Abstract
Numerous G protein-coupled receptors (GPCRs) have been shown to form heteromeric receptors in cell-based assays. Among the many heteromers reported in the opioid receptor family are μ/κ, κ/δ, and μ/δ. However, the in vivo physiological and behavioral relevance for the proposed heteromers have not yet been established. Here we report a unique example of a ligand, N-naphthoyl-β-naltrexamine (NNTA) that selectively activates heteromeric μ/κ-opioid receptors in HEK-293 cells and induces potent antinociception in mice. NNTA was an exceptionally potent agonist in cells expressing μ/κ-opioid receptors. Intriguingly, it was found to be a potent antagonist in cells expressing only μ-receptors. In the mouse tail-flick assay, intrathecal (i.t.) NNTA produced antinociception that was ~100-fold greater than by intracerebroventricular (i.c.v.) administration. The κ-antagonist, norBNI, decreased the i.t. potency, and the activity was virtually abolished in μ-opioid receptor knockout mice. No tolerance was induced i.t., but marginal tolerance (3-fold) was observed via the i.c.v. route. Moreover, NNTA produced neither significant physical dependence nor place preference in the ED50 dose range. Taken together, this work provides an important pharmacologic tool for investigating the in vivo functional relevance of heteromeric μ/κ-opioid receptors and suggests an approach to potent analgesics with fewer deleterious side effects.
Keywords: pain
Opioid receptors are class A members of the G protein-coupled receptor (GPCR) superfamily. There is high amino acid homology (~60%) within the opioid receptor family that constitutes a group of four receptor types: MOP (μ), DOP (δ), KOP (κ), and NOP (nociceptin, orphanin FQ, ORL1) (1, 2). Opioid receptors are present in the central nervous system and peripherally, including immune cells, and are believed to function as neuromodulators or immunomodulators. Morphine is among the best known clinically used analgesics that activate opioid receptors.
Although the concept of opioid receptor dimers was proposed nearly 30 y ago (3, 4), classical models of GPCRs, including opioid receptors, were generally based on the assumption that they are organized and function as monomers (5). However, in view of burgeoning evidence for the existence of heteromeric GPCRs, which includes at least 12 different heteromeric opioid receptors in cultured cells (6–17), it seems likely that constitutive oligomerization of GPCRs may be the general rule rather than the exception (18–21).
It appears that heteromers are not artifacts of overexpression, inasmuch as it has been shown that constitutively expressed receptor heteromers are secreted from the endoplasmic reticulum before being expressed on the cell surface (22–24). Moreover, it has been shown that μ-opioid and CCK2 receptors do not form constitutive heteromers when overexpressed in Chinese hamster ovary (CHO) cells, but can be induced to form heteromers in the presence of a bivalent ligand containing μ-agonist and CCK antagonist pharmacophores (25). Thus, heteromerization does not appear to be an artifact related to receptor overexpression in cultured cells.
Although the results in cultured cells provide insight into the propensity for constitutive heteromerization, extrapolation to in vivo systems cannot be easily made without reliable pharmacological tools. Selective pharmacological tools that span the divide between cultured cells and in vivo systems can clarify the functional roles and localization of heteromeric opioid receptors in experimental animals and lead to the development of potent analgesics devoid of side effects. For example, studies with a series of bivalent ligands containing μ-agonist and δ-antagonist pharmacophores have led to the proposal for an in vivo role of heteromeric μ/δ-opioid receptors in opioid dependence (26, 27).
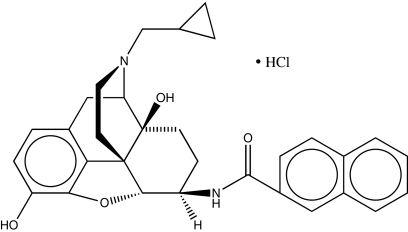
Here we report a unique example of a monovalent ligand, N-naphthoyl-β-naltrexamine (NNTA) (Fig. 1) that selectively activates heteromeric μ/κ-opioid receptors in HEK-293 cells. Moreover, NNTA exhibits potent antinociception and lack of physical dependence or conditioned place preference in mice (28–30).
Fig. 1.
Molecular structure of N-Naphthoyl-β-naltrexamine (NNTA).
Results
Immunofluorescence.
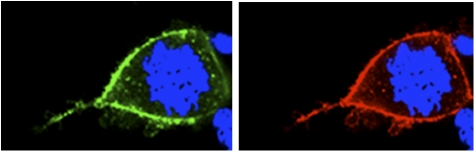
HEK-293 cells stably expressing hemagglutinin-tagged μ (HA-μ) or FLAG-tagged κ (FL-κ) receptors were used in the immunofluorescence assay to determine their relative densities. Cells were stained with anti-HA and anti-FLAG primary and respective secondary antibodies and imaged. High-resolution confocal images reveal both μ- and κ-opioid receptors to be extensively colocalized and similarly expressed on the cell surface of HEK-293 cells (Fig. 2).
