Abstract
Increasing evidence indicates that cerebrovascular dysfunction plays a pathogenic role in Alzheimer's dementia (AD). Amyloid-β (Aβ), a peptide central to the pathogenesis of AD, has profound vascular effects mediated, for the most part, by reactive oxygen species produced by the enzyme NADPH oxidase. The mechanisms linking Aβ to NADPH oxidase-dependent vascular oxidative stress have not been identified, however. We report that the scavenger receptor CD36, a membrane glycoprotein that binds Aβ, is essential for the vascular oxidative stress and neurovascular dysfunction induced by Aβ1–40. Thus, topical application of Aβ1–40 onto the somatosensory cortex attenuates the increase in cerebral blood flow elicited by neural activity or by endothelium-dependent vasodilators in WT mice but not in CD36-null mice (CD360/0). The cerebrovascular effects of infusion of Aβ1–40 into cerebral arteries are not observed in mice pretreated with CD36 blocking antibodies or in CD360/0 mice. Furthermore, CD36 deficiency prevents the neurovascular dysfunction observed in transgenic mice overexpressing the Swedish mutation of the amyloid precursor protein Tg2576 despite elevated levels of brain Aβ1–40. CD36 is also required for the vascular oxidative stress induced by exogenous Aβ1–40 or observed in Tg2576 mice. These observations establish CD36 as a key link between Aβ1–40 and the NADPH oxidase-dependent vascular oxidative stress underlying the neurovascular dysfunction and suggest that CD36 is a potential therapeutical target to counteract the cerebrovascular dysfunction associated with Aβ.
Keywords: Alzheimer's disease, functional hyperemia, Nox2, receptor for advanced glycation end-products
There is increasing evidence that vascular factors play a significant role in the pathogenesis of Alzheimer's dementia (AD) (1, 2). Whereas the structure of cerebral blood vessels is altered in AD, cerebral blood flow (CBF) is reduced even in the early stages of the disease (3). Epidemiological studies have shown that cerebrovascular diseases and AD share similar risk factors, suggesting a pathogenic commonality (4). Furthermore, small ischemic lesions amplify the cognitive dysfunction linked to amyloid plaques and neurofibrillary tangles, the neuropathological hallmarks of AD (5, 6). These observations have suggested an interaction between vascular insufficiency and the clinical expression of AD, raising the possibility that cerebrovascular dysfunction promotes the disease process underlying the dementia (1, 4, 7).
Amyloid-β (Aβ), the amyloid precursor protein (APP)-derived peptide central to AD pathogenesis, has profound cerebrovascular effects that disrupt the brain's blood supply and render the brain more vulnerable to injury (3). The survival of the brain depends on continuous and well-regulated delivery of blood to ensure that its energy requirements are met and that deleterious metabolic byproducts are cleared from the neuronal microenvironment (8). Consequently, sophisticated vascular control mechanisms ensure that the brain receives a sufficient amount of blood flow at all times (9). For example, functional hyperemia matches the delivery of blood flow with the metabolic demands imposed by neural activity, whereas vasoactive agents released from endothelial cells regulate microvascular flow (9). Aβ1–40, but not Aβ1–42, disrupts these vital homeostatic mechanisms, leading to neurovascular dysfunction and increasing the susceptibility of the brain to injury (3).
A key mechanism through which Aβ exerts its deleterious vascular effects involves the production of reactive oxygen species (ROS) by the enzyme NADPH oxidase, a multiunit enzyme that requires the small GTPase Rac for activation (10, 11). The mechanisms linking Aβ to vascular NADPH oxidase activation have not been defined, however. In monocytic cells, the scavenger receptor CD36, a membrane glycoprotein that binds a wide variety of ligands, including Aβ (10), is part of a molecular complex mediating Aβ-induced ROS production through a signaling pathway culminating in Rac activation (11–13). Therefore, we sought to determine whether CD36 is involved in the vascular oxidative stress and neurovascular dysfunction induced by Aβ. We found that the powerful effects of Aβ1–40 on endothelial vasoactivity and neurovascular coupling are prevented by CD36 blocking antibodies and are not observed in CD36-null (CD360/0) mice. Furthermore, mice overexpressing mutated APP (Tg2576) but lacking CD36 did not exhibit impairment in neurovascular regulation. These effects were associated with suppression of vascular oxidative stress and were independent of changes in brain Aβ levels in Tg2576 mice. These findings establish CD36 as an absolute requirement for the NADPH oxidase-dependent oxidative stress underlying the neurovascular dysfunction induced by Aβ1–40 and identify this scavenger receptor as a target for preventing the deleterious vascular effects of the Aβ peptide.
Results
CD36 Is Present in Brain Microvascular Endothelia, and Its Expression Is Enhanced in Tg2576 Mice.
