Abstract
Neurons in the central nervous system (CNS) fail to regenerate axons after injuries due to the diminished intrinsic axon growth capacity of mature neurons and the hostile extrinsic environment composed of a milieu of inhibitory factors. Recent studies revealed that targeting a particular group of extracellular inhibitory factors is insufficient to trigger long-distance axon regeneration. Instead of antagonizing the growing list of impediments, tackling a common target that mediates axon growth inhibition offers an alternative strategy to promote axon regeneration. Neuronal growth cone, the machinery that derives axon extension, is the final converging target of most, if not all, growth impediments in the CNS. In this study, we aim to promote axon growth by directly targeting the growth cone. Here we report that pharmacological inhibition or genetic silencing of nonmuscle myosin II (NMII) markedly accelerates axon growth over permissive and nonpermissive substrates, including major CNS inhibitors such as chondroitin sulfate proteoglycans and myelin-associated inhibitors. We find that NMII inhibition leads to the reorganization of both actin and microtubules (MTs) in the growth cone, resulting in MT reorganization that allows rapid axon extension over inhibitory substrates. In addition to enhancing axon extension, we show that local blockade of NMII activity in axons is sufficient to trigger axons to grow across the permissive–inhibitory border. Together, our study proposes NMII and growth cone cytoskeletal components as effective targets for promoting axon regeneration.
Keywords: myelin, glial scar, multi-compartment neuronal culture chamber
The inability of mature neurons to accomplish long-distance axon regeneration accounts for devastating and permanent disabilities after neural injury. Because regeneration failure is attributed in part to the hostile environment, extensive studies have focused on identification of the axon growth impediments and elucidation of the inhibitory mechanisms (1–5). As a result, multiple axon growth inhibitors have been identified, among which myelin-derived inhibitors and chondroitin sulfate proteoglycans (CSPGs) in the glial scar are the most studied (6, 7). However, although some findings remain a subject of continuous debate, genetic ablation of all three major myelin inhibitors (8) or receptors for myelin or CSPGs (9, 10) failed to induce long-distance axon regeneration, suggesting that counteracting individual inhibitory components alone is insufficient to trigger extensive axon growth.
The neuronal growth cone is a sensory-motile structure located at the tip of an extending axon. The growth cone is not only the machinery that drives axon extension, but also the converging target of axon growth regulatory signals, including inhibitory signals in the central nervous system (CNS) (11, 12). However, few studies to date have attempted to promote axon growth, especially over inhibitory molecules, by directly manipulating the growth cone. An important feature of an advancing growth cone is that a subset of microtubules (MTs) extends into the growth cone periphery, so that they can probe intracellular space and respond to extracellular signals (13, 14). In contrast to advancing growth cones, injured axons form retraction bulbs, which contain disorganized MTs that fail to extend into the periphery (15, 16). Thus, reorganization of the MT network and subsequent formation of an advancing growth cone might promote axon growth and overcome multiple inhibitory signals.
Here we show that pharmacological inhibition or genetic silencing of nonmuscle myosin II (NMII), which powers retrograde actin flow in the growth cone, markedly enhances axon growth over potent CNS inhibitory substrates via reorganization of the growth cone cytoskeleton. We also demonstrate that local blockade of NMII activity in the axon allows axons to grow across the permissive–inhibitory border.
Results
NMII Inhibition Promotes Robust Axon Growth over CSPGs and CNS Myelin.