Fig. 2.
High-magnification confocal images of double-labeling immunofluorescence for μ- and κ-opioid receptors on HEK-293 cells. The identical cells are shown labeled for μ (green fluorescence) and κ (red fluorescence). DAPI counterstaining is shown in blue.
Binding of NNTA in Stably Expressing HEK-293 Cells.
The affinity of NNTA for the different opioid receptor types was investigated in cells stably transfected with the different types of opioid receptors. In a competition binding assay using [3H]diprenorphine (Table S1), NNTA was found to bind with very high affinity to cells that express μ- (Ki = 0.077 pM) or κ- (Ki = 0.084 pM) opioid receptors. NNTA possessed substantially lower binding affinity for δ-opioid receptors (Ki = 1.39 nM).
The selective radioligands, [3H]DAMGO and [3H]U69593, were used for μ- and κ-opioid receptors, respectively, in cells containing μ- or κ-receptors alone and in μ/κ-coexpressing cells (Table S2). Competition binding with these radioligands afforded Ki values that were essentially identical between the cells coexpressing μ/κ-receptors and cells singly expressing μ- or κ-receptors.
NNTA Selectively Activates μ/κ-Heteromeric Opioid Receptors.
The selectivity of NNTA for the different opioid receptors was determined using intracellular Ca2+ release experiments in HEK-293 cells stably expressing opioid receptors. Six different cell lines containing singly expressed μ-, κ-, and δ-receptors or coexpressed as μ/κ, κ/δ, and μ/δ were used for the experiments. It is important to note here that the same coexpressed cell lines used in our studies have been previously established to be expressing heterodimeric receptors using coimmunoprecipitation (31, 32). The six different stably expressing cell lines were transiently transfected with Δ6-Gαqi4-myr (33), a chimeric Gα subunit to measure the extent of intracellular Ca2+ ion release due to receptor activation. The chimeric G protein has already been characterized previously and used for different Gi/Go G protein-coupled receptors, including the opioid receptors (31, 33, 34). Prior comparisons between the Δ6-Gαqi4-myr method and [35S]GTPγS binding have given consistent results (34).
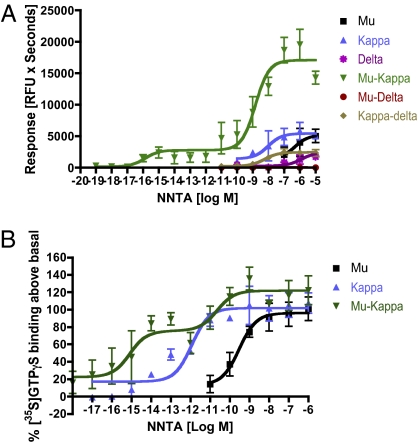
NNTA was substantially more efficacious in the cells coexpressing μ/κ-opioid receptors [AUCpeak = 15,947 relative fluorescent units (RFU) × seconds] compared with other cell lines (Fig. 3A and Tables S3 and S4). Interestingly, the concentration response curve of NNTA at the μ/κ-opioid receptor was biphasic with activation observed at concentrations as low as 10−16 M (Fig. 3A). The different cell lines were evaluated for consistent receptor expression and activation using the standard ligands, DAMGO (μ), U69593 (κ), and DPDPE (δ), as controls (Tables S3 and S4).
Fig. 3.
NNTA is a highly selective agonist at the μ/κ-opioid receptor heteromers in (A) intracellular calcium release and (B) [35S]GTPγS experiments. The concentration response curve showed a characteristic biphasic response in the HEK-293 cells coexpressing μ/κ-opioid receptor heteromers.
To ensure the reproducibility of the results obtained from the Ca2+ release experiments, we evaluated NNTA using the [35S]GTPγS assay in membranes isolated from HEK-293 cells stably expressing μ-, κ-, and μ/κ-opioid receptors. It is important to note here that for this experiment, the binding of [35S]GTPγS to the endogenous pool of G proteins was measured because the chimeric G protein was not transfected. Thus, the data observed was independent of the calcium release experiments to reassess NNTA's unique selectivity using a different experimental technique. NNTA was again most potent at the μ/κ-opioid heteromers (EC50 1 = 0.81 fM; EC50 2 = 22 pM) compared with κ (1.1 pM) and μ (0.3 nM). The biphasic concentration response was even more pronounced with greater activation in the sub-pM range (Fig. 3B) using this alternate method.