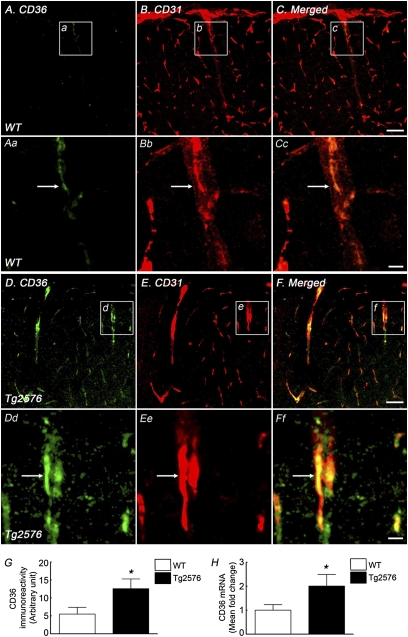
First, we used immunocytochemistry to determine whether CD36 is expressed in the somatosensory cortex in 3-mo-old Tg2576 mice and WT littermates. We found that CD36 immunoreactivity is colocalized predominantly with the endothelial marker CD31 in WT mice (Fig. 1). In particular, CD36 was observed in CD31+ penetrating arterioles (Fig. 1 A–F) but not in MAP2+ neurons (Fig. S1 A–C). CD36 immunoreactivity was not detected in cells expressing the microglial/macrophage marker Iba1, but it was seen in cultured microglia (Fig. S2 A–C and J–L). In Tg2576 mice, CD36 immunolabel (Fig. 1 A–G) and mRNA (Fig. 1H) were increased in cerebral blood vessels, whereas neurons and microglia remained unlabeled (Figs. S1 and S2). No CD36 immunoreactivity was observed in CD360/0 mice, attesting to the specificity of the label (Figs. S1 and S2). Thus, CD36 is present predominantly in cerebral endothelial cells and its expression is increased in Tg2576 mice.
Fig. 1.
CD36 immunoreactivity is present in WT mice and is increased in Tg2576 mice. In WT and Tg2576 mice (aged 3–4 mo), CD36 expression (A and Aa for WT, D and Dd for Tg2576) is observed in endothelial cells immunolabeled with CD31 (B and Bb for WT, E and Ee for Tg2576), as shown by merged images (C and Cc for WT, F and Ff for Tg2576). Images in Aa–Ff are magnifications of boxed areas, indicated in a–f and A–F, respectively. The CD36 expression level in endothelial cells in Tg2576 mice (D–F and Dd–Ff) is higher than that in WT mice (A–C and Aa–Cc). CD36 immunoreactivity (G) and mRNA (H) are increased in Tg2576 mice. Arrows in Aa–Ff indicate colocalization between CD36 and CD31. n = 4–6 per group. *P < 0.05. (Scale bars: C and F, 50 μm; Cc and Ff, 10 μm.)
Neurovascular Dysfunction Induced by Aβ1–40 Is Not Observed in CD360/0 Mice.
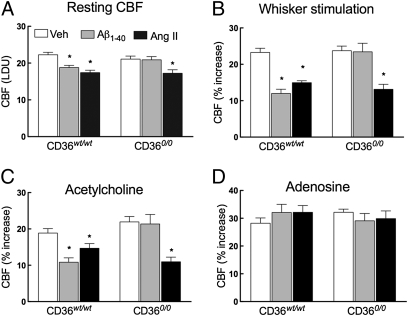
Next, we used CD360/0 mice to determine whether this scavenger receptor is involved in the neurovascular dysfunction induced by Aβ1–40. All cerebrovascular responses tested, including the CBF increase evoked by whisker stimulation or by topical application of the endothelium-dependent vasodilators ACh and bradykinin and the Ca2+ ionophore A23187, as well as responses to the smooth muscle relaxant adenosine or to hypercapnia, did not differ between mice with WT CD36 (CD36wt/wt) and CD360/0 mice (Fig. 2 and Fig. S3A). In CD36wt/wt mice, neocortical superfusion with Aβ1–40 attenuated resting CBF and the increase in CBF produced by whisker stimulation or by endothelium-dependent vasodilators, attesting to the disruptive effects of Aβ1–40 on CBF regulation (Fig. 2 A–C and Fig. S3A). As in previous studies, responses to adenosine and hypercapnia were not altered (Fig. 2D and Fig. S3A), indicating that selected vascular responses are preserved. In contrast to WT mice, Aβ1–40 did not alter cerebrovascular responses in CD360/0 mice (Fig. 2 and Fig. S3A). To determine whether the lack of effect of Aβ1–40 in CD360/0 mice was specific for this peptide, we examined the cerebrovascular effects of neocortical superfusion with angiotensin II (AngII), an octapeptide, which, like Aβ1–40, disrupts neurovascular function through NADPH oxidase-derived ROS (14). Unlike Aβ1–40, AngII attenuated cerebrovascular responses equally in CD360/0 and CD36wt/wt mice (Fig. 2 A–C). Therefore, CD36 is required for the cerebrovascular alterations induced by Aβ1–40 but not by AngII.
