP, where recording is done at the DG, but not in the Schaffer collaterals, where recording is done at the CA1. This was evident by a parallel in vivo recording from the CA1 region that failed to show a similar increase in LTP response despite a similar level of VEGF induction in both regions and a comparable angiogenic response (Fig. S9).
Fig. 5.
VEGF GOF enhances LTP whereas VEGF LOF ablates LTP in the DG. (A) In vivo LTP in the DG of anesthetized adult animals. VEGF on mice (n = 6) switched for 5 to 15 d before the experiment showed a significantly enhanced LTP. Similar switch of I-sVEGF-R1 (n = 8) demonstrate inability to obtain LTP. In the VEGF on > off (n = 7) and I-sVEGF-R1 on > off (n = 7) groups, the respective transgene was induced for 4 wk followed by 4 wk in the off mode. Results showed complete or partial reversibility, respectively. Representative traces are presented for control before high-frequency stimulation (HFS) (a), VEGF after HFS (b), control after HFS (c), and I-sVEGF-R1 after HFS (d).
Prompted by the results described here that have demonstrated a requirement for ongoing VEGF to maintain cognitive gain, we wished to determine whether switching off the respective transgene will similarly lead to reversal of the LTP change. As shown in Fig. 5, switching off VEGF indeed resulted in return to an LTP response that is indistinguishable from control, and terminating VEGF inhibition resulted in a partial rescue of LTP. These results not only uncovered a role for VEGF in synaptic modulation, but also suggest that VEGF-induced synaptic strengthening may contribute to VEGF-induced memory facilitation.
Discussion
This study uncovered a surprising role for VEGF in neuronal plasticity, namely modulating plasticity of mature neurons. The study not only extends the list of nonangiogenic functions for VEGF, but may also provide insights on complex neuronal/vascular cross-talk in general, and on the question of how components of the vascular system may modify neuronal activity in particular.
Uncoupling multiple functions of VEGF in the adult brain has been hampered by the lack of appropriate genetic systems suitable for conditional VEGF manipulations in a tightly controlled spatial and temporal manner. Here, we developed systems of conditional VEGF GOF or LOF in brain regions implicated in adult neurogenesis and memory. Furthermore, the systems we developed allowed for reversal of the respective VEGF manipulation at will, thereby allowing us to determine whether ongoing VEGF signaling is also required to maintain the respective VEGF-induced phenotype. With the aid of these systems, we were able to corroborate and extend previous studies that used exogenous administration of VEGF or a VEGF inhibitor to demonstrate VEGF-enhanced hippocampal neurogenesis (4–6) and memory (5).
Traditionally, it has been difficult to distinguish neuronal phenotypes resulting from impaired perfusion, which is an anticipated consequence of VEGF LOF, from perfusion-independent effects. The ability to inhibit VEGF signaling at times when hippocampal vessels are no longer dependent on VEGF allowed us to conclude that neuronal phenotypes resulting from VEGF LOF are indeed not caused by impaired perfusion, which remained unchanged (Fig. 2 and Fig. S4). Because VEGF is primarily an angiogenic factor and its overexpression indeed led to increased vascular density in the hippocampus, we could directly evaluate the effect of hyperperfusion on neurogenesis and memory. Interestingly, an elevated level of basal neurogenesis accompanying the increase in vascular density (Fig. 1) persisted even after the return of VEGF to its normal level (following switching off of transgene expression; Fig. 4 and Fig. S7), suggesting that expansion of the hippocampal vasculature alone is sufficient for a sustained increase in basal neurogenesis without a need for ongoing VEGF signaling. Intriguingly, under these conditions of augmented hippocampal perfusion, increased neurogenesis, and normal VEGF levels, the cognitive gain and enhanced LTP returned to control levels.
The latter result has implications beyond VEGF biology by virtue of addressing the general debate regarding the relative contribution of newly added neurons to learning and memory. Findings reported here suggest that increased neurogenesis by itself is not sufficient for enhancing memory, at least in the fear-conditioning paradigm, nor enhancing LTP. Two additional lines of evidence argue against a major contribution of newly added neurons to memory processes: First, VEGF LOF compromised memory and LTP without reducing neurogenesis (Figs. 2 and 5). We further reason that, even if newborn neurons that were added in the setting of VEGF LOF were functionally impaired, their relatively small population could not have accounted for complete abrogation of memory and LTP. Second, the VEGF-induced enhancement of memory and LTP—and, conversely, memory and LTP deficits under conditions of VEGF inhibition—were already evident within 5 d from onset, i.e., at an early time when newly made neurons could have not possibly become functional (Figs. 3 and 5).