NNTA Is a Potent Antagonist at Homomeric μ-Opioid Receptors.
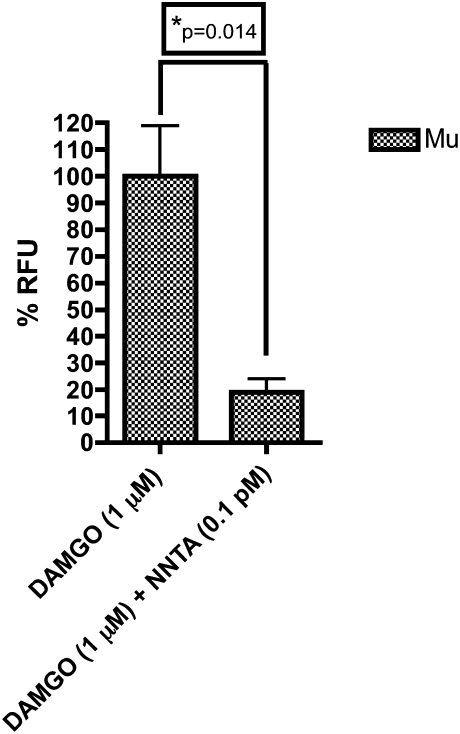
Given that NNTA contains a pharmacophore derived from the μ-selective opioid antagonist, naltrexone, we wished to evaluate the possibility of antagonism at μ-opioid receptors. We therefore evaluated NNTA antagonism of the standard μ-agonist, DAMGO, in the μ-opioid receptor cell line. NNTA (0.1 pM) was observed to significantly antagonize (~80%) the effect produced by 1 μM of DAMGO (Fig. 4).
Fig. 4.
NNTA antagonized the effect of DAMGO in HEK-293 cells expressing μ-opioid receptors in the intracellular calcium release assay. There was ~80% inhibition of DAMGO (1 μM) by NNTA (0.1 pM) that was determined to be significant by an unpaired t test (two-tailed, P < 0.05).
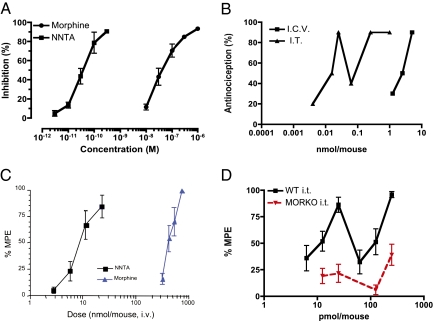
NNTA Is a Highly Potent Agonist in the Guinea Pig Ileum and Mice.
NNTA was tested for agonistic activity in the Hartley guinea pig ileum (Charles River Laboratories). In comparison with morphine (IC50 = 50.6 nM), NNTA (IC50 = 0.038 nM) was 1,225 times more potent (Fig. 5A). In ICR-CD1 mice (Harlan Laboratories), NNTA produced potent antinociception through both intrathecal (i.t.) and intracerebroventricular (i.c.v.) routes when tested using the tail-flick assay (Fig. 5B). Interestingly, NNTA produced a biphasic dose–response relationship when administered i.t. The antinociceptive ED50 (95% CI) values were 18.7 (10.3–32.8) pmol per mouse and 101.7 (75.3–137.5) for the i.t. route and 2.06 (1.09–3.27) nmol per mouse for the i.c.v. injections. Thus, NNTA was at least 110-fold more potent when administered spinally than when given supraspinally.
Fig. 5.
NNTA is a potent agonist in vivo but lacks spinal activity in MORKO mice. (A) NNTA was an ~1200-fold more potent agonist compared with morphine in the guinea pig ileum. (B) In the ICR-CD1 mouse tail-flick experiments (Quantal method) a biphasic antinocicpetive dose–response was observed after intrathecal (i.t.) administration (ED50 = 9.8 pmol per mouse and 101.7 pmol per mouse); NNTA was >200-fold more potent i.t. than i.c.v. (ED50 = 2.1 nmol per mouse). (C) NNTA (ED50 = 8.8 nmol per mouse, CI: 6.8–11.5) was at least 50-fold more potent compared with morphine (ED50 = 420 nmol per mouse, CI: 378–469) when administered i.v. (D) NNTA did not produce significant antinociception when administered intrathecally in the MORKO mice at doses that produced strong antinociception in wild-type mice. MPE, maximal possible effect.
NNTA is also a potent antinociceptive agonist when administered by the i.v. route (Fig. 5C), with an ED50 of 8.8 (6.8–11.5) nmol per mouse. By comparison, the i.v. morphine ED50 value was 420 (378–469) nmol per mouse or ~50-fold more potent than morphine after systemic administration. NNTA was also active via the oral route with an ED50 of 2.68 mg/kg (1.74–4.29).