Fig. 2.
Cerebrovascular effects of Aβ1–40 superfusion are not observed in CD360/0 mice. In CD36wt/wt mice, both Aβ1–40 (5 μM) and AngII (50 nM) attenuate resting CBF (A) and dampen the increase in CBF evoked by whisker stimulation (B) or ACh (10 μM) (C). (A–C) In CD360/0 mice, Aβ1–40 does not attenuate vascular responses, whereas AngII does. (D) CBF response to adenosine (400 μM) is not affected in either group. LDU, relative laser-Doppler perfusion units; Veh, vehicle. n = 5 per group. *P < 0.05 from Veh by ANOVA, and Tukey's test.
CD36 Is Involved in the Neurovascular Dysfunction Induced by Circulating Aβ1–40.
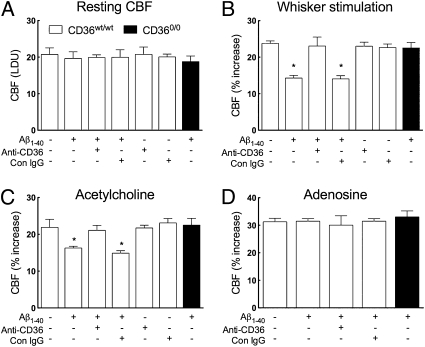
CD36 is expressed on cerebral endothelial cells (Fig. 1) and could interact with circulating Aβ1–40 to induce neurovascular dysfunction. Considering that plasma Aβ1–40 alters cerebrovascular reactivity (15–17), we examined the role of CD36 in the cerebrovascular dysfunction induced by circulating Aβ1–40. To this end, we infused human Aβ1–40 into the internal carotid artery ipsilateral to the cortical site at which CBF was measured. Infusion rate and Aβ1–40 concentration were adjusted to achieve steady-state plasma Aβ1–40 levels comparable to those of Tg2576 mice (Fig. S3B). In WT mice, intracarotid (i.c.) infusion of Aβ1–40 attenuated the increase in CBF induced by functional hyperemia or ACh, without altering resting CBF or the CBF response to adenosine (Fig. 3 A–D). Infusion of CD36 blocking antibodies, but not control IgG, before Aβ1–40 prevented the cerebrovascular alterations, without reducing plasma levels of Aβ1–40 (Fig. 3 B and C and Fig. S3B). Antibodies against the receptor for advanced glycation end-products (RAGE) also attenuated the CBF effects of i.c. Aβ1–40 (Fig. S4A), as reported by others (17). In CD360/0 mice, i.c. administration of Aβ1–40 elevated plasma Aβ1–40 but failed to induce cerebrovascular dysfunction (Fig. 3 A–D and Fig. S3B). Thus, the cerebrovascular effects of circulating Aβ1–40 require CD36.
Fig. 3.
Cerebrovascular effects of i.c. infusion of Aβ1–40 are prevented by CD36 blocking antibodies and are not observed in CD360/0 mice. In CD36wt/wt mice, i.c. infusion of Aβ1–40 (1 μM, 150 μL/h) attenuates the increase in CBF induced by whisker stimulation (B) or ACh (C) but not by adenosine (D). (A–C) Anti-CD36 antibodies do not affect resting CBF or baseline cerebrovascular responses but prevent the dysfunction induced by Aβ1–40. (A–D) Control (Con) IgG had no effect. The cerebrovascular dysfunction is not observed in CD360/0 mice. n = 5 per group. *P < 0.05 from no treatment by ANOVA and Tukey's test.
Tg2576 Mice Lacking CD36 Do Not Develop Neurovascular Dysfunction.
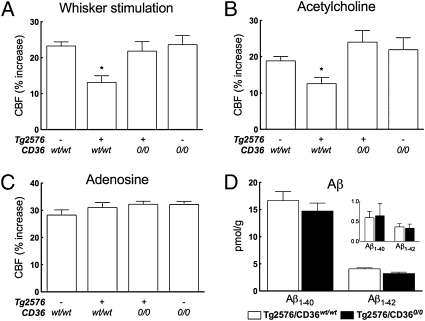
To determine whether CD36 is also involved in the cerebrovascular dysfunction in mice overexpressing APP in which endogenous levels of Aβ are elevated, we generated Tg2576 mice lacking CD36. To avoid secondary cerebrovascular effects of the pathological changes induced by amyloid plaques, experiments were performed in littermates aged 3–4 mo when amyloid deposition and behavioral deficits are not yet present (18). In Tg2576 mice expressing WT CD36, cerebrovascular responses to functional hyperemia and to endothelium-dependent vasodilators were attenuated (Fig. 4 A and B and Fig. S4B). In contrast, in Tg2576 mice lacking CD36, these cerebrovascular responses were completely normal (Fig. 4 A–C and Fig. S4B). Remarkably, the protection from cerebrovascular dysfunction conferred by CD36 deficiency in Tg2576 mice was not associated with a reduction in the brain level of Aβ1–40 or Aβ1–42 (Fig. 4D). Therefore, lack of CD36 protects Tg2576 mice from cerebrovascular dysfunction.