Although VEGF manipulations were used in the present study to uncouple adult neurogenesis and learning, the question remains to what extent the relatively minor contribution of neurogenesis to learning can be generalized. Previous studies have shown that newborn neurons are essential for memory and that they are more responsive to novel stimuli than existing neurons (8–15). The different conclusions drawn from these studies and the present study could also reflect methodological differences. Notably, the previous studies used selective ablation of newborn neurons (based on antimitotic agents or ablation of nestin- or GFAP-positive cells), which could have affected other cells as well, whereas the present study circumvented any cellular damage and is mostly based on a kinetic argument.
It is not known whether the newly discovered effect of VEGF on neuronal plasticity is direct, taking place via VEGF binding to neuronally expressed VEGF receptors, or indirect, i.e., via inducing endothelial or glial cells to secrete some factor acting on neuronal cells. A precedent for an indirect effect vis-à-vis VEGF-induced neurogenesis is the seasonal addition of new neurons to the high vocal center (HVC) of male songbirds, in which testosterone-induced VEGF was found to stimulate nearby endothelial cells to secrete BDNF that, in turn, promotes recruitment of neurons from the HVC ventricular zone (24). The notion of a direct response of neurons to VEGF is supported by reports on expression of the high-affinity VEGF receptors FLK1 and FLT1 and of the auxiliary nonsignaling receptor neuropilin on neuronal cells (6, 25–27). Also consistent with a direct mechanism are findings showing that VEGF can induce neurite growth in cultured cortical neurons (28–30). In vivo, however, a rigorous distinction between direct and indirect mechanisms may necessitate neuronal-, glial-, and endothelial-specific ablation (or functional knockdown) of each VEGF receptor. Supporting an indirect mechanism, we detected endothelial-specific expression of VEGF tyrosine kinase receptors FLK1 and FLT1 in the hippocampus but could not detect their expression on hippocampal neurons (Fig. S2). However, we cannot exclude the possibility of a low level of expression that is below our detection threshold.
The requirement for ongoing VEGF signaling for proper memory may have implications for anti-VEGF–based cancer therapy, as a prolonged, systemic treatment with VEGF-neutralizing antibodies may potentially impair cognitive functioning.
In conclusion, this study uncovered a function of the highly pleiotropic factor VEGF, linking the brain vasculature to neuronal functioning in a more complex manner than previously thought.
Materials and Methods
Animals.
All animal procedures were approved by the animal care and use committee of the Hebrew University. The transgenic CamKIIα-tTa brain-specific mouse driver line was purchased from Jackson Labs (19). pTET-VEGF164 responder line (20) and pTET-I-sVEGF-R1 responder line were described previously (31). For switching-off of VEGF or I-sVEGF-R1, water was supplemented by 500 mg/L tetracycline (Tevacycline; Teva) and 3% sucrose. For switching on the transgenes, tetracycline-supplemented water was replaced by fresh water for the desired time. SI Materials and Methods provides additional details.
Fear-Conditioning Assay.
Context and cued conditioning were measured as described (32). The detailed protocol is provided in SI Materials and Methods.
In Vivo LTP Measurements.
PP-evoked responses in the DG were measured in vivo as described (32). The detailed protocol is provided in SI Materials and Methods.
Immunohistochemistry.
For immunohistochemistry, we used formalin-fixed paraffin-embedded tissues (dissected to 3 μm) and 4% paraformaldehyde-fixed frozen 50-μm floating sections. SI Materials and Methods includes a list of antibodies and detailed immunohistochemistry protocols, along with additional details on methods.
Supplementary Material
Acknowledgments
We thank Andras Nagy and Janet Rossant for mice and Rinnat Porat, Nicola Maggio, and Ahuva Itin for technical assistance. This work was supported by the Israel Science Foundation and the German–Israeli Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007640108/-/DCSupplemental.
References
- 1.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 2.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schänzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 6.Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 8.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 10.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 12.Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 13.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 15.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 16.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 17.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 18.Sahay A, et al. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25:3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 20.Dor Y, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May D, et al. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci USA. 2008;105:282–287. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 25.Mani N, Khaibullina A, Krum JM, Rosenstein JM. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Exp Neurol. 2005;192:394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer MH, Tripps WK, Feldmann RE, Jr, Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003;344:165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148:59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- 31.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Avital A, et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.