NNTA Is Antagonized by the κ-Selective Antagonist, norBNI, in Mice and HEK-293 Cells Coexpressing μ- and κ-Receptors.
Selective antagonists were used to investigate possible mechanisms for the agonism of NNTA in ICR-CD1 mice. Antagonist ED50 ratios were determined for selective μ- and κ-antagonists β-funaltrexamine (β-FNA) (35) and norbinaltorphimine (norBNI) (36), respectively. Whereas β-FNA did not significantly antagonize NNTA i.c.v., norBNI produced weak antagonism by that route (ED50 ratio = 5.63). More potent antagonism was observed upon i.t. administration (ED50 ratio = 38.26).
Because β-FNA induced significant calcium release in HEK-293 cells coexpressing μ- and κ-receptors, we used the μ-antagonist d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) and norBNI to antagonize NNTA (Fig S2). CTOP did not antagonize NNTA, whereas norBNI significantly antagonized NNTA, corroborating the in vivo data.
NNTA Is Not Spinally Active in μ-Knockout (MORKO) Mice.
Given the potent and biphasic spinal activity of NNTA in wild-type mice, its cell-based antagonistic activity at μ-opioid receptors and highly potent activity at μ/κ-receptors, these data suggested that the spinal activity was mediated via μ/κ-heteromeric receptors rather than homomeric κ-receptors in vivo. Hence, we determined the activity of NNTA in μ-knockout mice (MORKO) at doses that produced antinociception (12.5, 25, 125, and 250 pmol per mouse) in the corresponding wild-type mice (B6129PF1/J). NNTA did not produce significant antinociception in MORKO mice for the dose ranges tested (Fig. 5D).
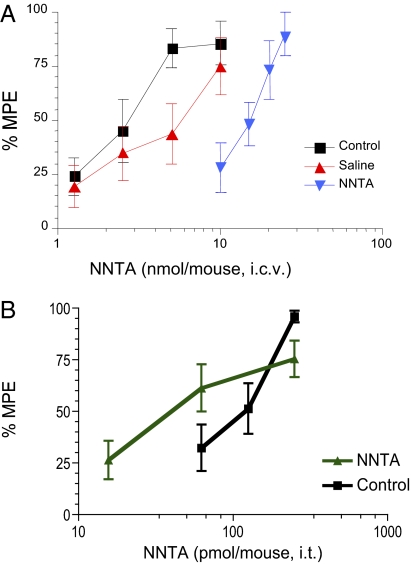
NNTA Produces No Intrathecal Tolerance or Physical Dependence.
The i.c.v. tolerance and physical dependence experiments were conducted as described previously (37) using osmotic minipumps to infuse NNTA or saline i.c.v. for 3 d. Results shown in Fig. 6A are the tail-flick dose–response curves obtained for NNTA administered i.c.v. to naïve control mice, mice infused i.c.v. with saline, and mice infused i.c.v. with NNTA. The ED50 values calculated from these curves are: control, 2.5 (1.8–3.6) nmol per mouse; saline infused, 4.8 (2.8–8.1) nmol per mouse; and NNTA infused, 14.1 (11.9–17.5) nmol per mouse. These results indicate that a threefold tolerance developed to NNTA-induced antinociception. Previous studies using morphine in the same protocol showed a sixfold development of tolerance to i.c.v. morphine (30).
Fig. 6.
NNTA i.c.v. and i.t. tolerance studies. (A) Saline or NNTA was infused for 3 d and the antinociceptive ED50’s were determined on the fourth day. The dose–response curve for NNTA i.c.v. infusion (blue triangles) is right shifted (threefold) compared with saline infused (red triangles) and acute NNTA treatment (control, black squares) in the mouse tail-flick assay, indicating the development of tolerance. (B) NNTA (250 pmol per mouse) was injected i.t. twice a day (5-h gap between injections) for 2 d and once on the third day after which, the tail-flick latencies were calculated. No significant tolerance was observed as the NNTA curve did not differ significantly from the acute NNTA treatment (control) after the repeated injections.
To determine i.t. tolerance, mice were injected i.t. with NNTA (250 pmol per mouse) twice daily (5-h gap between injections) for 2 d and once on the third day, after which the antinociceptive ED50 was determined. NNTA did not induce any significant tolerance (Fig. 6B), as the control ED50 of 101.7 pmol per mouse (75.27–137.4) did not significantly differ from the NNTA ED50 of 46.3 pmol per mouse (22.91–93.42).