Fig. 4.
Tg2576 mice lacking CD36 are protected from the neurovascular dysfunction. (A and B) Increase in CBF evoked by whisker simulation or ACh is attenuated in Tg2576 mice expressing WT CD36. These cerebrovascular responses are not altered in Tg2576 mice lacking CD36, however. (C) CBF responses produced by adenosine are not affected in all groups of mice. (D) Brain concentrations of SDS-soluble and SDS-insoluble (Inset) Aβ1–40 and Aβ1–42 are comparable in Tg2576 mice expressing WT CD36 (Tg2576/CD36wt/wt) and lacking CD36 (Tg2576/CD360/0). n = 5–6 per group. *P < 0.05 from transgene-negative CD36wt/wt, Tg2576-CD360/0, and transgene-negative CD360/0 by ANOVA and Tukey's test.
CD36 Is Required for the Cerebrovascular Oxidative Stress Induced by Aβ1–40 and Observed in Tg2576 Mice.
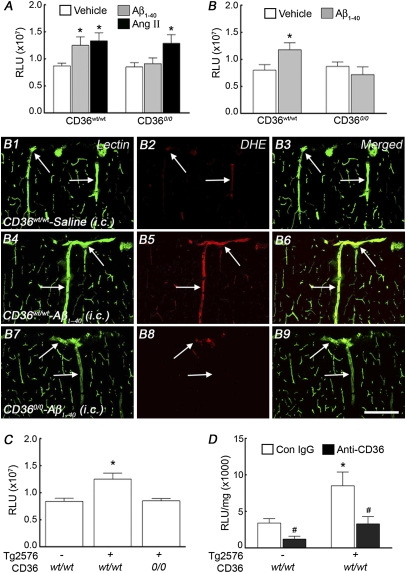
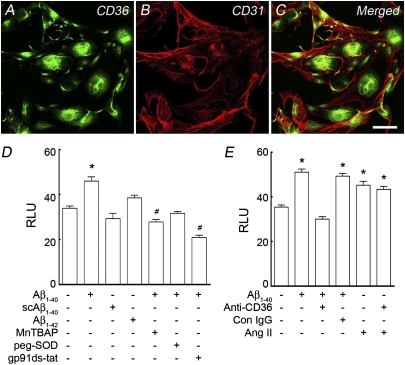
Next, we used dihydroethidine (DHE) to study whether CD36 is involved in Aβ1–40-induced vascular oxidative stress. In WT mice, neocortical application of Aβ1–40 increased ROS-dependent DHE fluorescence, an effect not observed in CD360/0 mice (Fig. 5A). Neocortical application of AngII was able to increase ROS in CD360/0 mice, however, attesting to the fact that these mice are capable of producing vascular ROS (Fig. 5A). In CD36wt/wt mice, i.c. administration of Aβ1–40 increased ROS production in endothelial cells of penetrating arterioles and capillaries (Fig. 5B and B1–B6). In CD360/0 mice, however, i.c. administration of Aβ1–40 failed to increase endothelial ROS (Fig. 5B and B7–B9). Similarly, ROS were not increased in Tg2576 mice lacking CD36 (Fig. 5C). Furthermore, CD36 blocking antibodies attenuated the ROS increase in cerebrovascular preparation from Tg2576 mice, assessed ex vivo by a luminol-based method (Fig. 5D). To determine whether Aβ1–40 could increase ROS in endothelial cells in a CD36-dependent manner, we used mouse cerebral endothelial cell cultures, which express CD36 (Fig. 6 A–C). Aβ1–40, but not a scrambled version of the peptide or Aβ1–42, increased ROS production assessed by DHE (Fig. 6D). The increase in ROS was attenuated by ROS scavengers and by the NADPH oxidase peptide inhibitor gp91ds-tat (Fig. 6D), confirming that NADPH oxidase was the source of the ROS in this model. The increase in ROS induced by Aβ1–40 was prevented by pretreatment with CD36 blocking antibodies but not control IgG (Fig. 6E). In contrast to Aβ1–40, AngII was able to increase ROS in culture pretreated with CD36 blocking antibodies (Fig. 6E). These observations implicate CD36 in the endothelial ROS production induced by Aβ1–40.
