Abstract
mRNA–tRNA translocation is a central and highly regulated process during translational elongation. Along with the mRNA, tRNA moves through the ribosome in a stepwise fashion. Using cryoelectron microscopy on ribosomes with a P-loop mutation, we have identified novel structural intermediates likely to exist transiently during translocation. Our observations suggest a mechanism by which the rate of translocation can be regulated.
The elongation cycle of protein synthesis is an iterative process that can be divided into three steps, tRNA incorporation, peptidyl transfer, and mRNA–tRNA translocation (1). During tRNA incorporation, an aminoacyl-tRNA (aa-tRNA) is delivered to the ribosomal A site as part of a ternary complex with elongation factor Tu (EF-Tu) and GTP. In the peptidyl-transfer reaction, the nascent peptide chain bound to the P-site tRNA is transferred to the N-terminus of the aminoacyl group of the A-site aa-tRNA, and thus the polypeptide chain is extended by one amino acid. The resulting ribosome complex bearing a peptidyl-tRNA in the A site and a deacylated tRNA in the P site is termed the pretranslocational (PRE) complex. In the translocation process, the mRNA, along with the tRNAs, are moved by one codon with respect to the ribosome. The resulting posttranslocation (POST) complex contains a peptidyl-tRNA at the P site and a deacylated tRNA at the E site. The new downstream mRNA codon is presented at the A site, which enables the incorporation of the next cognate aa-tRNA. Translocation is catalyzed by elongation factor G (EF-G). However, factor-free, spontaneous translocation occurs, as well, albeit at much lower rates (2).
To a first approximation translocation of tRNA and mRNA substrates takes place in two steps: First, the acceptor arms of the A-site and P-site tRNAs move to the P site and E site on the 50S subunit, respectively, forming the so-called hybrid A/P and P/E tRNA configurations; second, the anticodon arms of the A/P and P/E tRNA, along with the mRNA, move to the 30S subunit’s P and E sites, respectively (3). The hybrid configurations of the tRNAs have been directly visualized in three-dimensional structures of the ribosome obtained by cryoelectron microscopy (cryo-EM) and single-particle reconstruction (4–6). Coupled with the formation of the hybrid tRNA configurations, as seen in these cryo-EM reconstructions, are a number of conformational changes of the ribosome, including the so-called ratchet-like motion (a counterclockwise rotation of the 30S subunit with respect to the 50S subunit), and the movement of the L1 stalk toward the main body of the ribosome where it makes contact with the elbow of the hybrid P/E tRNA. These conformational changes of the ribosome are thought to play a direct role in the translocation mechanism, by helping break and reestablish substrate-ribosome interactions during the movement (5, 7). Recently, the structure of the PRE ribosome, with tRNAs replaced by anticodon stem loops, was solved by crystallography in two intermediate conformations (8).
Increasingly, the technique of single-molecule fluorescence resonance energy transfer (sm-FRET) is being used to obtain real-time dynamic information about the translocation process (9). These studies have established that ribosome and tRNA substrates fluctuate between two or more structurally and kinetically distinct states (10–14). In particular, one sm-FRET study provided evidence that the tRNA duplex in the PRE complex oscillates between three states: (i) the classical state (A/A and P/P), (ii) the hybrid state (A/P and P/E), and (iii) a previously unidentified partially hybrid state (A/A and P/E), in which only one tRNA has moved (11). Occupancy of this additional state was enriched by introducing a point mutation, G2252C, on the P-loop of the peptidyl-transferase center on the 23S rRNA. This mutation has been shown to promote the formation of the P/E hybrid tRNA, and at the same time prevent the formation of the A/P hybrid tRNA configuration (15).
Here, we used cryo-EM and single-particle reconstruction to study this G2252C PRE complex. We set out to visualize intermediate states of the tRNAs in an attempt to understand how the global conformation of the ribosome changes as the tRNAs switch between those states. As a result, we were able to identify two intermediate states in which the tRNAs are in unique positions, with the P/E tRNA apparently primed to latch on to the L1 stalk. We also show that the observed positions of the L1 stalk can be characterized as the result of different directions of movement, each of which is associated with a different tRNA configuration. These observations lead to a hypothesis of how the L1 stalk may be employed to regulate the translocation of tRNA.