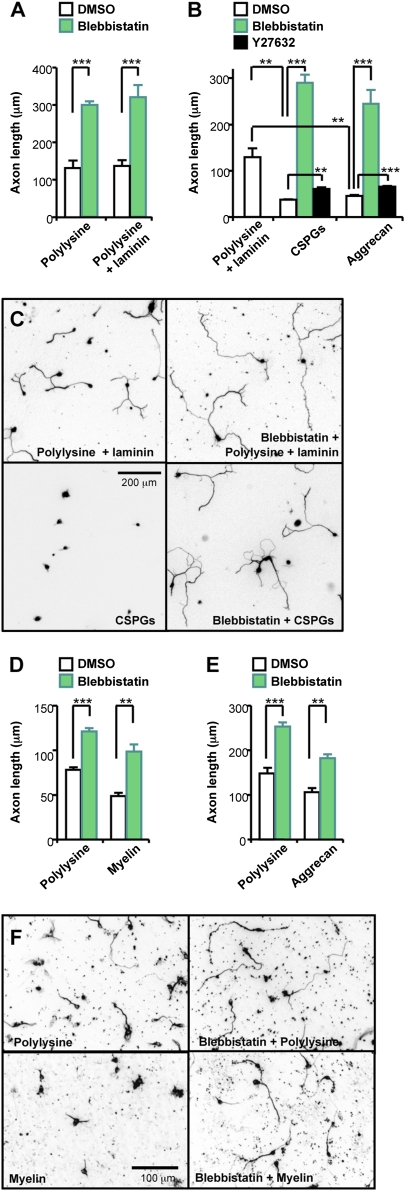
MT extension into the growth cone periphery is an essential process for axon growth. Because MTs are transported away from the leading edge when coupled to a retrograde flow of actin filaments (17, 18), we tested whether axon growth could be enhanced by inhibiting NMII activity that powers retrograde actin flow. For this purpose, we applied blebbistatin, a specific inhibitor of NMII ATPase activity (19–21). When NMII activity was inhibited, we found that axon growth of embryonic dorsal root ganglion (DRG) neurons was enhanced in a dose-dependent manner on permissive substrates (100 μg/mL polylysine plus 5–10 μg/mL laminin) (Fig. S1 and Fig. 1A). On the basis of the dose curve, in subsequent experiments blebbistatin was applied at a concentration of 25 μM, the lowest concentration that produced maximum effect, to reduce any possible nonspecific effects of the drug. To our surprise, blockade of NMII activity enabled neurons to completely overcome axon growth inhibition induced by CSPGs (Fig. 1 B and C), a group of inhibitory molecules that potently prevent axon growth both in vitro and in vivo (6, 22). Furthermore, when NMII activity was inhibited, axons on inhibitory substrates became longer than those on permissive substrates (Fig. 1 B and C). Previous studies have shown that CSPGs activate Rho GTPase and that blockade of Rho kinase (ROCK) activity with Y27632 reverses CSPG inhibition (23, 24). We also observed that the inhibitory effects of CSPGs and aggrecan, a member of CSPGs, were partially alleviated by Y27632 (Fig. 1B) to the extent comparable to previous reports (23, 24). However, it should be noted that axon growth promotion was marginal and that CSPGs still potently blocked axon growth even in the presence of Y27632. In addition to CSPGs and aggrecan, NMII inhibition also markedly enhanced axon growth of cerebellar granule neurons on CNS myelin extracts (Fig. 1 D–F), which comprise another major class of axon growth impediments.
Fig. 1.
Blockade of NMII activity promotes axon growth. (A–C) Embryonic DRG neurons were cultured on permissive (polylysine alone or polylysine plus laminin) or inhibitory substrates (CSPGs or aggrecan) in the presence or absence of blebbistatin or Y27632, as indicated. Neurons were fixed (at 20–22 h after plating on polylysine; at 12–14 h after plating on all other substrates) and immunostained with anti-TuJ1 antibodies. Quantification of axon length (A and B) and representative images (C, inverted images from TuJ1 immunostaining) are shown. (D–F) Postnatal cerebellar granule neurons were cultured on polylysine, aggrecan, or myelin extracts in the presence or absence of blebbistatin, as indicated. Quantification of axon length (D and E) and representative images (F) are shown. **P < 0.01; ***P < 0.001.
Knocking Down Different NMII Isoforms Has Distinct Effects on Regenerative Axon Growth over Permissive and Inhibitory Substrates.
