Abstract
Nitric oxide (NO) is a signaling molecule that can trigger adaptive (physiological) or maladaptive (pathological) responses to stress stimuli in a context-dependent manner. We have previously reported that NO may signal axonal injury to neighboring glial cells. In this study, we show that mice deficient in neuronal nitric oxide synthase (nNOS−/−) are more vulnerable than WT mice to toxin-induced peripheral neuropathy. The administration of NO donors to primary dorsal root ganglion cultures prevents axonal degeneration induced by acrylamide in a dose-dependent manner. We demonstrate that NO-induced axonal protection is dependent on hypoxia-inducible factor (HIF)-1–mediated transcription of erythropoietin (EPO) within glial (Schwann) cells present in the cultures. Transduction of Schwann cells with adenovirus AdCA5 encoding a constitutively active form of HIF-1α results in amelioration of acrylamide-induced axonal degeneration in an EPO-dependent manner. Mice that are partially deficient in HIF-1α (HIF-1α+/−) are also more susceptible than WT littermates to toxic neuropathy. Our results indicate that NO→HIF-1→EPO signaling represents an adaptive mechanism that protects against axonal degeneration.
The transcriptional activator hypoxia-inducible factor (HIF)-1 functions as a global regulator of oxygen homeostasis that facilitates both oxygen delivery and adaptation to oxygen deprivation (1). HIF-1 is a heterodimer composed of a HIF-1β subunit, which is constitutively expressed, and a HIF-1α subunit, the expression and transcriptional activity of which are precisely regulated by the cellular oxygen concentration (1–3). In nonhypoxic conditions, HIF-1α is subject to ubiquitination, via the von Hippel Lindau protein E3 ubiquitin ligase, and undergoes subsequent rapid proteasomal degradation (1, 3). For HIF-1α to interact with von Hippel Lindau protein, it must first be hydroxylated by HIF prolyl hydoxylases, which are oxygen-dependent enzymes (1, 3). In addition to hypoxia, iron chelators, cobalt chloride, and nitric oxide (NO) have been shown to induce HIF-1 activation via stabilization of HIF-1α (1–4).
In both brain (5, 6) and heart (7, 8), HIF-1 activation is critical to ischemic preconditioning, which represents the endogenous biological phenomenon in which a sublethal hypoxic-ischemic insult induces protection against future lethal insults. HIF-1 is the transcriptional activator of several genes that potentially may be involved in the neuroprotection associated with ischemic preconditioning, including erythropoietin (EPO), vascular endothelial growth factor, glycolytic enzymes, heme oxygenase, glucose transporters, and neuroglobin (9). However, several studies have suggested the critical importance of endogenous EPO production for ischemic tolerance, via EPO-induced JAK-2, STAT5, and NF-κB phosphorylation within neurons (6, 10–12). Astrocytes are the major source of EPO production within the brain (12), whereas Schwann cells seem to be the major source in the peripheral nervous system (13, 14).
The ability of EPO to prevent neuronal death from a variety of insults is well established (10, 15–18). In addition, EPO has been shown to robustly prevent axonal degeneration, independent of neuronal death (14, 19). This is therapeutically relevant, given that axonal loss is a prominent cause of disability in several human neurologic diseases, including diabetic peripheral neuropathy, multiple sclerosis, and Alzheimer's disease, and often precedes neuronal death by several years in these diseases (20). We recently described an endogenous axonal-protective pathway whereby injured axons induce EPO production and release by neighboring glial cells (14). Our in vitro data suggested that neuronal nitric oxide synthase (nNOS)-derived NO serves as an important axonal injury signal. We performed the studies reported herein to build on these previous observations. These studies suggest that NO at particular dosage ranges prevents toxin-induced peripheral axonal degeneration via the stimulation of HIF-1–mediated transcription of EPO within Schwann cells.
Results
Increased Susceptibility of nNOS−/− Mice to Acrylamide-Induced Neuropathy.