After 3 d of NNTA infusion, the naloxone-induced jumping method failed to reveal any physical dependence. Naloxone did not induce significant jumping in either saline (1.57 ± 0.7 jumps) or NNTA-infused mice (1.0 ± 0.7 jumps) over a 10-min period. Under identical conditions in prior studies (31), naloxone induced 100 jumps in i.c.v. morphine-infused mice, whereas only ~5 jumps were observed for the saline-infused mice. Thus, in contrast to the robust tolerance and physical dependence that develops after chronic i.c.v. morphine infusion (26), no withdrawal syndrome was effected with naloxone after chronic i.c.v NNTA treatment.
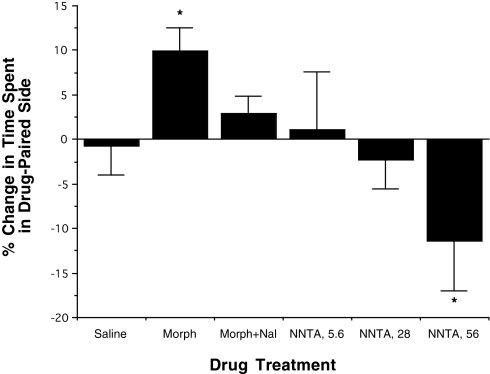
Conditioned place preference studies corroborated the absence of reward learning and drug-seeking behavior of NNTA. In the conditioned place preference experiments set up as described previously (27), morphine (1,200 nmol/kg) produced a place preference that was blocked by naloxone (300 nmol/kg). The two lower doses of NNTA (5.6 and 28 nmol) did not produce a significant effect on the time that the mice spent in the drug-paired chamber (Fig. 7). However, at the 10× ED50 dose (56 nmol), NNTA produced a significant place aversion effect (P < 0.1).
Fig. 7.
NNTA does not produce dependence in mouse conditioned place preference studies. NNTA at the ED50 dose (5.6 nmol/kg, i.v.) and 5× ED50 dose (28 nmol/kg, i.v.) did not produce any significant place preference, suggesting a lack of reward due to chronic NNTA treatment. At the 10× ED50 dose (56 nmol/kg, i.v.) considerable place aversion was observed. On the other hand, morphine (1,200 nmol/kg) in the same protocol produced robust place preference that could be reversed by naloxone (300 nmol/kg).
Discussion
The report by Wang et al. (9) that demonstrated the association of μ- and κ-opioid receptors as heteromers in cultured cells renewed our interest in a ligand, NNTA (Fig. 1), prepared in our laboratory a decade ago. In view of its extremely high potency (1,200-fold greater than morphine) in the guinea pig ileum preparation (Fig. 5A) and sub-pM binding affinity for μ- and κ-receptors (Tables S1 and S2), we entertained the possibility that NNTA might activate μ/κ-heteromeric receptors (28, 29). In view of the pressing need for selective pharmacologic tools for the study of heteromeric opioid receptors in vivo, we investigated the properties of NNTA in cultured cells and mice.
Experiments with HEK-293 cells containing stably expressed homomeric (μ-, κ-, and δ-) and heteromeric-opioid receptors (μ/κ-, μ/δ-, and δ/κ) using fluorometric Ca2+ ion release methodology and [35S]GTPγS binding assays as an index of receptor activation revealed results that were consistent with selective activation of heteromeric μ-κ (Fig. 3). In this regard, treatment of the μ/κ-cell line with NNTA led to remarkably potent activation at concentrations consistent with the binding data. Significantly, interaction of NNTA with cell lines that contained other heteromeric opioid receptors or homomeric receptors afforded substantially lower activity.
The remarkable ~1,000-fold enhancement of agonist potency exhibited in the μ/κ-cell line compared with singly expressed κ-receptors conceivably may be due to allosterically induced amplification of activation of the κ-receptor due to heteromer formation. From this perspective, and in view of the comparable affinity of NNTA for μ- and κ-receptors, one possible scenario is that a conformational change induced in the μ-receptor protomer leads to enhanced activation via its NNTA-bound κ-receptor partner in the heteromeric μ/κ-receptors. The high μ-receptor antagonist potency of NNTA in the μ-receptor cell line would be consistent with this view (Fig. 4).
Because the results of experiments with the HEK-293 cell lines were consistent with the selective activation of μ/κ-heteromers by NNTA, we investigated its antinociceptive properties using the mouse tail-flick assay (Fig. 5 B and C). By the i.t. route, NNTA exhibited a biphasic dose–response relationship (ED50 = 18.7 pmol and 101.7 pmol), which was over 100-fold greater than by i.c.v. administration. The large difference raised the possibility that NNTA might be activating phenotypic opioid receptors that are more responsive in the cord compared with those in the brain.