Fig. 5.
Aβ1–40-induced vascular oxidative stress requires CD36. ROS generation was assessed by DHE microfluorography in the somatosensory cortex (A–C) and by a luminol-based assay in pial cerebrovascular preparations (D). (A) Neocortical superfusion with Aβ1–40 (5 μM) increases ROS production in CD36wt/wt mice but not in CD360/0 mice, whereas AngII increases ROS in both groups. (B) i.c. infusion of Aβ1–40 increases cerebrovascular ROS in CD36wt/wt mice but not in CD360/0 mice. (B) Effect of Aβ1–40 i.c. infusion on ROS production in penetrating arterioles of the somatosensory cortex outlined with the endothelial marker tomato lectin (B1, B4, and B7). (B1–B3) In CD36wt/wt mice, minimal vascular ROS signal is observed (arrows) with vehicle infusion. (B4–B6) Aβ1–40 infusion increases the ROS signal in penetrating arterioles (arrows), however. (B7–B9) In CD360/0 mice, Aβ1–40 infusion fails to increase vascular ROS (arrows). (C) ROS are elevated in Tg2576 mice (aged 3–4 mo) but not in Tg2576 mice lacking CD36. n = 5–6 per group.*P < 0.05 from vehicle by ANOVA and Tukey's test. (Scale bar: 200 μm.) (D) Anti-CD36 antibodies attenuate the increase in ROS observed in isolated cerebral vessels of Tg2576 mice. n = 4–5 per group. *P < 0.05 from wt/wt; #P < 0.05 from Con IgG. Con, control; RLU, relative fluorescent light unit; Veh, vehicle.
Fig. 6.
Aβ1–40-induced endothelial oxidative stress requires CD36. (A–C) CD36 is expressed in CD31+ cerebral endothelial cell cultures (bEND.3). (D) In these cultures Aβ1–40 (300 nM) but not scrambled Aβ1–40 (scAβ1–40, 300 nM) or Aβ1–42 (5 μM) increases the ROS signal, an effect attenuated by the ROS scavengers MnTBAP (30 μM) and PEG-SOD (50 U/mL) or by the NADPH oxidase peptide inhibitor gp91ds-tat (1 μM). (E) Increase in ROS induced by Aβ1–40 but not AngII (1 μM) is attenuated by CD36 blocking antibodies (Anti-CD36; 2.5 μg/mL) but not control (Con) IgG.(Scale bar: 50 μm.) RLU, relative fluorescent light unit. n = 18–50 cells per group. *P < 0.05 (significantly increased from no treatment); #P < 0.05 (significantly decreased from no treatment) by ANOVA and Tukey's test.
Discussion
We have demonstrated that the scavenger receptor CD36 is an absolute requirement for the cerebrovascular alterations induced by Aβ1–40. First, we established that CD36 is present in cerebral endothelial cells in WT mice and that its expression is increased in Tg2576 mice. We then examined the role of this receptor in the cerebrovascular dysfunction induced by Aβ1–40. We found that the deleterious cerebrovascular effects of Aβ1–40 are not observed in CD360/0 mice. Thus, the increase in CBF induced by neural activity or by endothelium-dependent vasodilators was preserved in CD360/0 mice in which Aβ1–40 was topically applied to the somatosensory cortex. In contrast, AngII, a peptide that induces cerebrovascular dysfunction through angiotensin type 1 receptors and vascular NADPH oxidase (14), was effective in CD360/0 mice, supporting the selectivity of the involvement of CD36 in Aβ1–40-mediated vascular responses. We then explored the role of CD36 in the cerebrovascular actions of plasma Aβ1–40 and found that the attenuation in vascular reactivity induced by Aβ1–40 infused into the internal carotid artery was prevented by CD36 blocking antibodies and was not observed in CD360/0 mice. To provide further evidence of the involvement of CD36 in the cerebrovascular effects of Aβ1–40, we investigated Tg2576 mice lacking CD36. As observed with administration of exogenous Aβ1–40, the cerebrovascular dysfunction was abrogated in Tg2576 mice lacking CD36 despite comparable elevations in brain Aβ1–40.
Next, we sought to identify the mechanisms by which CD36 participates in the vascular effects of Aβ1–40. The cerebrovascular dysfunction induced by Aβ1–40 is mediated, in large part, by ROS generated by NADPH oxidase, which induce vascular dysfunction through multiple mechanisms, including reduced bioavailability of nitric oxide (10, 11). Because CD36 binds Aβ and increases NADPH oxidase-dependent ROS production in cells of monocytic lineage (11–13), we hypothesized that CD36 is also required for the vascular oxidative stress induced by Aβ1–40. Consistent with this hypothesis, Aβ1–40, either applied to the neocortex or infused into the carotid artery, failed to increase vascular ROS in CD360/0 mice. CD36 blocking antibodies attenuated the ROS increase observed ex vivo in cerebral vessels of Tg2576 mice or induced by Aβ1–40 in cerebral endothelial cultures. Furthermore, Tg2576 mice lacking CD36 did not exhibit vascular ROS increases. Collectively, these findings unveil a previously unrecognized role of CD36 in the vascular oxidative stress and cerebrovascular dysfunction induced by Aβ1–40.