Results
Observation of Multiple Conformations of the PRE Complex.
We collected approximately 100,000 projection images of the G2252C PRE complex. A large degree of conformational heterogeneity was anticipated because of the dynamic nature of this complex, as characterized by the sm-FRET study (11). Thus, we applied maximum likelihood (ML) classification (16) to generate seven classes (see Methods). We then obtained a reconstruction from projections falling in each of the classes using standard angular refinement. Among the resulting density maps, one showed incomplete density for the tRNA, suggesting the existence of residual heterogeneity. This map was discarded in further analysis. In the remaining six density maps, two pairs were found to be structurally quite similar, and thus the underlying data subsets were pairwise combined for reconstruction. In this way we obtained a total of four density maps of the G2252C PRE complex (Fig. 1 and Table S1). Finally, we applied real-space refinement (17) to obtain atomic models that optimally fit into the maps.
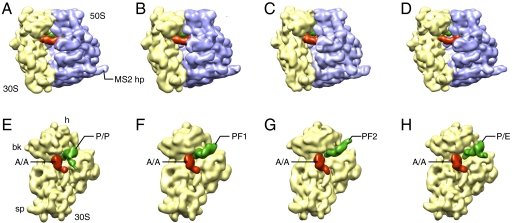
Fig. 1.
The four cryo-EM density maps of the G2252C PRE complex produced by maximum-likelihood classification in XMIPPS followed by SPIDER refinement (Table S1). (Upper) Density maps of the 70S structures. (Lower) Density maps of the 30S subunit and intersubunit ligands isolated computationally. (A) and (E) Density map of the nonrotated structure (NRS). (B) and (F) Intermediate rotated structure 1 (IRS1). (C) and (G) Intermediate rotated structure 2 (IRS2). (D) and (H) Rotated structure (RS). Landmarks: bk, beak; sp, spur; MS2 hp: MS2-binding hairpin inserted into helix 98 of the large subunit for purification purpose.
Using the crystal structure of the Escherichia coli ribosome [Protein Data Bank (PDB) IDs 212U and 212V (18)] as reference, we measured the extent of the counterclockwise rotation of the 30S subunit with respect to the 50S subunit (Fig. 2 A–C and Table 1). We term the structure with the least amount of rotation (approximately 1.0°) the nonrotated structure (NRS), the one with 3.2° of rotation the intermediate rotated structure 1 (IRS1), with 4.9°, the intermediate rotated structure 2 (IRS2), and, with 7.5°, the rotated structure (RS). We compared these structures with those found by X-ray crystallography of the ribosome bound with tRNA anticodon stem loop fragments (8). The two intermediate structures (3I1Q/3I1R and 3I1Z/3I20) obtained by these authors have rotation angles that are similar to those of IRS2 (Table 1).
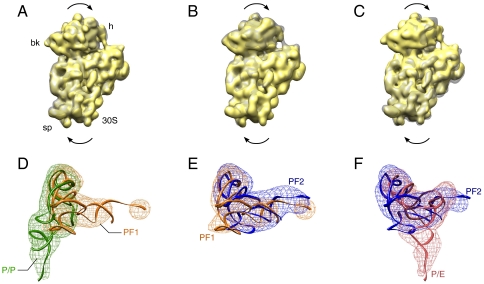
Fig. 2.
Comparison of the different states of theG2252C PRE complex. (Upper) Superimposition of the density maps of the 30S subunit, depicting the rotation of the 30S. (Lower) Superimposition of the density maps of the P-site tRNA, depicting the conformational change of the P-site tRNA. (A) and (D) Comparison between the nonrotated structure (NRS) and intermediate rotated structure 1 (IRS1). (B) and (E) Comparison between the intermediate rotated structure 1 (IRS1) and the intermediate rotated structure 2 (IRS2). (C) and (F) Comparison between the intermediate rotated structure 2 (IRS2) and the rotated structure (RS).
Table 1.