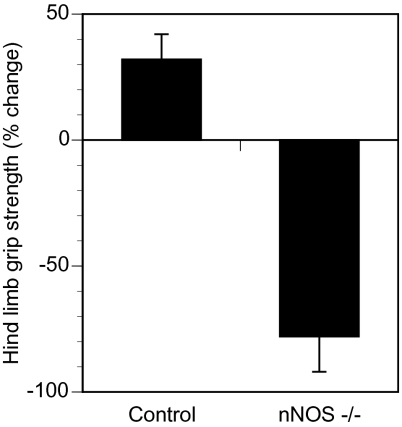
Acrylamide-induced peripheral neuropathy is a well-established, reproducible rodent model of distal axonal degeneration (21). Acrylamide is known to interfere with slow axonal transport (22). Oral administration of acrylamide to rodents causes distal degeneration of motor axons, resulting in distal limb weakness (14, 23). The administration of acrylamide at 200 ppm in drinking water did not cause distal limb weakness in WT mice, whereas this dose was sufficient to cause significant distal limb weakness in nNOS-deficient mice, as demonstrated by the mean change in hindlimb grip strength after 1 wk of acrylamide exposure (Fig. 1).
Fig. 1.
Increased susceptibility of nNOS−/− mice to acrylamide-induced peripheral neuropathy. Eight-week-old control (WT) and nNOS−/− mice (n = 5 each) were exposed to 200 ppm acrylamide in their drinking water for 1 wk. Hind limb grip strength was measured before and after acrylamide exposure. The percent change (mean ± SEM) at the end of the study compared with baseline is shown. The difference between the WT (Control) and nNOS-deficient (nNOS−/−) mice was statistically significant (P < 0.05).
NO Donor Administration Prevents Axonal Degeneration Ex Vivo.
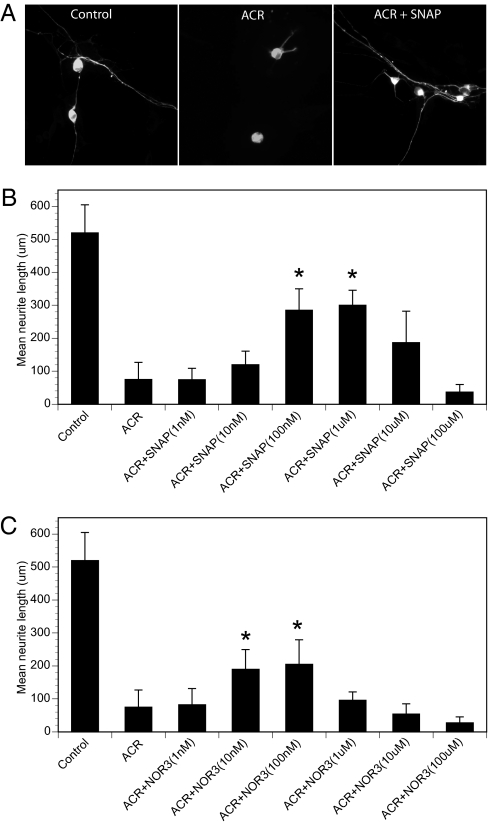
Administration of acrylamide to primary rat dorsal root ganglion (DRG) cultures with established axons results in morphological evidence of axonal degeneration, which begins distally and proceeds centripetally. This is reflected in a significantly shorter mean axonal length in DRG cultures after 36 h of acrylamide exposure. Administration of the NO donor S-nitroso-N-acetyl-dl-penicillamine (SNAP) 30 min before acrylamide administration produced dose-dependent protection against acrylamide-induced axonal degeneration (Fig. 2 A and B). SNAP doses of 100 nM–1 μM were protective, but, consistent with the published literature, SNAP doses of ≥100 μM independently caused axonal degeneration (data not shown) and exacerbated acrylamide-induced axonal toxicity (Fig. 2B). Similar dose-dependent axonal protection was observed for another NO donor, 4-ethyl-2-[(E)-hydroxy imino]-5-nitro-3-hexenamide (NOR3), but the protective dose range was lower (Fig. 2C). Axonal toxicity was also seen at high micromolar doses of NOR3.
Fig. 2.
NO donor prevents axonal degeneration in DRG cultures in a dose-dependent manner. (A and B) Primary rat DRG cultures with established axons were exposed to acrylamide (ACR) at a concentration of 1 mM for 36 h, either alone or 30 min after pretreatment with the NO donor SNAP at concentrations ranging from 1 nM to 100 μM. The cultures were fixed and stained with anti-βIII tubulin monoclonal antibody. The longest axons of at least 15 neurons per coverslip were measured under fluorescent microscopy (A), and mean ± SEM lengths are plotted (B). *P < 0.05 vs. ACR. (C) The experiment was repeated using the NO donor NOR3. *P < 0.05 vs. ACR.
