Abstract
Graft loss from chronic rejection has become the major obstacle to the long-term success of whole organ transplantation. In cardiac allografts, chronic rejection is manifested as a diffuse and accelerated form of arteriosclerosis, termed cardiac allograft vasculopathy. It has been suggested that T-cell recognition of processed alloantigens (allopeptides) presented by recipient antigen-presenting cells through the indirect pathway of allorecognition plays a critical role in the development and progression of chronic rejection. However, definitive preclinical evidence to support this hypothesis is lacking. To examine the role of indirect allorecognition in a clinically relevant large animal model of cardiac allograft vasculopathy, we immunized MHC inbred miniature swine with synthetic polymorphic peptides spanning the α1 domain of an allogeneic donor-derived swine leukocyte antigen class I gene. Pigs immunized with swine leukocyte antigen class I allopeptides showed in vitro proliferative responses and in vivo delayed-type hypersensitivity responses to the allogeneic peptides. Donor MHC class I disparate hearts transplanted into peptide-immunized cyclosporine-treated pigs not only rejected faster than unimmunized cyclosporine-treated controls (mean survival time = 5.5 +/−1.7 vs. 54.7 +/−3.8 days, P < 0.001), but they also developed obstructive fibroproliferative coronary artery lesions much earlier than unimmunized controls (<9 vs. >30 days). These results definitively link indirect allorecognition and cardiac allograft vasculopathy.
Graft loss from chronic rejection affects all organs to varying degrees and has become the major obstacle to the long-term success of whole organ transplantation. In cardiac allografts, chronic rejection is manifested as a diffuse and accelerated form of atherosclerosis, termed cardiac allograft vasculopathy (CAV). Hearts are particularly susceptible to chronic rejection, because the vascular lesions usually progress to vessel occlusion, myocardial infarction, and graft failure. At present, the immunobiological mechanisms underlying CAV are unknown, and an effective means of preventing this disease is not available.
After organ transplantation, there are two distinct yet nonmutually exclusive pathways by which T cells recognize allogeneic MHC antigens and initiate the rejection process (1). Direct allorecognition occurs when host CD4+ T cells recognize intact allo-MHC molecules on the surface of donor antigen-presenting cells (APCs). Indirect recognition occurs when host CD4+ T cells respond to processed alloantigen presented as peptides bound to self-MHC class II molecules on self-APCs.
One theory of chronic rejection suggests that after transplantation, a small number of host CD4+ T cells are indirectly primed against a restricted repertoire of immunodominant peptides. Early posttransplant, the actions of these self-MHC-restricted T cells are overshadowed by the larger number of T lymphocytes that are directly primed by professional APCs present in the newly engrafted tissue (i.e., donor passenger leukocytes) (2). Over time, however, donor passenger leukocytes are depleted from the allograft (3), whereas recipient APCs continually infiltrate the allograft and process/present shed donor allopeptides. This process results in the diminishing importance of alloresponses mediated by directly primed T cells (4) and the predominance of a lingering low-grade alloresponse mediated by indirectly primed T cells. It is generally believed that endothelial cell injury is the final pathway to CAV, similar to nontransplant atherosclerosis (5). Indirectly primed CD4+ T cells could facilitate endothelial injury by providing help for alloantibody formation, by promoting lymphokine secretion required for macrophage and cytotoxic T-cell activity, and/or by producing growth factors for smooth muscle cells (6–8).
The important role of indirect allorecognition in chronic rejection is supported by two relevant observations in human organ allograft recipients. First, T cells from renal, cardiac, and lung transplant recipients with chronic rejection show evidence of reactivity to donor HLA allopeptides (6, 9–11). Second, patients with CAV demonstrate evidence of donor-specific hyporesponsiveness to directly presented but not indirectly presented donor HLA antigens (6, 9).
Although the indirect allorecognition theory is compelling, definitive experimental evidence linking indirect allorecognition and chronic rejection in a large animal system is lacking. In this article, we use a unique and clinically relevant large animal model of chronic rejection to show that indirect allorecognition of donor antigen by host T cells not only can induce but also can accelerate CAV. Our observations define a mechanistic link between indirect alloreactivity and chronic rejection and provide the rationale to develop novel and rational therapies to prevent this process in human transplant recipients.