Conformational changes observed in the four structures and related crystal structures (see ref. 8)
| Rotation angle (°) | L1 stalk movement | Distance between the tRNAs (Å) | Distance between L1 and the tRNA (Å) | ||
| Inward rotation (°) | Forward rotation (°) | ||||
| 2I2U-2I2V | 0 | 0 | 0 | ||
| NR | 1.0 | 7 | −10 | 41 | 93 |
| IRS1 | 3.2 | 12 | −10 | 59 | 69 |
| IRS2 | 4.9 | 21 | −5 | 66 | 60 |
| RS | 7.5 | 35 | 12 | 69 | 37 |
| 3I1O -3I1P | 0.8 | 1 | −9 | N/A | |
| 3I1M -3I1N | 6.3 | −4 | 2 | ||
| 3I1S -3I1T | −0.9 | 3 | −3 | ||
| 3I1Q -3I1R | 5.0 | 0 | 0 | ||
| 3I21 -3I22 | −0.6 | 3 | −5 | ||
| 3I1Z -3I20 | 5.1 | 0 | 0 | ||
“Rotation angle” refers to the counterclockwise rotation of the 30S subunit with respect to the 50S subunit, viewed from the 30S subunit’s solvent side. “Larger angle” means larger degree of rotation. The “inward rotation” refers to the inward movement of the L1 stalk toward the main body of the ribosome. The “forward rotation” refers to the movement of the L1 stalk toward the 30S subunit of the ribosome. “Distance between tRNAs” refers to the distance between residue 47 of the A-site tRNA and residue 8 of the P-site tRNA, measured based on atomic models obtained trough real-space refinement. “Distance between L1 and the P-site tRNA” refers to the distance between residue 55 of the L1 stalk protein and residue 8 of the P-site tRNA. The model of the L1 stalk protein was obtained by aligning the L1 stalk of 3KNI, which contains the L1 stalk protein, with the atomic models of L1 stalk base obtained by real-space refinement.
The movement of the L1 stalk can be characterized by a combination of two directions of rotation around a hinge point located approximately at residue 2,197 of helix 76, one in the plane separating the subunits, and the other perpendicular to that plane, toward the 30S subunit (Fig. 3). We measured the angles of these two rotations (Table 1). It can be readily seen that the L1 stalk displays a gradual movement inward, into the intersubunit space, and the degree of this movement increases with the extent of the ratchet-like intersubunit rotation. The perpendicular movement, toward the 30S subunit, is observed in the IRS2 and RS structures. The L1 stalks in the crystal structures of the intermediate states (3I1M/3I1N, 3I1Q/3I1R, and 3I1Z/3I20, (8) do not display the inward rotation, but, compared to the nonrotated structures (3I1O/3I1P, 3I1S/3I1T, 3I21/3I22), they also display a rotation toward the 30S subunit (Table 1).
Fig. 3.
Interaction of the L1 stalk with the P-site tRNA in the intermediate rotated structure 1 (IRS1) and the intermediate rotated structure 2 (IRS2). (A) and (C) Interaction of the L1 stalk with the P-flipped 1 (PF1) tRNA observed in the intermediate rotated structure 1 (IRS1). (B) and (D) Interaction of the L1 stalk with the P-flipped 2 (PF2) tRNA observed in the intermediate rotated structure 2 (IRS2). The L1 stalk is depicted in blue, and tRNA in green. Two columns show the interactions from two different views. The thumbnail on the upper left corner of each column indicates the view and region from which the structures are shown.
Novel Configurations of the P-site tRNA.
The A-site tRNA remains at the A site in all four structures, whereas the P-site tRNA moves from the P-site to the P/E-hybrid position through intermediate steps. In NRS, the P-site tRNA remains at the classical P/P position (Fig. 1A). In IRS1, even though part of the tRNA density is weak, the orientation of the tRNA is unambiguous, providing basis for fitting the crystal structures of tRNA into the cryo-EM density. Compared to the classical P/P position, the tRNA has rotated clockwise (as viewed from the 50S subunit’s intersubunit side) around the anticodon arm by approximately 95°, having flipped the acceptor arm toward the E site on the 50S subunit (Figs 2A and 3A). We term this position P-flip 1 (PF1). The tip of the CCA end, as shown in the atomic model generated by real-space refinement, lies underneath helices 78 and 77, which are situated above helix 76 of 23S rRNA (Fig. 3).
The displacement of the tRNA in IRS2, compared to that in IRS1, can be characterized by an approximately 15° tilt toward the E site, with the anticodon stem loop fixed on the 30S subunit’s E site, and an approximately 13° clockwise rotation (viewed from the 50S intersubunit side) around the anticodon arm (Figs. 1C and 2B). We term this structure P-flipped 2 (PF2).