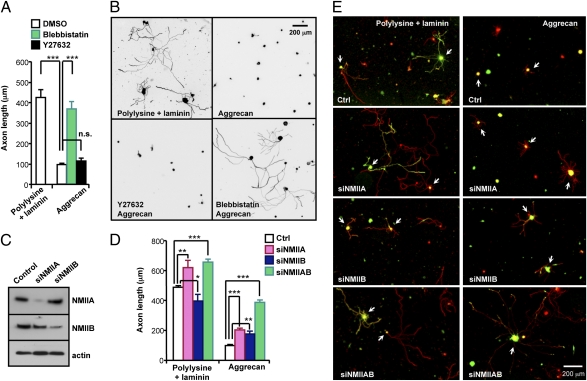
We next examined if inhibition of NMII could enhance axon growth from adult neurons, which would be more relevant to axon regeneration. As in embryonic DRG neurons, aggrecan drastically prevented axon growth from adult DRG neurons. Remarkably, when NMII activity was inhibited, adult DRG neurons grew axons robustly over aggrecan, comparable to the extent on permissive substrates, whereas inhibition of ROCK had little effect (Fig. 2 A and B). In growth cones, NMIIA and NMIIB are the two major NMII isoforms (25, 26), which are inhibited by blebbistatin with similar potency (20, 21). To investigate which of the isoform(s) is responsible for the axon growth-promoting effects, we transfected adult DRG neurons with siRNAs against NMIIA (siNMIIA) and/or NMIIB (siNMIIB) (Fig. 2C). As in NMIIB knockout mice (27), knocking down NMIIB inhibited axon growth on permissive substrates. By contrast, depletion of NMIIA enhanced axon growth (Fig. 2 D and E), suggesting opposing roles of NMII isoforms in the regulation of axon growth on permissive substrates. On aggrecan, knocking down either NMIIA or NMIIB promoted axon growth, and knocking down both isoforms accounted for nearly all of the axon growth-promoting effect of blebbistatin (Fig. 2 D and E), ruling out the possible role of NMIIC, which is also expressed in DRG growth cones (28). These results suggest that, on permissive substrates, blockade of NMIIA activity is responsible for the axon growth-promoting effect of blebbistatin, whereas on inhibitory substrates axon growth promotion is attributed to inhibition of both NMIIA and NMIIB. However, it should be noted that a replating procedure used in this study might not be the same as inhibiting NMII expression in neurons with continuously growing axons or neurons with long axons that have stalled because the turnover of NMII in growth cones may be different.
Fig. 2.
NMII inhibition induces robust axon growth from adult DRG neurons on aggrecan. (A and B) Adult DRG neurons from conditioning lesioned mice were cultured on polylysine plus laminin or aggrecan in the presence or absence of blebbistatin or Y27632, as indicated. Quantification of axon length (A) and representative images (B) are shown. (C–E) Adult DRG neurons were transfected with Venus and siRNAs against NMIIA (siNMIIA) and/or IIB (siNMIIB), as indicated. At 4 d after transfection, neurons were harvested for immunoblotting (C) or replated on polylysine plus laminin or aggrecan to allow axon growth anew (D and E). Representative immunoblots (C) and images of replated neurons transfected with siRNAs and Venus (arrows in E) are shown. Quantification of axon length is shown in D. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
NMII Inhibition Promotes Axon Growth over CSPGs via Reorganization of the Growth Cone Cytoskeletal Structure.