NO Donors Induce EPO Production in Schwann Cells via Activation of HIF-1.
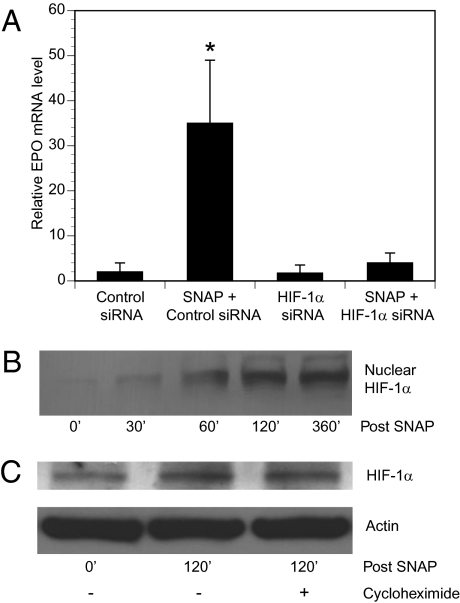
Our previous work suggested that NO donors could stimulate EPO production from Schwann cells, the predominant glial cells present in DRG cultures (14). As shown in Fig. 3A, the addition of an axonal-protective dose of SNAP (1 μM) increased EPO mRNA levels in pure Schwann cell cultures. The ability of SNAP to induce EPO mRNA production in Schwann cells was dependent on HIF-1α; previous transfection of Schwann cells with short interfering RNA (siRNA) directed against HIF-1α prevented SNAP-induced EPO mRNA production, whereas a control nontargeting siRNA had no effect (Fig. 3A). Exposure of Schwann cells to desferrioxamine, which increases HIF-1 activity (24), also induced EPO mRNA production in pure Schwann cell cultures (data not shown).
Fig. 3.
NO donors induce EPO production in Schwann cells via stimulation of HIF-1 activity. (A) Pure Schwann cell cultures were transfected with nontargeting siRNA (control) or siRNA targeting HIF-1α for 24 h and then treated with SNAP (1 μM) for 6 h. EPO mRNA levels were determined by quantitative real-time RT-PCR relative to control (mean ± SEM). *P < 0.05 vs. control. (B) Schwann cells were exposed to SNAP (1 μM) for the indicated time (in minutes). Nuclear extracts were prepared and HIF-1α protein levels were by determined by immunoblot assay. (C) Schwann cell cultures were pretreated with vehicle (−) or cycloheximide (+) and exposed to SNAP for the indicated times, after which whole cell lysates were prepared. HIF-1α and actin levels were determined by immunoblot assay.
We next investigated the mechanism by which SNAP increased HIF-1α levels in Schwann cells. After the addition of SNAP (1 μM) to pure Schwann cell cultures, HIF-1α protein was observed to accumulate within nuclear extracts over time (Fig. 3B). This accumulation was not associated with increased HIF-1α mRNA levels as determined by RT-PCR experiments (data not shown). In addition, cycloheximide administration did not prevent SNAP-induced increases in Schwann cell HIF-1α protein levels (Fig. 3C). Thus, our data suggest that a posttranslational mechanism mediated the increase in Schwann cell HIF-1α levels induced by SNAP, as has been reported in other cell types (4).
HIF-1α and EPO Expression Are Required for the Protective Effect of NO Donors.
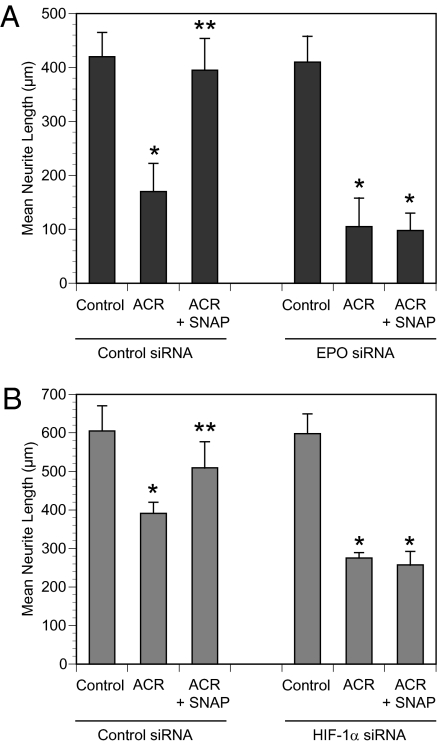
To determine whether HIF-1–mediated transcription of EPO is necessary for SNAP-mediated protection against acrylamide-induced axonal degeneration, we transfected Schwann cells in DRG cultures bearing established axons with EPO siRNA or a control siRNA before treatment with SNAP (1 μM) and/or acrylamide. As shown in Fig. 4A, we found that SNAP did not prevent acrylamide-induced axonal degeneration after Schwann cell transfection with EPO siRNA. We obtained similar results after transfection of Schwann cells with HIF-1α siRNA (Fig. 4B). These data indicate that expression of both HIF-1α and EPO in Schwann cells is necessary for SNAP-induced protection against axonal degeneration in DRG cultures.