In RS, the P-site tRNA is in the hybrid P/E position (Fig. 1D) that has been observed in previous cryo-EM studies (5, 6). Because the A-site tRNA is in its classical A/A position, the configuration of the tRNA duplex in RS is thus a combination of A/A and P/E hybrid positions, just as suggested by the previous FRET study of the G2252C mutant (11).
Correlation with the sm-FRET Results on the G2252C PRE Complex.
We measured the distance between the residues where the fluorescent labels were attached to the tRNA (a distance we term dtRNAs) in the related sm-FRET study (11). The distances are 41 Å in NRS, 59 Å in IRS1, 66 Å in IRS2, and around 69 Å in RS. These measurements agree very well with the three-states situation inferred in the sm-FRET study: the dtRNAs measured for IRS2 and RS are very similar; thus, the low-FRET state observed in the sm-FRET study may be an aggregate of signals from IRS2 and RS. Moreover, the small differences in dtRNAs between the IRS1, IRS2, and RS configurations are consistent with the appearance in the histogram of the low-FRET population, which has a wide distribution. We also measured dtRNAs for the A/P and P/E tRNA duplex, based on the atomic model generated in a previous cryo-EM study (6). Here it is around 64 Å, which is similar to those in the IRS1, IRS2, and RS states of the ribosome.
Another sm-FRET study, showing that the motions of the L1 stalk and the tRNAs are not tightly coupled (19), is also consistent with our experimental results and interpretation. We inferred the position of L1 stalk protein by aligning the crystal structure of the helices 76–78 of 23S rRNA, along with that of the L1 protein in a ternary complex [PDB ID 3FIK (20)], to the atomic models of NRS, IRS1, IRS2, and RS obtained by real-space refinement. We then measured the distance between the L1 stalk protein (residue 55) and the P-site tRNA elbow where the dye is attached. This distance, which we denote as dL1-tRNA, is found to be 93 Å in NRS, 69 Å in IRS1, 60 Å in IRS2, and 37 Å in RS. This set of distances agrees well with the interpretation from the sm-FRET study, which identified four FRET states (11). By correlating the measurements of dL1-tRNA with dtRNAs (Table 1), we can see that their variations are indeed only loosely coupled. It is worth noting, though, that the L1 stalk protein may change its conformation and relative position with respect to the L1 stalk, potentially introducing further complexity to the behavior of dL1-tRNA.
Discussion
Through the investigation of a purified mutant ribosomal complex bearing the G2252C mutation in the P loop of the 23S rRNA, we have obtained structures of the ribosome in three previously uncharacterized intermediate states, in which the 30S ribosomal subunit is in intermediate rotational positions and the tRNAs are in previously uncharacterized positions. By establishing a correlation between tRNA positions and defined ribosome configurations, our observations lend further credence to the notion that tRNA repositioning goes hand in hand with distinct conformational changes of the ribosome.
One of the unique structures contains the (A/A, P/E) tRNA duplex, a configuration previously suggested by sm-FRET experiments on the G2252C PRE sample (11). Whereas it cannot be ruled out that this intermediate configuration is attained solely as the consequence of the G2252C mutation, sm-FRET studies of both wild-type and mutant PRE complexes suggest such intermediate configuration exists in the wild-type, as well, and that the reason we observe it by cryo-EM is that it is more populated in the G2252C mutant. There are two possible reasons why this (A/A, P/E) configuration has not been observed before by cryo-EM. First, the structural difference between tRNAs in A/A versus A/P configurations is rather small (6). Second, the difference in the angle of ratchet-like rotation between RS (approximately 7.5°) and the PRE complex bearing the A/P and P/E duplex (approximately 8.5°) is quite small, as well. Thus, it would be difficult to extract the RS state we observed here by less sensitive classification methods. Our interpretation of the unique configurations is that the point mutation may just decrease the frequency or likelihood of transitions of RS into the fully rotated hybrid state, characterized by tRNAs situated in A/P and P/E.