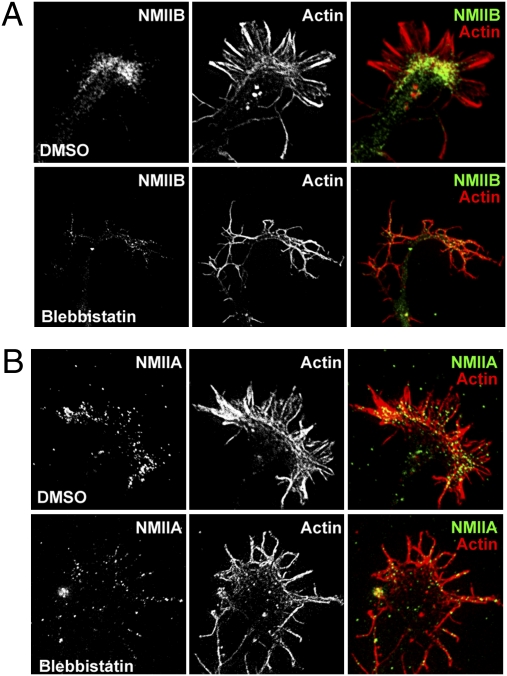
To understand the cellular mechanism by which NMII regulates axon growth, we first examined the localization of NMII isoforms in the growth cone. Distribution of NMII in the growth cone has been examined by several groups, reporting NMIIB in the growth cone periphery (29, 30) or in the central and the transitional domains (17, 26). When neurons were simultaneously fixed and detergent-extracted to remove soluble proteins, we observed a clear band of NMIIB remaining bound specifically in the transitional domain of the growth cone, colocalizing with the actin arc structure (Fig. 3A). The specific NMIIB localization shown here is similar to an observation made in Aplysia growth cones that used similar fixation methods (17). NMIIA immunostaining in the growth cone was less prominent compared with NMIIB. The localization of NMIIA in the transition zone was similar to that of NMIIB, but NMIIA appeared to be associated with actin filaments in the peripheral region as well (Fig. 3B). The localization of NMIIA and NMIIB in the transition zone was lost within 20 min of blebbistatin application, but the association between NMIIA and actin bundles in the peripheral domain seemed to be more resistant to blebbistatin treatment. Specific localization of NMII isoforms in the transitional domain is consistent with their role in mediating retrograde actin flow in the growth cone (17), and the loss of the distinct localization of NMII in response to blebbistatin provides a structural basis for the effect of blebbistatin.
Fig. 3.
Localization of NMIIA and NMIIB in growth cones. (A and B) Embryonic DRG neurons cultured on polylysine plus laminin were stained with phalloidin and anti-NMIIA (B) or NMIIB (A) antibodies. Note that the actin arc structure and the translational localization of NMII isoforms are lost by application of blebbistatin (25 μM, 20 min).
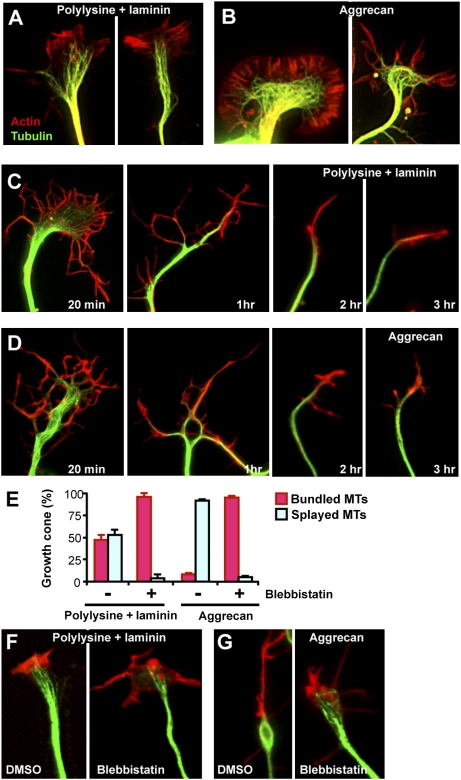
To further address the mechanism by which NMII inhibition led to axon growth promotion, we examined growth cone cytoskeletal structures and their reorganization in response to blebbistatin. Tightly bundled axonal MTs often get dispersed as they enter the growth cone, where they display complex and dynamic configurations, such as looping, bundling, and splaying. On permissive substrates, we observed that, in about half of the embryonic DRG growth cones, MTs splayed out as they entered the growth cone, whereas in the other half MTs remained bundled (Fig. 4A). By contrast, on inhibitory substrates MTs were spread in the vast majority (92%) of growth cones, often forming loops in the central domain (Fig. 4B). Similar to an observation made in Aplysia growth cones (31), blebbistatin induced de-bundling of MTs in the neck region. However, this effect was transient and after treatment with blebbistatin for a longer period MTs became bundled into tight arrays in growth cones on both permissive and inhibitory substrates (Fig. 4 C–E). Y27632, by contrast, had little effect on growth cone MT organization (Fig. S2), consistent with its modest effect on axon growth over CSPGs. Unlike embryonic DRG neurons, most growth cones of adult DRG neurons from conditioning lesioned mice contained straight and bundled MTs with their tips pointing toward the growth cone leading edge on permissive substrates (Fig. 4F). On aggrecan, these neurons formed dystrophic growth cones in which MTs were confined to the central zone with little extension into the periphery (Fig. 4G). Inhibition of NMII released the MTs from a compressed state and allowed MT extension toward the leading edge, resulting in a growth cone cytoskeletal structure similar to that on permissive substrates (Fig. 4 F and G).