Fig. 4.
NO prevents axonal degeneration via HIF-1α and EPO. Schwann cells in DRG cultures were transfected with nontargeting (control) siRNA or siRNA targeting EPO (A) or HIF-1α (B) and exposed to SNAP (1 μM) and/or ACR (1 mM) as indicated. Axon length (mean ± SEM) is shown. *P < 0.05 vs. control; **P < 0.05 vs. ACR.
Schwann Cell Overexpression of HIF-1α Prevents Axonal Degeneration via EPO.
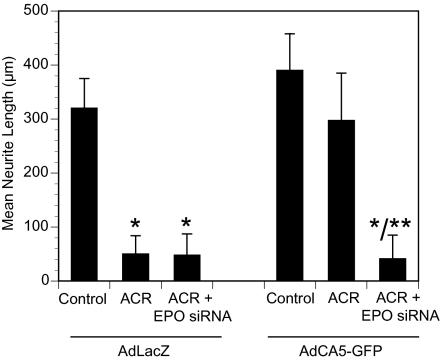
To determine the effect of HIF-1 gain of function, we exposed DRG cultures bearing established axons to recombinant adenoviral vector encoding either β-galactosidase (AdLacZ) or a constitutively active form of HIF-1α (AdCA5) containing amino acid deletions and substitutions that inhibit degradation of the protein (25). We performed preliminary dosing studies using AdCA5 (which also expresses GFP) to determine the number of plaque-forming units per cell required to achieve >90% infection of Schwann cells with <1% infection of neurons in DRG cultures. Acrylamide administration induced significant axonal degeneration in AdLacZ-infected cultures, but failed to induce significant axonal degeneration in AdCA5-infected cultures (Fig. 5). The protection afforded by AdCA5 transduction was completely abolished by previous transfection of DRG cultures with EPO siRNA.
Fig. 5.
Overexpression of HIF-1α in Schwann cells prevents axonal degeneration via EPO. DRG cultures and EPO siRNA-transfected DRG cultures were transduced with recombinant adenovirus encoding a constitutively active form of HIF-1α (AdCA5) or Escherichia coli β-galactosidase (AdLacZ) and exposed to 1 mM acrylamide (ACR). Axon length was measured, and mean ± SEM values (n = 3) are plotted. *P < 0.05 vs. control; **P < 0.05 vs. ACR.
Partial HIF-1α Deficiency Increases Susceptibility to Toxin-Induced Neuropathy.
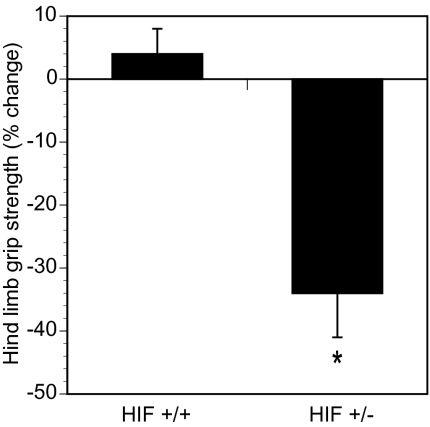
Although HIF-1α+/− heterozygous mice develop normally, partial HIF-1α deficiency results in significantly impaired responses to hypoxia and ischemia (1, 7, 15, 26). The administration of 200 ppm of acrylamide in drinking water for 1 wk did not cause distal limb weakness in WT HIF-1α+/+ mice, whereas this dose was sufficient to cause significant distal limb weakness in HIF-1α+/− littermate mice (Fig. 6). Thus, both gain-of-function and loss-of-function studies demonstrate a critical role for HIF-1 in the prevention of acrylamide-induced peripheral neuropathy.