In the other two unique structures revealed in our study, the deacylated tRNA adopts distinct, flipped configurations, in which the acceptor arm is rotated toward the E site around the anticodon arm. This poses a problem because the tRNA in the flipped position evidently must maintain its codon-anticodon interaction. Crystallography studies showed that the anticodon loop of the tRNA could adopt conformations that would accommodate some degree of rotation of the P-site tRNA (21–23), though the size of this variation falls short of what we observe. Another possible source of flexibility comes from the mRNA, which has also been shown by crystallography studies to have some degree of flexibility (24).
Because the movement of the P-site tRNA goes hand in hand with conformational changes of the ribosome, it can be concluded that these unique configurations are the result of discrete intermediate steps that occur between the classical P-site position of the deacylated tRNA and formation of the P/E hybrid tRNA configuration. As the G2252C point mutation is unlikely to change the trajectory of the tRNA movement, we interpret the two observed intermediate positions as being physiologically relevant. These states, which have escaped detection in previous structural studies, may be more populated as a consequence of the mutation through destabilization of the classical, P/P configuration. As a result, the number of molecules occupying normally short-lived intermediate states would be increased. Importantly, the flipped P-site tRNA configurations are present in the partially rotated structures, and part of the tRNA density is not well resolved, suggesting that the motions of the tRNA and the ribosome may not be perfectly coupled. Thus, the flipped P-site tRNA configurations would likely be averaged out when traditional supervised classification methods are used, which compare particles to either preselected, classical/nonrotated, or hybrid/rotated states reference structures.
Probably of critical importance for the present findings, the Mg2+ concentration in our specimen is 15 mM. High Mg2+ concentrations reduce the rates of tRNA dynamics in the wild-type PRE complex (25) and favor classical A- and P-site tRNA configurations in the wild-type PRE complex (11, 25, 26) and hybrid state tRNA configurations in the G2252C PRE complex (11).
The L1 stalk plays an important role in translocation (5, 10, 11). As far as the structure is concerned, cryo-EM studies have shown that the L1 stalk interacts with the elbow of the P/E hybrid tRNA and thereby serves to stabilize the hybrid position (5). Here, we show that, through its CCA ends, the tRNA interacts with the L1 stalk even before it forms the P/E position (Fig. 3). We believe that these interactions might be an important prerequisite for the formation of the P/E hybrid state tRNA. As described above, in IRS1, helices 77 and 78 of the 23S rRNA latch on to the PF1 tRNA above the tip of the CCA end (Fig. 3A). This interaction may stabilize the tRNA in the intermediate, flipped position, which would readily explain why this state is sufficiently populated to be captured by classification. In the ISR2 structure, the position of the L1 stalk (Fig. 3B), still allows the CCA end of the PF2 tRNA to interact closely with the L1 stalk. Further L1 stalk movement in the direction toward the 30S subunit would allow helices 77 and 78 to slide over the CCA end of the tRNA, disengaging these interactions, presumably to allow the P-site tRNA to reach the P/E hybrid state, as observed in the RS structure. The existence of discernible tRNA-L1 stalk interactions, combined with destabilization of the classical P/P state as a consequence of the G2252C mutation, may explain why flipped states of the P-site tRNA are sufficiently populated (i.e., in the 10% range) to be captured by classification.
Combining our results with those from previous sm-FRET experiments (10, 11, 19, 27), we propose a mechanism for the L1 stalk inducing the formation of the hybrid state (see Fig. S1). In this mechanism, the formation of the hybrid state is the consequence of a productive interaction between the L1 stalk and the CCA end of the tRNA, as seen in configurations IRS1 and IRS2. Before this productive interaction, the random motion of the L1 stalk in and out of the intersubunit space provides opportunities for chance encounters with the tRNA that fluctuates independently, but these encounters do not result in binding events with high probability. The tRNA is committed to form the hybrid P/E position only when its CCA end is latched onto the L1 stalk. One piece of evidence supporting this interpretation comes from the related sm-FRET study (11). In the L1-stalk depleted molecules, the rate of transitions of the tRNA duplex from the classical to the hybrid state is strongly decreased, suggesting that the L1 stalk may not only stabilize the P/E state of the tRNA, but also induce its formation. The increased population in the hybrid state in the G2252C mutant can be explained by the increase of the chances of encounters between the tRNA and the L1 stalk: The mutation has the effect that the PF1 configuration is sampled more frequently by the deacylated tRNA, increasing the chances for the L1 stalk to catch the CCA end of the tRNA, and thus increasing the probability of the ribosome transiting to the second setting.