Because a similar phenomenon has been reported previously (31, 38) in connection with differential populations of putative heteromeric opioid receptors in the spinal cord and brain, we were interested in pharmacologically characterizing the receptors that were activated by NNTA. This involved determining the ED50 ratios for antagonism using selective antagonists for κ- (norBNI) and μ- (β-FNA) opioid receptors. These data revealed that only norBNI antagonized the effect of NNTA, which suggested involvement of κ-receptors in the antinociceptive response.
In view of reports for the colocalization of δ- and κ-receptors in rodent spinal cord (39) and the presence of putative heteromeric δ/κ-receptors (31, 38), taken together with our cell-based data that show NNTA to be substantially more efficacious in activating μ/κ-heteromers, the exceptional potency of i.t. NNTA tends to support the concept that the observed spinal antinociception is mediated principally via μ/κ-heteromers. This concept was further supported by the lack of significant spinal antinociception in μ-opioid receptor knockout mice (Fig. 5D), demonstrating that κ-receptors alone are unable to mediate the potent spinal activity of NNTA. These data are also consistent with the significantly lower activity of NNTA in cells expressing only κ-opioid receptors in the intracellular calcium release assay (Fig. 3).
Given the unique properties of NNTA, it was evaluated for tolerance and physical dependence. Antinociceptive tolerance was dependent on the route of administration of NNTA. Some tolerance was observed after 3 d of infusion by the i.c.v. route (Fig. 6A), although it was substantially less robust compared with morphine (34). Significantly, no tolerance was observed when NNTA was administered i.t. (Fig. 6B). These results, coupled with the potent spinal antinociception of NNTA in wild-type mice and lack of spinal activity in μ-knockout mice, suggest that there may be a tissue-dependent localization of different phenotypic receptor populations as suggested from other studies (31, 38, 40). Because the in vitro data showed NNTA to be highly selective and active at heteromeric μ/κ-opioid receptors, these receptors may be the predominant target for NNTA in the cord.
Our finding that naloxone did not precipitate naloxone-induced jumping in mice infused with NNTA for 3 d suggested the absence of physical dependence. Moreover, conditioned place preference (CPP) studies indicated the absence of reward learning or drug-seeking behavior through the administration of NNTA (Fig. 7). At the ED50 dose (5.6 nmol) and 5× ED50 dose (28 nmol) of NNTA, there was no significant CPP. However, there was a significant dose-related trend for aversion at doses of NNTA that produced antinociception in 100% of the mice. The aversion of NNTA at high dose is of interest because members of the clinically used mixed agonist–antagonist class (2) of opioids that have κ-agonist and μ-antagonist components, are known to produce dysphoria in humans (2). Whether the dysphoria is mediated primarily through activation of μ/κ-heteromers, homomeric κ-receptors or other κ-receptor–containing heteromers remains to be investigated.
In conclusion, the present study has revealed that the highly potent opioid ligand, NNTA, selectively activates heteromeric μ/κ in cultured cells, and therefore may be a useful pharmacological tool for investigating the role of such receptors in vitro and in vivo. As NNTA produces unusually potent, biphasic spinal (i.t.) antinociception that is two orders of magnitude greater than its supraspinal (i.c.v.) effect in mice, and given its very high potency in cultured cells, the mouse spinal cord may possibly contain μ/κ-heteromers. The potent spinal activity is abolished in MORKO mice, further corroborating the high degree of functional coupling between μ- and κ-receptors in the cord. Because the unusually high potency of NNTA was initially uncovered through screening on the electrically stimulated guinea pig ileum, this preparation also may possibly contain a subpopulation of μ/κ-heteromers. This is of interest because the guinea pig ileum (GPI) has been widely used in the screening of opioid agonists. The absence of physical dependence in mice, the low degree of antinociceptive tolerance upon chronic i.c.v administration, and the lack of significant CPP at its ED50 dose, suggest that μ/κ-opioid receptors may be a viable target for the development of analgesics devoid of the undesirable side effects associated with morphine.
Materials and Methods
Materials.
β-FNA and norBNI were synthesized as described previously (35, 36). cDNAs encoding human κ-, μ-, and murine δ-opioid receptors were inserted separately into the mammalian expression vector pcDNA3 (Invitrogen) and these were used to generate the singly expressing stable cell lines. HEK-293 cells stably coexpressing μ/κ-, κ/δ-, and μ/δ-receptors were obtained from Jennifer Whistler (Ernest Gallo Clinic and Research Center, University of California, San Francisco) and their construction has been previously explained and verified (31, 32, 34). The chimeric G protein, Δ6-Gαqi4-myr, was graciously provided by Evi Kostenis (University of Bonn, Bonn) and its construction has been previously described (33).