Fig. 6.
Mice partially deficient for HIF-1α are more susceptible to toxin-induced neuropathy than WT littermates. HIF-1α+/+ (HIF+/+) and HIF-1α+/− (HIF+/−) littermates were exposed to drinking water containing 200 ppm acrylamide. Hind limb grip strength was measured before and after 1 wk. The percent change (mean ± SEM) is plotted. *P < 0.05 vs. HIF+/+.
Discussion
The dual nature of NO with respect to neurotoxicity and neuroprotection has been commented on in the literature (27). There is a large body of literature describing NO-induced neuronal apoptosis via both caspase-dependent and caspase-independent [e.g., poly (ADP-ribose) polymerase-1 mediated] mechanisms (28). In addition, NO-induced axonal injury is suggested to play a role in the axonal degeneration that occurs in multiple sclerosis (29). In contrast, NO-mediated axonal protection has not been comprehensively studied. It is known that nNOS expression is significantly increased in DRG neurons within 4 h after ipsilateral sciatic nerve injury (30). In previous work, we demonstrated that (i) a variety of toxic agents causing a retrograde pattern of axonal degeneration, including acrylamide, HIV-1 gp120, and dideoxycytosine, also induced NOS activity within DRG neurons; (ii) nNOS inhibition exacerbated axonal degeneration induced by these toxins; (iii) NO donors robustly induced EPO mRNA and protein production by Schwann cells; and (iv) nNOS inhibition prevented the increase in Schwann cell EPO production that was observed in DRG cultures exposed to axonal toxins. We postulated that NO served as an important axonal injury signal that stimulated neighboring glial cells to produce EPO, which would then protect neurons against axonal degeneration.
In this study, we show that nNOS−/− mice are more susceptible to acrylamide-induced peripheral neuropathy than their WT counterparts. Acrylamide ingestion robustly causes a retrograde pattern of sensorimotor axonal loss in rodents, and we and others have previously shown that the extent of this distal axonal loss closely correlates with the severity of hind limb grip weakness (4, 21, 23, 27). In subsequent ex vivo experiments, we noted that relatively low doses of the NO donors SNAP and NOR3 prevented axonal degeneration in DRG cultures induced by acrylamide. However, consistent with previous studies, addition of high doses of the same NO donors induced axonal degeneration in primary DRG cultures.
Our data demonstrate that the ability of NO to prevent axonal degeneration was dependent on its ability to activate HIF-1 by increasing HIF-1α protein levels within the Schwann cells present in DRG cultures. Incubation of Schwann cells with SNAP resulted in nuclear accumulation of HIF-1α that was not associated with an increase in HIF-1α mRNA levels and did not require de novo synthesis of HIF-1α protein. Thus, our data indicate that NO stabilizes HIF-1α protein in Schwann cells. This is consistent with previous reports of HIF-1α stabilization by chemically diverse NO donors in a variety of mammalian cell types, although differences in the kinetics of induction have been noted (4).
HIF-1 transcriptionally activates hundreds of target genes (1), including several that may have neuroprotective properties. Transfection of Schwann cells with a constitutively active form of HIF-1α resulted in significant axonal protection in DRG cultures. Of note, this HIF-1α–mediated axonal protection, as well as that induced by SNAP administration, was almost completely abrogated by EPO siRNA transfection of the cultures. This suggests that EPO is a critical transcriptional target for HIF-1–mediated axonal protection. The ability of EPO to prevent axonal degeneration by a variety of toxins has been described in the literature, although the signaling mechanism has yet to be defined (19, 31–33). It should be noted that HIF-2α, which is a vertebrate paralog of HIF-1α, has been shown to play an essential role in the hepatic, renal, and CNS production of EPO that maintains erythropoiesis (34, 35). On the other hand, recent studies indicate that expression of inducible NO synthase is selectively activated by HIF-1α (36). Thus, further studies are required to determine whether, in addition to HIF-1α, HIF-2α is required for the NO-induced EPO production by Schwann cells, which serves to prevent axonal degeneration.