As this study was completed, we became aware of a study of back-translocation by Fischer et al. (28), in which multiple intermediate conformational states of the ribosome–tRNA complex were identified. None of the states identified in the present study of forward-translocation has a counterpart in the results presented by Fischer and et al. although, conceivably, there might be a partial overlap in the states visited during the two processes.
Methods
Preparation of Ribosome Complexes.
Ribosome complex was prepared as previously described (11).
Electron Microscopy and Image Processing.
The ribosome specimen was diluted into a final concentration of 42 nM. A cryogrid (carbon-coated Quantifoil 2/4 grid, Quantifoil Micro Tools GmbH) was prepared as previous described (29). Microscopy was performed under low-dose conditions on an FEI Tecnai Polara instrument operating at 300 kV and a nominal magnification of 59,000×. Micrographs were recorded on a 4k × 4k 16-bit TVIPS TemCam-F415 CCD camera with a physical pixel size of 15 μm by using the automated data collection system AutoEMation (30). The effective magnification on the CCD was 100,000× due to a postmagnification ratio of 1.7, thus making the pixel size 1.5 Å on the object scale. Micrographs were subsequently decimated to a 3 Å pixel size to boost the low-spatial frequency signal and reduce the processing time. Reconstruction was done following standard SPIDER protocols for reference-based reconstruction (31). Maximum-likelihood classification (16) was performed using the XMIPP package.
Real-space refinement fitting was done as described previously (17, 32). The initial atomic models were from the crystal structure of the E. coli ribosome [PDB IDs 2AVY and 2AW4 (33)]. The head of H38 was from PDB structure 1PNY (34). The initial atomic model of A-site tRNA used was from PDB structure 2WDG (35).
Supplementary Material
Acknowledgments.
We thank Jianlin Lei and Bob Grassucci for assistance in the microscopy, Lila Iino-Rubinstein and Melissa Thomas for assistance in the preparation of the illustrations, Eduard Schreiner for calculating the 30S rotation angles in Table 1, and Jesper Pallesen and Yaser Hashem for a critical reading of the manuscript. This work was supported by the Howard Hughes Medical Institute and National Institutes of Health R01 GM29169 (J. Frank), P01 GM064692 (to Robert M. Glaeser), and R01 GM079238 (to S.C.B.)
Footnotes
The authors declare no conflict of interest.
Data deposition: The cryo-EM maps reported in this paper have been deposited with the Electron Microscopy Data Bank, http://emdatabank.org/ (accession no. EMD-5262).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101503108/-/DCSupplemental.
References
- 1.Dunkle JA, Cate JH. Ribosome structure and dynamics during translocation and termination. Annu Rev Biophys. 2010;39:227–244. doi: 10.1146/annurev.biophys.37.032807.125954. [DOI] [PubMed] [Google Scholar]
- 2.Pestka S. Studies on the formation of trensfer ribonucleic acid-ribosome complexes. V. On the function of a soluble transfer factor in protein synthesis. Proc Natl Acad Sci USA. 1968;61:726–733. doi: 10.1073/pnas.61.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 4.Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 5.Valle M, et al. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 6.Agirrezabala X, et al. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol Cell. 2008;32:190–197. doi: 10.1016/j.molcel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank J, Gao H, Sengupta J, Gao N, Taylor DJ. The process of mRNA-tRNA translocation. Proc Natl Acad Sci USA. 2007;104:19671–19678. doi: 10.1073/pnas.0708517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Dunkle JA, Cate JH. Structures of the ribosome in intermediate states of ratcheting. Science. 2009;325:1014–1017. doi: 10.1126/science.1175275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard SC. Single-molecule observations of ribosome function. Curr Opin Struc Biol. 2009;19:103–109. doi: 10.1016/j.sbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fei J, Kosuri P, MacDougall DD, Gonzalez RL., Jr Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol Cell. 2008;30:348–359. doi: 10.1016/j.molcel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Munro JB, Altman RB, O’Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munro JB, et al. Spontaneous formation of the unlocked state of the ribosome is a multistep process. Proc Natl Acad Sci USA. 2009;107:709–714. doi: 10.1073/pnas.0908597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornish PV, Ermolenko DN, Noller HF, Ha T. Spontaneous intersubunit rotation in single ribosomes. Mol Cell. 2008;30:578–588. doi: 10.1016/j.molcel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ermolenko DN, et al. Observation of intersubunit movement of the ribosome in solution using FRET. J Mol Biol. 2007;370:530–540. doi: 10.1016/j.jmb.