Aβ binds several cell surface proteins, including, for example, integrins, the α7 subunit of the nicotinic ACh receptor, the insulin receptor, the serpin enzyme complex, and different scavenger receptors (19). Of these putative Aβ “receptors,” CD36 has been found to be part of a macromolecular complex mediating the increase in ROS by activating NADPH oxidase in microglial cells (11–13). Our data suggest that the interaction of Aβ1–40 with CD36 in endothelial cells is also critical for its cerebrovascular effects. This conclusion is supported by the finding that CD36 is expressed in endothelial cells and is required for the cerebrovascular dysfunction induced by Aβ1–40 either applied to the cortex or administered intravascularly. Because neocortically applied Aβ1–40 may reach cerebral vessels from the abluminal side and intravascular Aβ1–40 may reach cerebral vessels from the luminal side, these findings would suggest expression of CD36 both on the luminal and abluminal endothelial cell membranes. It remains to be established whether the subcellular localization of endothelial CD36 follows this pattern. Microglial cells in culture express CD36 and are a source of NADPH-derived ROS (12, 13, 20). We did not observe CD36 immunoreactivity in microglia in the mouse brain, however. Although we cannot rule out that CD36 expression levels are below the sensitivity of immunocytochemistry, the data do not point to a major role of microglia in this model.
CD36 has previously been reported to interact with fibrillary forms of Aβ and to trigger NADPH oxidase-dependent ROS production in monocytic cells (12, 21). Our in vivo and in vitro studies suggest that Aβ1–40, a peptide not as fibrillogenic as Aβ1–42 (2), is able to induce CD36-dependent ROS production and cerebrovascular dysfunction, however. Thus, fibril formation may not be required for the functional interaction of Aβ1–40 with CD36. Indeed, Aβ1–42 is not vasoactive (22) and, as anticipated, does not induce endothelial oxidative stress (present study). Whether oligomeric Aβ1–40 is involved in the response remains unclear. Whatever the aggregation state of exogenous Aβ1–40, the findings in Tg2576 mice lacking CD36 attest to the critical role of this scavenger receptor in the vascular effects of Aβ1–40. Another caveat is that the plasma and brain concentrations of Aβ1–40 in AD models are higher than those observed in AD (23). Nevertheless, the fact that AD is associated with cerebrovascular dysfunction (1, 7) supports the relevance of the present observations to the pathobiology of AD.
CD36 deficiency in Tg2576 mice did not alter brain levels of Aβ. This observation suggests that CD36 is not involved in the mechanisms regulating Aβ homeostasis, at least in 3- to 4-mo-old Tg2576 mice. Interestingly, brain Aβ levels are also not reduced in Tg2576 mice lacking the key NADPH oxidase subunit NOX2 (24), indicating that NADPH oxidase-derived ROS do not influence brain Aβ levels. Approaches that reduce ROS independent of their sources, such as ROS scavengers, have been shown to reduce brain Aβ levels, however (25–27). Therefore, the participation of other sources of radicals in brain Aβ production cannot be excluded.
CD36 can signal either independently or as part of a larger molecular complex including Toll-like receptors (TLRs), the class A scavenger receptor, α6-β1 integrin, and CD47 (12, 13, 23). Our observation that blocking RAGE also attenuates the cerebrovascular dysfunction supports the involvement of this scavenger receptor in the signaling pathway (17), but its relationships with CD36 await further exploration. Concerning TLRs, some of the proinflammatory effects of CD36 have been attributed to the association with TLR2/1, TLR2/6, or TLR4/6 heterodimers, leading to NF-κβ activation and proinflammatory gene expression through the canonical MyD88-TIRAP-TRIP-TRAM pathway (28–31). In particular, the proinflammatory signaling of Aβ depends on the interaction of CD36 with TLR4/6 heterodimers (30). Nevertheless, considering the rapidity with which Aβ1–40 increases ROS, this transcriptional pathway is unlikely to induce the oxidative stress mediating the cerebrovascular effects of Aβ1–40. Rather, the activation of NADPH oxidase by CD36 may depend on GTP loading of Rac1 mediated by the guanine nucleotide exchange factor vav (11), an effect requiring the cooperative signaling of CD36 with TLRs, α6-β1 integrin, and associated molecules (32). It remains to be established whether a similar pathway is involved in the CD36-dependent NADPH oxidase activation induced by Aβ1–40 in cerebral endothelial cells.