Fig. 4.
NMII inhibition induces reorganization of growth cone cytoskeletal structures. (A–E) Embryonic DRG neurons cultured on polylysine plus laminin (A and C) or aggrecan (B and D) in the presence (C and D) or the absence (A and B) of blebbistatin were stained with phalloidin and anti-tubulin antibodies. The exposure time has been readjusted for each picture to increase the visibility of the actin structure. Representative images (A–D) and quantification of growth cone MT structures (E) are shown. (F and G) Representative images of adult DRG growth cones. Adult DRG neurons were cultured on polylysine plus laminin (F) or aggrecan (G) in the presence or absence of blebbistatin, as indicated, and stained with phalloidin and anti-tubulin antibodies.
Axon Growth Promotion over CSPGs Induced by NMII Inhibition Requires the Interplay Between Actin and MTs.
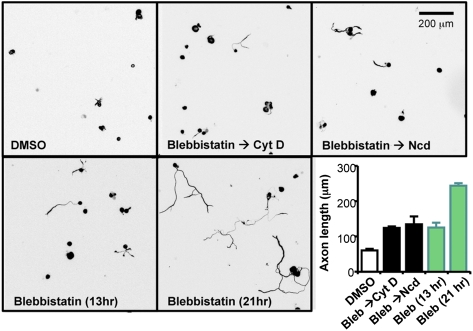
In addition to the changes in MT configuration, NMII inhibition led to the marked reorganization of actin structure (Fig. 4 A–D). Rapid depletion of actin arcs in the transitional zone was noted (Fig. 3), and there was a dramatic reduction in the growth cone lamellipodial areas in response to blebbistatin. By contrast, filopodial structures became more prominent, reminiscent of the growth cone structures of the NMIIB knockout mice (27) and the changes in F-actin structure induced by NMIIB inhibition in chicken DRG neurons (32). The structural reorganization was accompanied by reduction in the total F-actin levels. To quantitatively assess the changes in F-actin contents, we performed time-course experiments and measured the mean fluorescent intensity of F-actin in the growth cone. These experiments revealed that blebbistatin markedly reduced F-actin levels in the growth cone on both permissive and inhibitory substrates (Fig. S3). By treating neurons with cytochalasin D at a concentration that dampens actin dynamics (33), we found that both the initiation (Fig. S4) and the elongation (Fig. 5) of axons promoted by blebbistatin were abolished. These results suggest that, although inhibition of NMII reduced F-actin levels, actin dynamics in the growth cone is still necessary for axon growth to occur. Similarly, when neurons were treated with nocodazole at a low concentration that specifically dampens MT dynamics (33), blebbistatin no longer promoted axon growth. Together, these results suggest that both actin and MT dynamics are required for the axon growth promotion induced by blebbistatin over inhibitory substrates, and perhaps it is the interplay between the two polymers that brings about the rapid axon extension in response to blebbistatin.
Fig. 5.
Axon growth promotion over CSPGs induced by NMII inhibition requires actin and MT dynamics. Adult DRG neurons from conditioning lesioned mice were plated on CSPGs and were first treated with blebbistatin or DMSO as a vehicle control. After 13 h of treatment, cytochalasin D (Cyt D) (100 nM) or nocodazole (Ncd) (50 nM) was added to the blebbistatin-containing culture media and cultured for another 8 h. Note that application of Cyt D or Ncd completely prevented the axon growth-promoting effect of blebbistatin when applied to actively growing axons (black bars), whereas those treated with blebbistatin only continued to grow (green bar).