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Dorner S, Brunelle JL, Sharma D, Green R. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat Struct Mol Biol. 2006;13:234–241. doi: 10.1038/nsmb1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheres SH, et al. Disentangling conformational states of macromolecules in 3D-EM through likelihood optimization. Nat Methods. 2006;4:27–29. doi: 10.1038/nmeth992. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, et al. Study of the structural dynamics of the E. coli 70S ribosome using real-space refinement. Cell. 2003;113:789–801. doi: 10.1016/s0092-8674(03)00427-6. [DOI] [PubMed] [Google Scholar]
- 18.Berk V, Zhang W, Pai RD, Cate JH. Structural basis for mRNA and tRNA positioning on the ribosome. Proc Natl Acad Sci USA. 2006;103:15830–15834. doi: 10.1073/pnas.0607541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munro JB, et al. Spontaneous formation of the unlocked state of the ribosome is a multistep process. Proc Natl Acad Sci USA. 2009;107:709–714. doi: 10.1073/pnas.0908597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa E, et al. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc Natl Acad Sci USA. 2009;106:1063–1068. doi: 10.1073/pnas.0811370106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barraud P, Schmitt E, Mechulam Y, Dardel F, Tisne C. A unique conformation of the anticodon stem-loop is associated with the capacity of tRNAfMet to initiate protein synthesis. Nucleic Acids Res. 2008;36:4894–4901. doi: 10.1093/nar/gkn462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuart JW, Koshlap KM, Guenther R, Agris PF. Naturally-occurring modification restricts the anticodon domain conformational space of tRNA(Phe) J Mol Biol. 2003;334:901–918. doi: 10.1016/j.jmb.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 23.Rees B, Cavarelli J, Moras D. Conformational flexibility of tRNA: structural changes in yeast tRNA(Asp) upon binding to aspartyl-tRNA synthetase. Biochimie. 1996;78:624–631. doi: 10.1016/s0300-9084(96)80008-3. [DOI] [PubMed] [Google Scholar]
- 24.Yusupova G, Jenner L, Rees B, Moras D, Yusupov M. Structural basis for messenger RNA movement on the ribosome. Nature. 2006;444:391–394. doi: 10.1038/nature05281. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard SC, Kim HD, Gonzalez RL, Jr, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HD, Puglisi JD, Chu S. Fluctuations of transfer RNAs between classical and hybrid states. Biophys J. 2007;93:3575–3582. doi: 10.1529/biophysj.107.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fei J, et al. Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation. Proc Natl Acad Sci USA. 2009;106:15702–15707. doi: 10.1073/pnas.0908077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 29.Grassucci RA, Taylor DJ, Frank J. Preparation of macromolecular complexes for cryo-electron microscopy. Nat Protoc. 2007;2:3239–3246. doi: 10.1038/nprot.2007.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei J, Frank J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J Struct Biol. 2005;150:69–80. doi: 10.1016/j.jsb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Shaikh TR, Trujillo R, LeBarron JS, Baxter WT, Frank J. Particle-verification for single-particle, reference-based reconstruction using multivariate data analysis and classification. J Struct Biol. 2008;164:41–48. doi: 10.1016/j.jsb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman M. Restrained real-space macromolecular atomic refinement using a new resolution-dependent electron-density function. Acta Crystallogr A. 1995;51:69–80. [Google Scholar]
- 33.Schuwirth BS, et al. Structures of the bacterial ribosome at 3.5 Å resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 34.Vila-Sanjurjo A, et al. X-ray crystal structures of the WT and a hyper-accurate ribosome from Escherichia coli. Proc Natl Acad Sci USA. 2003;100:8682–8687. doi: 10.1073/pnas.1133380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voorhees RM, Weixlbaumer A, Loakes D, Kelley AC, Ramakrishnan V. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat Struct Mol Biol. 2009;16:528–533. doi: 10.1038/nsmb.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.