The relevance of endogenous HIF-1 activity to axonal protection in vivo is demonstrated by our experiments showing that HIF-1α+/− mice with partial HIF-1α deficiency are significantly more vulnerable to acrylamide-induced peripheral neuropathy than their WT littermates. Thus, our ex vivo and in vivo data suggest that inducing endogenous HIF-1 activity may be beneficial in neurologic diseases in which axonal degeneration is a prominent pathological feature. In this context, HIF prolyl hydroxylase inhibitors, which stabilize HIF-1α, are currently in development (37, 38). An alternative strategy is the administration of recombinant human EPO, which has the advantage of being used extensively to treat anemia. Clinical trials are needed to determine whether either of these therapeutic strategies will result in neurologic improvement without dose-limiting polycythemia in patients with peripheral neuropathy.
Materials and Methods
Animal Studies.
All procedures involving animals were approved by The Johns Hopkins University Animal Care and Use Committee.
DRG and Pure Schwann Cell Cultures.
Mixed DRG neuronal and pure Schwann cell cultures were prepared as described previously (31, 38). In brief, spinal DRGs were dissected from decapitated gestational day 15 Sprague–Dawley rat embryos and enzymatically dissociated with 0.25% trypsin in L-15 medium. Neurons were maintained in Neurobasal medium (Gibco Invitrogen) supplemented with 10% FBS, penicillin (1 U/L), streptomycin (1 U/L), and nerve growth factor (10 ng/mL). Experiments were carried out in 24-well plates with the cells grown on collagen-coated glass coverslips. Neurons were allowed to establish long axons and were then treated with reagents as described in Results. After 36 h, cells were fixed with 4% paraformaldehyde and stained with 1:1,000 anti-βIII tubulin monoclonal antibody (Promega). The longest axons of at least 15 neurons per coverslip were measured using a nonbiased sampling method and analyzed as described previously (31, 39). All experiments were performed in triplicate and repeated at least once. Schwann cells were prepared from 1-d-old Sprague–Dawley rat pups and purified (40). Cells were passaged by trypsinization and used in experiments when they were subconfluent.
Immunoblot Assays.
Pure Schwann cell cultures were treated with 1 μM SNAP (Sigma-Aldrich), and at various times whole cell lysates and nuclear extracts were prepared as described previously (14). Equal amounts of total protein were loaded into each lane, fractionated by SDS/PAGE, and blotted onto PVDF membranes. After blocking of nonspecific binding, membranes were incubated with anti–HIF-1α antibody (1:200 dilution; Santa Cruz Biotechnology) overnight at 4 °C and visualized using the ECL-Plus chemiluminescence kit (Amersham). To inhibit protein synthesis, cells were pretreated with 100 μM cycloheximide (Sigma-Aldrich) before the addition of SNAP.
Quantitative Real-Time RT-PCR Assays.
Control or HIF-1α siRNA constructs (Ambion) were delivered to pure Schwann cell cultures using Lipofectamine 2000 (Invitrogen) as described previously (14). After 24 h, the cells were treated with SNAP (1 μM) for 6 h. Total RNA was isolated using TRIzol, and the cDNA was synthesized using 2 μg of total RNA in the presence of Ready-to-Go You Prime First Strand beads (Amersham) and random primers. mRNA levels were measured by real-time RT-PCR using the two-color DNA Engine Opticon System (M.J. Research), and the relative amount of EPO mRNA was normalized to the internal control (GAPDH mRNA) in the same PCR run. To avoid the possibility of amplifying contaminating genomic DNA, primers for EPO (5′-agtcgcgttctggagaggta-3′ and 5′-tgcagaaagtatccgctgtg-3′) were designed to span an intron, RT-PCR was performed with template-free control samples, and gel electrophoresis was performed to confirm the correct size of the amplicon and the absence of nonspecific bands. Data were analyzed by ANOVA with post hoc correction for multiple comparisons.
HIF-1α and EPO siRNA Transfection.
Down-regulation of HIF-1α and EPO expression in Schwann cells was accomplished using RNA interference. HIF-1α and EPO siRNA and a nontargeting control were purchased from Ambion. Pure Schwann cell cultures were transfected with Lipofectamine 2000 for 24 h, and cells were washed before plating with DRG neurons. The efficiency of siRNA transfection was evaluated by determining the proportion of neurons and Schwann cells labeled with Cy3-labeled siRNA constructs. No neurons within the DRG cultures were Cy3-labeled, whereas >90% of Schwann cells were Cy3-labeled. Cells were treated with acrylamide (1 mM; Sigma-Aldrich) or acrylamide and SNAP (1 μM) before fixing and staining with anti-βIII tubulin antibody. Axonal lengths were measured as described above.