In conclusion, we have demonstrated that CD36 is an essential requirement for the cerebrovascular actions of Aβ1–40. CD36, localized mainly to endothelial cells, may interact with Aβ1–40 to activate NADPH oxidase, triggering vascular oxidative stress and the attendant cerebrovascular dysfunction. CD36 is required for the vascular dysfunction induced by Aβ1–40 but not by AngII, supporting the selectivity of the involvement of CD36 in the vascular effects of Aβ1–40. The fact that CD36 does not contribute to Aβ1–40 production in the brain of Tg2576 mice suggests that the vasoprotective effects deriving from its genetic inactivation are not attributable to reduced Aβ1–40 levels. The identification of CD36 as a key link between Aβ1–40 and vascular oxidative stress offers the prospect of targeted interventions aimed at ameliorating cerebrovascular function by suppressing Aβ-induced CD36 signaling.
Materials and Methods
A more detailed description of the methods used in this study is presented in SI Materials and Methods.
Mice.
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Studies were performed in 3- to 4-mo-old C57BL6/J or CD360/0 male mice. Transgenic mice overexpressing the Swedish mutation of the APP Tg2576 (18) were crossed with CD360/0 mice (33). To minimize confounding effects of background heterogeneity and genetic modifiers, experiments were performed in age-matched littermates.
General Surgical Procedures.
As described in detail elsewhere (10, 11, 28, 34) and in SI Materials and Methods, mice were anesthetized with isoflurane (1–2% vol/vol), followed by urethane (750 mg/kg administered i.p.) and α-chloralose (50 mg/kg administered i.p.). A femoral artery was cannulated for recording of arterial pressure and collection of blood samples. In some studies, the external carotid artery ipsilateral to the cranial window was catheterized for infusion of selected agents into the internal carotid artery (see below) (17). Mean arterial blood pressure, blood gases, and rectal temperature were monitored and controlled (Tables S1–S4).
Monitoring of CBF.
A 2 × 2-mm opening was drilled through the parietal bone, the dura was removed, and the site was superfused with modified Ringer's solution (37 °C, pH 7.3–7.4) (10, 11, 28, 34). Relative CBF was continuously monitored at the site of superfusion with a laser-Doppler flow probe (Vasamedic).
Detection of ROS by DHE Fluoromicrography.
ROS production was measured using DHE (Molecular Probes) fluoromicrography (10, 11, 35). DHE (2 μM) was superfused on the somatosensory cortex for 60 min. In some studies, DHE superfusion was followed by cosuperfusion with human Aβ1–40 (5 μM; rPeptide). Aβ1–40 was dissolved in DMSO to minimize subsequent fibril formation (22). The Aβ1–40 concentration achieved in the brain is likely to be lower because of diffusion limitations (22). At the end of the superfusion, the brain was removed, sectioned (thickness of 20 μm), and processed for detection of ROS-dependent fluorescence by confocal microscopy (10, 11, 35). To study ROS production in endothelial cells following i.c. infusion of Aβ1–40, DHE was superfused cortically for 30 min, followed by a 30-min i.c. infusion of Aβ1–40. FITC-conjugated Lycopersicon esculentum (tomato) lectin (100 μg per 100 μL; Vector Laboratories), an endothelial marker, was then infused i.c. over 5 min (36). Brain sections were cut and processed for determination of ROS-dependent fluorescence in the cells labeled with FITC–tomato lectin. Mouse brain endothelial cells (bEND.3 cells; American Type Culture Collection) were incubated with DHE (2 μM, 30 min) and exposed to Aβ1–40 (300 nM; rPeptide), scrambled Aβ1–40 (300 nM; rPeptide), Aβ1–42 (5 μM; rPeptide), or AngII (1 μM; Sigma) for 15 min. Cultures were pretreated with vehicle or with the ROS scavengers MnTBAP (30 μM; Calbiochem) and peg-SOD (50 U/mL; Sigma). In other studies, cultures were pretreated with CD36 blocking antibodies (2.5 μg/mL, Clone FA6-152; StemCell Technologies, Inc.), control IgG (2.5 μg/mL), or the NADPH oxidase peptide inhibitor gp91ds-tat (1 μM). ROS-dependent fluorescence was quantified by confocal microscopy as previously described (SI Materials and Methods).
CD36 Real-Time PCR and ROS Measurement in Isolated Cerebral Vessels.
The pia mater was stripped from the brain surface, and pial cerebral blood vessels were processed for CD36 real-time PCR or assayed for ROS production using the luminol-based indicator L012 (34) (SI Materials and Methods).
Measurement of Plasma and Brain Aβ.
Brain or plasma Aβ levels were determined using ELISA-based assays, as described previously (24, 27).