Materials and Methods
Animals.
Swine leukocyte antigen (SLA)gg (class Ic, class IId) donors and class I disparate SLAdd (class Id, class IId) recipients between the ages of 2 and 3 months were generously provided by David H. Sachs (Massachusetts General Hospital) from his herd of partially inbred MGH Miniature Swine (12). All animal care and procedures were performed in compliance with both the Principles of Laboratory Animal Care (40) and the Guide for the Care and Use of Laboratory Animals (41).
Synthetic SLA Class Ic Peptides.
Most of the polymorphic sites of two known class I SLA loci in the pig (designated P1 and P14) are contained within the hypervariable regions of the α1 and α2 domains, as demonstrated by the comparison of the SLA class Ic PC14 α1 sequence to the corresponding SLA class Id PC14 α1 sequence (13).
Three peptides spanning the full length of the hypervariable regions of
the SLA class Ic PC14 α1 helix were synthesized and
labeled peptide 1 [amino acids (aa) 3–27], peptide 2 (aa 45–59),
and peptide 3 (aa 60–85). An allogeneic class II peptide,
DRβ (aa 24–42), was synthesized for use as a
negative-control peptide. Peptide purity was >90%, as verified by
HPLC and mass spectrometry.
(aa 24–42), was synthesized for use as a
negative-control peptide. Peptide purity was >90%, as verified by
HPLC and mass spectrometry.
Heterotopic Cardiac Transplantation.
Heterotopic cardiac transplantation was performed and allograft function monitored as previously described (14). Cyclosporine (CyA), generously provided by Novartis (Hanover, NJ), was administered intravenously to selected recipients at 10–13 mg/kg/day beginning on the day of surgery (POD 0) and continuing until POD 11, on the basis of earlier results (15).
Experimental Design.
Naïve SLAdd
(Id, IId) miniature swine
were s.c. immunized with a mixture of the three allogeneic SLA class
Ic PC14 peptides (500 μg of each peptide in
complete Freund's adjuvant) approximately 3 weeks before receiving
heterotopic class I mismatched SLAgg
(Ic, IId) hearts and 12
days of CyA. Two weeks after immunization and 1 week before
transplantation, splenocytes from the prospective recipients were
tested for in vitro proliferative responses against
individual allogeneic peptides. At the same time, the immunized pigs
were rechallenged with individual peptides to evaluate in
vivo delayed-type hypersensitivity (DTH) responses. Three control
groups included, (i) two SLAdd
(Id, IId) recipients of
class I mismatched SLAgg
(Ic, IId) hearts that were
not immunized and did not receive CyA; (ii) three
SLAdd (Id,
IId) recipients of class I mismatched
SLAgg (Ic,
IId) hearts that were not immunized but received
CyA; and (iii) two SLAdd
(Id, IId) recipients of
class I mismatched SLAgg
(Ic, IId) hearts that were
immunized with the irrelevant class II DRβ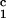 (aa
24–42) peptide and received CyA.
(aa
24–42) peptide and received CyA.
DTH Responses.
DTH responses were evaluated approximately 2 weeks after immunization with peptides by injecting 200 μg of individual peptide in 0.1 ml PBS intradermally into the neck of the pig. PBS was used as a negative control, whereas 100 μg of Mycobacterium tuberculosis H37 RA was used as a positive control. Width of induration was measured at 48 h after injection by blinded observers by using calipers. Positive responses were >10 mm, indeterminate responses were >5 mm and <10 mm, and negative responses were <5 mm of induration.
Peptide Proliferation Assay.