To examine if cytoskeletal reorganization induced by NMII inhibition affected axon growth rate, we analyzed axon growth by time-lapse microscopy. Inhibition of NMII activity markedly increased axon growth rate of embryonic DRG neurons on permissive substrates (5 μg/mL laminin + 100 μg/mL polylysine) (Fig. S5A). Time-lapse microscopy revealed that, in contrast to control neurons that underwent alternating phases of elongation, pause, and retraction, blebbistatin-treated neurons continued to grow axons at a relatively constant rate without appreciable pausing or retracting phases over the entire recording period (Fig. S5C). Adult DRG neurons from conditioning lesioned mice grew axons at a much faster rate than their embryonic counterparts, and the growth rate was further accelerated by inhibition of NMII (Fig. S5B). On inhibitory substrates, there was little axon growth as expected, but on exposure to blebbistatin, axon growth was observed within minutes, which lasted without appreciable pausing or retracting phases over the entire recording period (Fig. S5D and Movies S1 and S2).
The immediate change in axon growth rate on CSPGs in response to blebbistatin (Fig. S5D) is consistent with the rapid organization of the growth cone cytoskeleton (Fig. 4 B and D). Blebbistatin has been shown to induce rapid and reversible reorganization of cytoskeletal components in the growth cone (17). To examine if the effect of this compound on axon growth is also reversible, we performed washout experiments in neurons that were actively sending out axons on CSPGs in response to blebbistatin. When blebbistatin was washed out after 15 h of treatment, axons stopped growing and the lengths of axons were maintained for the next 6 h, as opposed to the actively growing axons that were continuously treated with blebbistatin (Fig. S6). Both the rapid response (Fig. S5D) and the reversibility (Fig. S6) support the notion that blebbistatin-induced axon growth promotion on CSPGs can be attributed to its direct effect on the growth cone cytoskeletal structures.
NMII Inhibition Triggers Axons to Grow Across the Permissive–Inhibitory Border in a Two-Compartment Culture System.
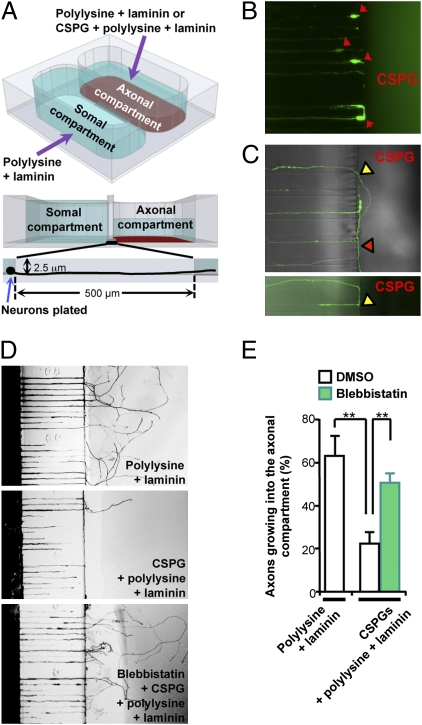
For successful regeneration, axon assembly must occur over inhibitory substrates, but before damaged axons enter the inhibitory zone, they encounter the border of the permissive–inhibitory environment. The ability to grow axons over uniformly coated inhibitory substrates may not necessarily translate into the ability to cross a permissive–inhibitory boundary. To address this issue, we modified the two-compartment culture platform (34) for more efficient and consistent coating with no shear stress on axons (Fig. 6A) (35). Adult DRG neurons were plated on the somal side coated with permissive substrates, and axon growth was guided into the CSPG-coated axonal side through the microchannels. When axons encountered the permissive–inhibitory border, axon growth into the axonal compartment was prevented and axons formed retraction bulb-like swellings at the border (Fig. 6B and Movie S3), similar to those encountering a lesion site in vivo (36). Notably, some axons that managed to exit the channels grew along the permissive–inhibitory border or turned back and re-entered the microchannels to avoid CSPGs (Fig. 6C and Movie S4). A compartmentalized chamber platform also allows treatments to be selectively applied to axons. In contrast to control axons that failed to enter the CSPG-coated axonal compartment, local administration of blebbistatin to the axonal side enabled axons to exit the channels and grow into the inhibitory terrain (Fig. 6 D and E and Movies S5 and S6), demonstrating that NMII inhibition promotes the growth of axons across the permissive–inhibitory boundary.
Fig. 6.