Immunocytochemistry:
Two-color immunofluorescence was used to analyze coexpression of μ- and κ-opioid receptors as previously described (34). Detailed description is provided in SI Materials and Methods.
Cell Culture and Intracellular Ca2+ Release Assay.
HEK-293 cells were cultured at 37 °C in Dulbelcco's modified Eagle's medium supplemented with 10% bovine calf serum, Pen/Strep antibiotics. For cells singly expressing opioid receptors, G418 was used as the selection antibiotic; G418 and Zeocin were used for selecting for cells coexpressing two opioid receptors. The protocol for the intracellular calcium release has been previously described in detail (34). Briefly, the stable opioid receptor cell lines were transiently transfected with 200 ng/20,000 cells of the chimeric G protein, Δ6-Gαqi4-myr, using OptiMEM medium (Invitrogen) and Lipofectamine 2000 (Invitrogen) reagent according to manufacturer protocol (1:2 wt/vol ratio for DNA:Lipofectamine). The cells were seeded into 96-well plates (half-area; Corning) at 20,000 cells per well after 24 h and assayed 48 h after transfection using the FLIPR calcium kit (Molecular Devices) in a Flexstation-III apparatus (Molecular Devices). The response was measured as area under the curve (relative fluorescence units × seconds) and plotted using nonlinear regression.
[35S]GTPγS Assay.
The assay was setup as described previously (34, 41) and is described in detail in SI Materials and Methods.
Animal Protocols.
Detailed descriptions of each assay are provided in SI Materials and Methods. For studies performed at the University of Minnesota, male ICR-CD1 mice (18–25 g or 30–35 g; Harlan), were housed in groups of eight in a temperature- and humidity-controlled environment with unlimited access to food and water and maintained on a 12-h light/dark cycle. The μ-opioid receptor knockout mice (BALB/C X C57BL/6 MORKO) were a generous gift from Sabita Roy (University of Minnesota, Minneapolis) and have been described previously (42). The corresponding wild-type mice (B6129PF1/J) were procured from The Jackson Laboratory. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
For studies performed at the Louisiana State University Health Sciences Center in Shreveport (LSUHSC-S), male ICR mice weighing 25–35 g (Harlan Sprague–Dawley) were used in the i.v. and chronic i.c.v. studies. The mice were housed in Association for Assessment and Accreditation of Laboratory Animals (AALAC)-accredited animal facilities with 12-h light/dark cycles and food and water available ad libitum. All procedures were approved by the IACUC at LSUHSC-S.
Supplementary Material
Acknowledgments
We thank Dr. Evi Kostenis for providing us with the cDNA for Δ6-Gαqi4-myr chimeric Gα subunit, Dr. Ping Yee Law and Ji Chu for access to the Flex apparatus and valuable advice, Dr. Tim Walseth for advice with the [35S]GTPγS experiments, Dr. Sabita Roy for the MORKO mice, and Dr. Jennifer Whistler for stable dual transfected HEK-293 cells. The excellent technical assistance of William LeBaron and Dr. Morgan Le Naour is greatly appreciated. This work was supported by National Institutes of Health Grant DA01533 from the National Institute on Drug Abuse.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016277108/-/DCSupplemental.
References
- 1.Cox BM, et al. Opioid receptors. International Union of Basic and Clinical Pharmacology database (IUPHAR-DB) http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=50. Accessed on February 21, 2011.