Two weeks after immunization, splenic tissue was harvested and splenocytes separated over a Ficoll gradient. T cells and antigen-presenting cells (APCs) were separated by nylon-wool adherence. To prevent contamination of responder cells with donor APCs, which may have migrated from the graft into the recipient spleen and thus may have provided a source for direct alloantigen presentation, nylon-wool nonadherent (thereby APC-depleted) splenocytes were used as responders and naïve nylon wool-adherent peripheral blood mononuclear cells (PBMCs) that were pulsed in vitro with class I peptides were used as APCs. Irradiated naïve nylon-wool adherent PBMCs to be used as APCs were preincubated with 50 μg/ml of individual allopeptides for 2 h at 37°C. The cells were then washed to remove excess peptides before being added to nylon-wool nonadherent T cells from spleen or PBMC (2 × 105) in a peptide proliferation assay for 5 days in triplicate plates, as previously described (16). [3H]Thymidine (1 μCi/well) was added for a 5- to 6-h period at the end of the culture, and [3H]thymidine incorporation was measured by β-scintillation counting. Stimulation index for each peptide equaled experimental cpm/media control cpm. For controls, splenocytes from 10 naïve nontransplanted SLAdd pigs were tested against each of the three allogeneic class I PC14 peptides. The average maximum stimulation index (SI) of all 30 naïve responses was 1.2 (Table 1.). Adding three standard errors resulted in a SI of 2.2. Therefore, a SI >2.3 was considered to be significant.
Table 1.
In vitro proliferative responses to SLA class Ic PC14 peptides
| Animal no. | Treatment | Graft survival, days | Posttransplant day of assay | Proliferative,
response SI/cpm
|
|||
|---|---|---|---|---|---|---|---|
| Peptide 1 | Peptide 2 | Peptide 3 | Media | ||||
| 13384 | None | 7 | 8 | 1.2/332 | 3.6/1034 | 4.7/1336 | 285 |
| 30 | 0.3/582 | 1.6/3305 | 3.9/7935 | 2043 | |||
| 168 | 7.3/3823 | 1.3/698 | 4.0/2101 | 525 | |||
| 13896 | None | 7 | 0 | 0.2/61 | 1.2/305 | 2.1/555 | 252 |
| 7 | 2.5/329 | 2.0/264 | 15.9/2096 | 132 | |||
| 13495 | CyA* | 52 | 59 | 2.1/655 | 2.2/676 | 2.4/721 | 306 |
| 13262 | CyA | 59 | 49 | 1.3/417 | 17.1/5338 | 4.8/1486 | 312 |
| † | None | 1.2 | 1.5 | 0.9 | |||
CyA 10–13 mg/kg IV on POD 0–11.
Average SI of 10 naïve SLAdd pigs.
Flow Cytometry.
Sera from animals were tested for antidonor IgM and IgG antibodies during the course of rejection, as previously described (16).
ELISA.
ELISA kits specific for porcine IFN-γ and IL-10 were purchased from BioSource International (Camarillo, CA). Supernatants harvested on day 2–3 of incubation were tested for IFN-γ and IL-10, following the manufacturer's instructions.
ELISPOT Assay.
ELISPOT plates (Immunospot M-200, Cellular Technologies, Cleveland, OH) were coated with anti-swine IFN-γ capture antibodies (Biosource International). After blocking and washing, responder cells (3 × 105) were added to duplicate wells with or without stimulators or antigens for 24 h. After washing, the anti-swine IFN-γ-biotinylated mAb detection antibody (Biosource International) was added, followed by streptavidin–horseradish peroxidase. The plates were developed by using 3-amino-9-ethylcarbazole (Sigma), and the resulting spots were counted on a computer-assisted ELISPOT image analyzer (Cellular Technologies).
Histology and Immunohistology.
Formalin-fixed tissue was stained with hematoxylin/eosin, Masson's trichrome stain, and Verhoeff stain, and evaluated by a blinded observer. Acute interstitial rejection was scored from 0 to 4 on the basis of the International Society for Heart and Lung Transplantation system (17). Vessel-wall thickening was scored as 0 (normal artery), 1 (<10% occlusion), 2 (>10% <50% occlusion), and 3 (>50% luminal occlusion) and the average score recorded (14). Frozen tissue sections were stained with anti-α smooth muscle actin mAb (clone 1A4, Sigma) and saturating concentrations of goat anti-swine IgM-FITC and IgG-FITC (Kirkegaard & Perry Laboratories).
Statistical Analysis.
Two-tail Student's t tests were used to compare graft survival times. Differences in survival time were deemed significant when P < 0.05.
Results
Indirect Allorecognition of Donor MHC Class I Peptides After Rejection of Cardiac Allografts.