Local blockade of NMII activity in the axonal compartment allows growth cones to cross the permissive–inhibitory border. (A) Schematic of the two-compartment chamber. (B) Representative images of dystrophic growth cones of adult DRG neurons facing the permissive–inhibitory border. (C) Representative images of axons at the permissive–inhibitory border. Note that axons exiting the microchannels did not grow into the CSPG-coated axonal compartment but rather grew along the border (red arrow) or turned back and re-entered the microchannels (yellow arrows) to avoid CSPGs. (D and E) Adult DRG neurons were cultured in two-compartment chambers, and blebbistatin was locally applied only in the axonal side. Neurons growing in different chambers were stained at the same time to quantify axons growing into the axonal compartment. Representative images (D) and quantification (E) of axons entering the axonal compartment are shown. **P < 0.01.
Discussion
Here we report an unprecedented, marked acceleration of axon growth over multiple CNS inhibitors by blockade of NMII activity in both CNS and peripheral nervous system neurons. Although NMII activity can be regulated by ROCK (37), the axon growth-promoting effect of NMII inhibition appears to be independent of the Rho-ROCK pathway. In striking contrast to NMII inhibition, the blockade of ROCK had little effect on axon growth over aggrecan or CSPGs, especially in mature neurons (Fig. 2). The Rho-ROCK pathway has been shown to mediate growth cone collapse in response to repulsive guidance cues and CNS inhibitors primarily by inducing rearrangement of the actin cytoskeleton, rather than MTs. It has widely been assumed that the chronic axon growth inhibitory effect of CNS inhibitors is reflected in the acute collapse response. However, a previous study (38) has clearly demonstrated that the acute growth cone collapse and the chronic axon growth inhibition are mechanistically distinct events, suggesting that MT- and actin-based growth cone responses and subsequent axon growth can be distinguished.
In the current study, we show that aggrecan prevents MT extension in the growth cone and that inhibition of NMII releases these MTs from a compressed state and induces MT extension toward the leading edge. Although blebbistatin has been shown to promote MT protrusion toward growth cone periphery on permissive substrates (39), how it regulates growth cone cytoskeletal structures and subsequent axon growth on CNS inhibitors, to our knowledge, has never been examined. On the basis of the reorganization of the actin structure and the reduction in F-actin levels in the growth cone, it is likely that the axon growth-promoting effect of NMII inhibition on inhibitory substrates occurs via increased MT extension toward the growth cone leading edge, which occurs as consequence of the attenuation of retrograde actin flow and the loss of actin arc structure (Fig. S7). Configuration of growth cone MTs is associated with the rate of axon extension. Splayed configuration of MTs on permissive substrates (Fig. 4A) is often observed in slowly advancing growth cones, whereas the looped configuration on aggrecan (Fig. 4C) is observed when growth cones are in a paused state (40, 41). The tightly bundled MT configuration that we observed in growth cones from conditioning lesioned mice and blebbistatin-treated neurons is characteristic of rapidly advancing growth cones (40). It is becoming increasingly apparent that during axon growth MTs function not only as structural scaffolds, but also as direct sensors and regulators that control growth cone dynamics. By changing MT configuration and dynamics, MTs actively interact and coordinate with other components of the cytoskeleton to alter axon growth (40).
On laminin, blebbistatin has been shown to reduce, rather than to promote axon growth of peripheral neurons (28, 39). The seemingly apparent discrepancy between previous studies (28, 39) and ours might be due to differences in the substratum. In our study, neurons were cultured on polylysine (100 μg/mL) plus 5–10 μg/mL of laminin, whereas other studies applied a higher concentration of laminin (25 μg/mL) without polylysine (39) or with a less adhesive substrate, polyornithine (28). The final outcome of NMII inhibition on axon growth over permissive substrates might depend on the adhesiveness of the substrates, but further studies are required to address this issue.
The primary strategy to repair the damaged axons is to bridge the lesion site by promoting long-distance axon regeneration. Regulating local axon assembly at the growth cone and gene expression in the soma are equally important in promoting axon growth. Because changes in the growth cone cytoskeleton have a direct impact on the rate of axon extension, approaches to regulate growth cone MTs provide a strategy that not only allows axon growth over multiple CNS inhibitors, but also enhances the efficiency of axon assembly. Thus, complementary to recent attempts aiming at enhancing the intrinsic growth capacity of neurons by modulation of gene expression (42, 43), our study provides an effective way to boost the intrinsic ability of axon assembly by direct regulation of growth cone motility.