- 2.Gutstein HB, Akil H. Opioid analgesics. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacologic Basis of Therapeutics. 11th Ed. New York: McGraw-Hill; 2006. Chap 21. [Google Scholar]
- 3.Erez M, Takemori AE, Portoghese PS. Narcotic antagonistic potency of bivalent ligands which contain beta-naltrexamine. Evidence for bridging between proximal recognition sites. J Med Chem. 1982;25:847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]
- 4.Portoghese PS, Ronsisvalle G, Larson D, Takemori AE. Synthesis and opioid antagonist bivalent ligands with conformationally restricted spacers. J Med Chem. 1986;28:1650–1653. doi: 10.1021/jm00159a014. [DOI] [PubMed] [Google Scholar]
- 5.Gershengorn MC, Osman R. Minireview: Insights into G protein-coupled receptor function using molecular models. Endocrinology. 2001;142:2–10. doi: 10.1210/endo.142.1.7919. [DOI] [PubMed] [Google Scholar]
- 6.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes I, et al. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci. 2000:RC110/1–RC110/5. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles AC, et al. Coexpression of delta-opioid receptors with micro receptors in GH3 cells changes the functional response to micro agonists from inhibitory to excitatory. Mol Pharmacol. 2003;63:89–95. doi: 10.1124/mol.63.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Sun X, Bohn LM, Sadée W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- 10.Wang HL, et al. Heterodimerization of opioid receptor-like 1 and mu-opioid receptors impairs the potency of micro receptor agonist. J Neurochem. 2005;92:1285–1294. doi: 10.1111/j.1471-4159.2004.02921.x. [DOI] [PubMed] [Google Scholar]
- 11.Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YQ, Limbird LE. Hetero-oligomers of alpha2A-adrenergic and mu-opioid receptors do not lead to transactivation of G-proteins or altered endocytosis profiles. Biochem Soc Trans. 2004;32:856–860. doi: 10.1042/BST0320856. [DOI] [PubMed] [Google Scholar]
- 13.Rios C, Gomes I, Devi LA. Interactions between delta opioid receptors and alpha-adrenoceptors. Clin Exp Pharmacol Physiol. 2004;31:833–836. doi: 10.1111/j.1440-1681.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer M, et al. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem. 2002;277:19762–19772. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer M, et al. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 16.Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci. 2005;77:1667–1673. doi: 10.1016/j.lfs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, et al. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Milligan G. G protein-coupled receptor dimerization: Function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 19.Rachid AJ, O'Dowd BF, George SR. Diversity and complexity of signaling though peptidergic G protein-coupled receptors. Endocrinology. 2005;145:2645–2652. doi: 10.1210/en.2004-0052. [DOI] [PubMed] [Google Scholar]
- 20.Angers S, Salahpour A, Bouvier M. Dimerization: An emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol. 2002;42:409–435. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 21.Gomes I, et al. G protein coupled receptor dimerization: Implications in modulating receptor function. J Mol Med. 2001;79:226–242. doi: 10.1007/s001090100219. [DOI] [PubMed] [Google Scholar]
- 22.Salahpour A, et al. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 23.Terrillon S, et al. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol. 2003;17:677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 24.Hasbi A, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: Coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, et al. Induction of heterodimerization of mu opioid (MOP) and type B cholecystokinin (CCKB) receptors by bivalent ligands. J Med Chem. 2009;52:247–258. doi: 10.1021/jm800174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels DJ, et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing opioid mu agonist and delta antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 28.Yekkirala AS, et al. San Diego, CA: 2007. NNTA is a highly selective activator of mu/kappa opioid receptor heterodimers. In 37th Annual meeting of the Society for Neuroscience. [Google Scholar]
- 29.Yekkirala AS, et al. Charleston, SC: 2008. NNTA is a highly selective activator of mu/kappa opioid receptor heterodimers that does not produce any tolerance or dependence. In Hot-Topics, International Narcotics Research Conference. [Google Scholar]
- 30.Li G, et al. Corrections to design, synthesis, and biological evaluation of 6α- and 6β-N-heterocyclic substituted naltrexamine derivatives as μ opioid receptor selective antagonists. J Med Chem. 2009;52:1416–1427. doi: 10.1021/jm801272c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldhoer M, et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rijn RM, Whistler JL. The δ(1) opioid receptor is a heterodimer that opposes the actions of the δ(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostenis E. Is Galpha16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol Sci. 2001;22:560–564. doi: 10.1016/s0165-6147(00)01810-1. [DOI] [PubMed] [Google Scholar]
- 34.Yekkirala AS, Kalyuznhy AE, Portoghese PS. Standard opioid agonists activate heteromeric opioid receptors: Evidence for morphine and [d-Ala2-MePhe4-Glyol5]enkephalin as selective μ−δ agonists ACS Chem. Neurosci. 2010;1:146–154. doi: 10.1021/cn9000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE. A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem. 1980;23:233–234. doi: 10.1021/jm00177a002. [DOI] [PubMed] [Google Scholar]
- 36.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 37.Lenard NR, Roerig SC. Development of antinociceptive tolerance and physical dependence following morphine i.c.v. infusion in mice. Eur J Pharmacol. 2005;527:71–76. doi: 10.1016/j.ejphar.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Lunzer MM, Portoghese PS. Selectivity of delta- and kappa-opioid ligands depends on the route of central administration in mice. J Pharmacol Exp Ther. 2007;322:166–171. doi: 10.1124/jpet.107.120279. [DOI] [PubMed] [Google Scholar]
- 39.Wessendorf MW, Dooyema J. Coexistence of kappa- and delta-opioid receptors in rat spinal cord axons. Neurosci Lett. 2001;298:151–154. doi: 10.1016/s0304-3940(00)01700-6. [DOI] [PubMed] [Google Scholar]
- 40.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 41.Mullaney I. G proteins and their identification. In: Milligan G, editor. Signal Transduction: A Practical Approach. 2nd Ed. London: Oxford Univ Press; 1999. pp. 100–101. [Google Scholar]
- 42.Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: Role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.