To determine whether self-restricted T-cell recognition of donor class I MHC peptides occurred during the acute rejection of a class I mismatched cardiac allograft, splenocytes were harvested from two untreated SLAdd pigs that had acutely rejected class I disparate SLAgg hearts. The recipient splenocytes were tested for in vitro proliferative responses directed against the three donor class Ic PC14 peptides. Table 1 demonstrates that immediately after acute rejection, APC-depleted splenocytes from both acutely rejecting swine displayed sensitization to donor class I peptides, presented in the context of self MHC class II molecules on self APCs. Stimulation indices were consistently positive (>2.3) against PC14 peptide 3 (SI = 4.7 and 15.9). Recipient no. 13384 also showed sensitization to PC14 peptide 2 (SI = 3.6), whereas no. 13896 had lower reactivity to PC14 peptide 1 (SI = 2.5). By POD 30, the proliferative responses in no. 13384 were limited to PC14 peptide 3 (SI = 3.9). By POD 168, reactivity in pig no. 13384 continued to be detected against PC14 peptide 3 (SI = 4.0); however, new reactivity developed against PC14 peptide 1 (SI = 7.3), suggesting that the specificity of T-cell responses to donor antigens changes during the progression of rejection (intramolecular epitope spreading), as has been demonstrated in humans with acute cardiac allograft rejection (18).
Reactivity against donor class I peptides was also examined in two SLAdd pigs that were treated with a 12-day course of CyA and rejected class I mismatched SLAgg hearts in a more chronic fashion after >50 days. Our previous studies have shown that CyA-treated recipients bearing class I disparate hearts developed CAV by POD 28 (15). By the time of allograft rejection, strong proliferative responses were detected in pig no. 13262 against PC14 peptides 2 (SI = 17.1) and 3 (SI = 4.8), whereas pig no. 13495 showed a weaker response to PC14 peptide 3 (SI = 2.4). These data demonstrate that after both acute and chronic rejection, swine transplanted with class I mismatched hearts were sensitized to polymorphic donor class I peptides through the indirect pathway of allorecognition.
DTH Responses to Allogeneic Class I MHC Peptides in Peptide-Immunized Swine.
To confirm indirect presentation in vivo, DTH
responses to the donor class I SLA peptides were analyzed in four pigs
immunized with the PC14 class Ic peptides. Table
2 shows that only PC14 peptide 3 elicited
a positive DTH response in the primed animals. All immunized pigs
showed brisk DTH responses to the M. tuberculosis H37 RA
positive control and negative responses to PBS control. These results
confirmed indirect alloantigen presentation in vivo and
validated the immunogenicity of specific class I SLA allopeptides. In
addition, two pigs immunized with the negative control peptide,
DRβ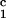 , demonstrated positive DTH responses directed
against the DR peptide (Table 2).
, demonstrated positive DTH responses directed
against the DR peptide (Table 2).
Table 2.
DTH responses in pigs immunized with class Ic PC14 or control peptides
| Animal | DTH
responses, mm of induration*
|
|||||
|---|---|---|---|---|---|---|
| Peptide 1 | Peptide 2 | Peptide 3 | PBS | DRβ1c † | MTB | |
| 13511§ | 0.0 | 0.0 | 20.0 | 0.0 | ND‡ | 19.0 |
| 13692§ | 0.0 | 0.0 | 28.0 | 0.0 | ND | 20.5 |
| 14071§ | 0.0 | 0.0 | 25.0 | 0.0 | ND | 16.0 |
| 14311§ | 0.0 | 0.0 | 25.5 | 0.0 | ND | 17.5 |
| 13914‖ | ND | ND | ND | 0.0 | 20.0 | 17.0 |
| 14146‖ | ND | ND | ND | 0.0 | 14.0 | 24.5 |
Measurements represent the average of two independent readings.
Control peptide.
Not done.
Immunized with PC14 peptides 1–3.
Immunized with DRβ1c control peptide.
Immunization of Recipient Pigs with Donor Class I MHC Peptides Promotes CAV in Class I Mismatched Hearts.