Although glial components potently block axon growth, the main function of the glial scar is to limit damage to the surrounding environment, which is essential for wound healing and provides protective functions (5). By targeting growth cone cytoskeletal components, this study suggests a possibility of inducing robust axon assembly over growth impediments without degrading the individual components that prevent axon growth.
Materials and Methods
Materials.
A full list of reagents and antibodies is provided in SI Materials and Methods.
Image Analysis and Statistics.
Axon length was measured with the “measure/curve” application of AxioVision 4.6 software (Carl Zeiss MicroImaging). For quantification of axon length, we restricted the analysis to neurons with processes equal to or longer than one cell body in diameter. The mean and SEM of neurite-bearing cells were calculated from at least three independent experiments. All error bars shown in the main and SI figures indicate SEM. To enable quantitative analysis of the MT structures in the growth cone, distance between two farthest MT plus ends was measured and normalized to the width of the growth cone neck. Growth cone MTs were categorized as “splayed” if the distance was more than three times longer than the width of the growth cone neck and as “bundled” otherwise. Student's t test was used to determine significance, which was set at a value of P < 0.05.
See SI Materials and Methods for details of substrate coating, transfection, immunofluorescence, microfabrication of the two-compartment chamber, and time-lapse video microscopy.
Supplementary Material
Acknowledgments
We thank Jai Madhok and Matt Fifer for critical reading of the manuscript, Stephen Dria for the preparation of schematics in Fig. 6A, Dr. Shuxin Li for myelin extracts, and Dr. Robert Adelstein for the NMIIA antibody. This work was supported by the Christopher and Dana Reeve Foundation (E.-M.H.), the Travis Roy Foundation (E.-M.H.), the Whitehall Foundation (F.-Q.Z.), the Basil O'Connor Starter Scholar Research Award of the March of Dimes (to F.-Q.Z.), the Maryland Stem Cell Research Fund (N.T. and I.H.Y.), and National Institutes of Health Grants RR020839 and GM072024 (to A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011258108/-/DCSupplemental.
References
- 1.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 2.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 4.Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15:R749–R753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 6.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JK, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng B, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, et al. PTP{sigma} is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: Receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertürk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci. 2007;27:9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. 2004;24:6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 18.Zhou FQ, Waterman-Storer CM, Cohan CS. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J Cell Biol. 2002;157:839–849. doi: 10.1083/jcb.200112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 20.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 21.Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 22.Davies SJ, et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 23.Borisoff JF, et al. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 24.Sivasankaran R, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 25.Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- 26.Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- 27.Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JA, Wysolmerski RB, Bridgman PC. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell. 2009;20:1167–1179. doi: 10.1091/mbc.E08-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng TP, Murakami N, Elzinga M. Localization of myosin IIB at the leading edge of growth cones from rat dorsal root ganglionic cells. FEBS Lett. 1992;311:91–94. doi: 10.1016/0014-5793(92)81374-u. [DOI] [PubMed] [Google Scholar]
- 30.Miller M, Bower E, Levitt P, Li D, Chantler PD. Myosin II distribution in neurons is consistent with a role in growth cone motility but not synaptic vesicle mobilization. Neuron. 1992;8:25–44. doi: 10.1016/0896-6273(92)90106-n. [DOI] [PubMed] [Google Scholar]
- 31.Burnette DT, et al. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diefenbach TJ, et al. Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J Cell Biol. 2002;158:1207–1217. doi: 10.1083/jcb.200202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor AM, et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang IH, Siddique R, Hosmane S, Thakor N, Höke A. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Exp Neurol. 2009;218:124–128. doi: 10.1016/j.expneurol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 37.Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 38.Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ketschek AR, Jones SL, Gallo G. Axon extension in the fast and slow lanes: substratum-dependent engagement of myosin II functions. Dev Neurobiol. 2007;67:1305–1320. doi: 10.1002/dneu.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore DL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.