Immunocytochemistry.
Mice were perfusion-fixed, and their brains were removed and sectioned (thickness of 40 μm). Sections were processed for double-labeling of anti-mouse CD36 IgA (1:500; BD Biosciences) with the neuronal marker MAP2 (1:500; Sigma), the endothelial cell marker CD31 (1:100; BD Biosciences), or the microglia/macrophage marker Iba1 (1 μg/mL; Wako Pure Chemical Industries). Sections were then incubated with anti-mouse IgA (CD36; BD Biosciences) and anti-rabbit IgG (MAP2 and Iba1) or anti-rat IgG (CD31) secondary antibodies (Jackson ImmunoResearch). Images were acquired using a confocal laser-scanning microscope (Leica). Procedures for quantification of immunofluorescence are described in SI Materials and Methods. Endothelial cell cultures were fixed, washed, and processed for CD36 and CD31 or Iba1 immunofluorescence. Confocal images were acquired as above.
Experimental Protocol for CBF Experiments.
CBF recordings were started after arterial pressure and blood gases were in a steady state (Tables S1–S4). To study the increase in CBF produced by somatosensory activation, the whiskers were mechanically stimulated for 60 s. The endothelium-dependent vasodilators ACh (10 μM; Sigma) and bradykinin (50 μM; Sigma) and the calcium ionophore A23187 (3 μM; Sigma) were superfused on the cortex, and the resulting changes in CBF were monitored. CBF responses to adenosine (400 μM; Sigma) or hypercapnia (Pco2, 50–60 mmHg) were also tested (22, 35). In some studies, responses were tested before and after superfusion with Ringer's solution containing human Aβ1–40 (5 μM) or AngII (50 nM) (22). In experiments with intravascular Aβ1–40 administration, responses were tested before and after i.c. infusion of vehicle or Aβ1–40 (1 μM, 150 μL/h) for 30 min. The Aβ1–40 concentration infused was chosen to increase plasma Aβ1–40 to a level similar to that of Tg2576/CD36wt/wt mice (Fig. S3B). In some studies, the effect of Aβ1–40 on CBF responses was tested before and 30 min after i.c. infusion (150 μL/h) of CD36 receptor or RAGE blocking antibodies (50 μg) or control IgG (50 μg).
Data Analysis.
Data are expressed as mean ± SEM. Two-group comparisons were analyzed by the two-tailed t test. Multiple comparisons were evaluated by ANOVA and Tukey's test. Differences were considered statistically significant for probability values less than 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants NS37853 (to C.I.), NS55118 (to W.E.V.N.), and NS41997 (to G.A.C.); and by American Heart Association Grant 09SDG2060701 (to L.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015413108/-/DCSupplemental.
References
- 1.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 4.de la Torre JC. Vascular risk factor detection and control may prevent Alzheimer's disease. Ageing Res Rev. 2010;9:218–225. doi: 10.1016/j.arr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke. 2010;41(Suppl):S144–S146. doi: 10.1161/STROKEAHA.110.598326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snowdon DA, Nun Study Healthy aging and dementia: findings from the Nun Study. Ann Intern Med. 2003;139:450–454. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolla MJ. The Cerebral Circulation. San Francisco: Morgan and Claypool; 2010. [PubMed] [Google Scholar]
- 10.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:1–8. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 12.Coraci IS, et al. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianca VD, Dusi S, Bianchini E, Dal Prà I, Rossi F. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J Biol Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- 14.Kazama K, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 15.Gurol ME, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 16.van Dijk EJ, et al. Plasma beta amyloid and impaired CO2-induced cerebral vasomotor reactivity. Neurobiol Aging. 2007;28:707–712. doi: 10.1016/j.neurobiolaging.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Deane R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 19.Verdier Y, Zarándi M, Penke B. Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: Binding sites and implications for Alzheimer's disease. J Pept Sci. 2004;10:229–248. doi: 10.1002/psc.573. [DOI] [PubMed] [Google Scholar]
- 20.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: Their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 21.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa K, Carlson GA, Iadecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Graff-Radford NR, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 24.Park L, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole GM, et al. NSAID and antioxidant prevention of Alzheimer's disease: Lessons from in vitro and animal models. Ann N Y Acad Sci. 2004;1035:68–84. doi: 10.1196/annals.1332.005. [DOI] [PubMed] [Google Scholar]
- 26.Lim GP, et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iadecola C, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 28.Abe T, et al. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoebe K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 30.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triantafilou M, et al. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 32.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar Abeta-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Febbraio M, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 34.Judkins CP, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 35.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, et al. Retroviral delivery of homeobox D3 gene induces cerebral angiogenesis in mice. J Cereb Blood Flow Metab. 2004;24:1280–1287. doi: 10.1097/01.WCB.0000141770.09022.AB. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.