Mouse Studies.
The nNOS−/− and WT strain-matched controls were purchased from the Jackson Laboratory. Information regarding derivation and characterization of these mice is available at http://jaxmice.jax.org. The generation of HIF-1α+/− mice on a C57BL/6J × 129 genetic background has been described previously (26, 41). Offspring of HIF-1α+/+ and HIF-1α+/− matings were genotyped by PCR, and 8-wk-old male HIF-1α+/− and HIF-1α+/+ littermates were studied. Acrylamide (electrophoresis grade; Sigma-Aldrich) was administered to mice in their drinking water at a concentration of 200 ppm for 1 wk. Hind limb grip strength testing was performed before acrylamide administration and repeated after 1 wk. The technician performing the grip strength assessments was blinded to the genotype of mice being tested. Statistical analysis was performed by ANOVA.
Acknowledgments
This work was supported by National Institutes of Heath Grants R01-NS43991 and R01-HL55338.
Footnotes
The authors declare no conflict of interest.
References
- 1.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Berchner-Pfannschmidt U, Tug S, Kirsch M, Fandrey J. Oxygen-sensing under the influence of nitric oxide. Cell Signal. 2010;22:349–356. doi: 10.1016/j.cellsig.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Narasimhan P, Yu F, Chan PH. Neuroprotection by hypoxic preconditioning involves oxidative stress-mediated expression of hypoxia-inducible factor and erythropoietin. Stroke. 2005;36:1264–1269. doi: 10.1161/01.STR.0000166180.91042.02. [DOI] [PubMed] [Google Scholar]
- 7.Cai Z, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc Res. 2008;77:463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 8.Eckle T, Köhler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 9.Zaman K, et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signaling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 11.Prass K, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 12.Ruscher K, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: Evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campana WM, Myers RR. Erythropoietin and erythropoietin receptors in the peripheral nervous system: Changes after nerve injury. FASEB J. 2001;15:1804–1806. doi: 10.1096/fj.00-0857fje. [DOI] [PubMed] [Google Scholar]
- 14.Keswani SC, et al. A novel endogenous erythropoietin-mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- 15.Cai Z, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 16.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 17.Gorio A, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirén AL, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keswani SC, Leitz GJ, Hoke A. Erythropoietin is neuroprotective in models of HIV sensory neuropathy. Neurosci Lett. 2004;371:102–105. doi: 10.1016/j.neulet.2004.08.080. [DOI] [PubMed] [Google Scholar]
- 20.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 21.Fullerton PM, Barnes JM. Peripheral neuropathy in rats produced by acrylamide. Br J Ind Med. 1966;23:210–221. doi: 10.1136/oem.23.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold BG, Griffin JW, Price DL. Slow axonal transport in acrylamide neuropathy: Different abnormalities produced by single-dose and continuous administration. J Neurosci. 1985;5:1755–1768. doi: 10.1523/JNEUROSCI.05-07-01755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LoPachin RM, Jr, Lehning EJ. Acrylamide-induced distal axon degeneration: A proposed mechanism of action. Neurotoxicology. 1994;15:247–259. [PubMed] [Google Scholar]
- 24.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- 25.Kelly BD, et al. Cell type–specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 26.Yu AY, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boje KM. Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci. 2004;9:763–776. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- 28.Dawson VL, Dawson TM. Deadly conversations: Nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36:287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- 29.Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 30.Abe S, et al. Nitric oxide synthase expressions in rat dorsal root ganglion after a hind limb tourniquet. Neuroreport. 2003;14:2267–2270. doi: 10.1097/00001756-200312020-00026. [DOI] [PubMed] [Google Scholar]
- 31.Keswani SC, et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi R, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA. 2004;101:823–828. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Höke A, Keswani SC. Neuroprotection in the PNS: Erythropoietin and immunophilin ligands. Ann NY Acad Sci. 2005;1053:491–501. doi: 10.1111/j.1749-6632.2005.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 34.Kapitsinou PP, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weidemann A, et al. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda N, et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratan RR, et al. Translation of ischemic preconditioning to the patient: Prolyl hydroxylase inhibition and hypoxia-inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35(Suppl 1):2687–2689. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- 38.Siddiq A, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition:A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keswani SC, et al. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann Neurol. 2003;53:57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- 40.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells, I: Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 41.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]