To determine the role of donor class I peptides in the development of
CAV, four SLAdd pigs immunized with the mixture
of PC14 class Ic peptides were transplanted with
class I mismatched SLAgg hearts under the cover
of a 12-day course of CyA. Control animals that were unimmunized but
treated with CyA rejected their class I mismatched hearts in 59, 53,
and 52 days (Table 3). Serial biopsies of
these allografts revealed the development of coronary artery intimal
proliferation but not until more than 4 weeks after transplantation
(Table 3), which is similar to our previous findings (15). In stark
contrast, recipients immunized with the mixture of PC14 class
Ic peptides rejected their
SLAgg hearts in an accelerated manner (POD 4, 5,
5, and 8), while still receiving CyA (P < 0.001, Table
3). Moreover, as early as POD 5, allografts from the PC14-immunized
recipients exhibited the characteristic fibroproliferative intimal
lesions of CAV. Indeed, three of the four hearts developed high-grade
intimal thickening (grade 2–3) between POD 5 and 8 (Table 3). The
characteristics of these arterial lesions were identical to those
observed in human heart transplant recipients undergoing chronic
rejection (19). The intima of the affected coronary arteries and
arterioles were thickened (Fig.
1a) because of collagen
deposition (Fig. 1b) and smooth muscle cell accumulation
(Fig. 1c). In many cases, this process resulted in complete
luminal occlusion (Fig. 1b). Immunization with the
irrelevant control peptide, DRβ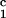 , did not accelerate
rejection of a class I disparate heart nor did it accelerate the
development of CAV (Table 3, Fig. 1d). However, immunization
of four additional pigs with a mixture of class
Ic peptides spanning the hypervariable region of
the α1 helix of the second classical swine
class I gene, PC1, also resulted in acceleration of intimal
proliferation (data not shown). Together, these data demonstrate that
indirect allorecognition of donor MHC peptides can induce and
accelerate the intimal proliferation characteristic of cardiac
allograft vasculopathy.
, did not accelerate
rejection of a class I disparate heart nor did it accelerate the
development of CAV (Table 3, Fig. 1d). However, immunization
of four additional pigs with a mixture of class
Ic peptides spanning the hypervariable region of
the α1 helix of the second classical swine
class I gene, PC1, also resulted in acceleration of intimal
proliferation (data not shown). Together, these data demonstrate that
indirect allorecognition of donor MHC peptides can induce and
accelerate the intimal proliferation characteristic of cardiac
allograft vasculopathy.
Table 3.
Graft survival and histology of class I disparate cardiac allografts
| Treatment | Animal | Graft survival, days | Histology at week*
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
| None | 13384 | 7 | Interstitial†: | 4 | |||||||
| Vascular‡: | 0 | ||||||||||
| None | 13896 | 7 | Interstitial: | 4 | |||||||
| Vascular: | 0 | ||||||||||
| CyA‡ | 13262 | 59 | Interstitial: | 1b | 3b | 1b | 3b | ||||
| Vascular: | 0 | 0 | 0 | 3 | |||||||
| CyA | 13397 | 53 | Interstitial: | 3a | 3b | 4 | |||||
| Vascular: | 0 | 0 | 3 | ||||||||
| CyA | 13495 | 52 | Interstitial: | 3a | 4 | ||||||
| Vascular: | 0 | 3 | |||||||||
| PC14 #1–3 + CyA | 13511 | 8 | Interstitial: | 4 | |||||||
| Vascular: | 2 | ||||||||||
| PC14 #1–3 + CyA | 13692 | 5 | Interstitial: | 4 | |||||||
| Vascular: | 3 | ||||||||||
| PC14 #1–3 + CyA | 14071 | 4 | Interstitial: | 4 | |||||||
| Vascular: | 1 | ||||||||||
| PC14 #1–3 + CyA | 14311 | 5 | Interstitial: | 4 | |||||||
| Vascular: | 3 | ||||||||||
| DRβ1c + CyA | 13914 | >60 | Interstitial: | 3a | 3a | 3a | 3a | 3a | 3a | ||
| Vascular: | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| DRβ1c + CyA | 14146 | >30 | Interstitial: | 3a | 3b | 3b | |||||
| Vascular: | 0 | 0 | 0 | ||||||||
Last datapoint in each row represents the postmortem specimen.
Grading based on the scoring system presented in Results.
CyA 10–13 mg/kg IV on POD 0–11.
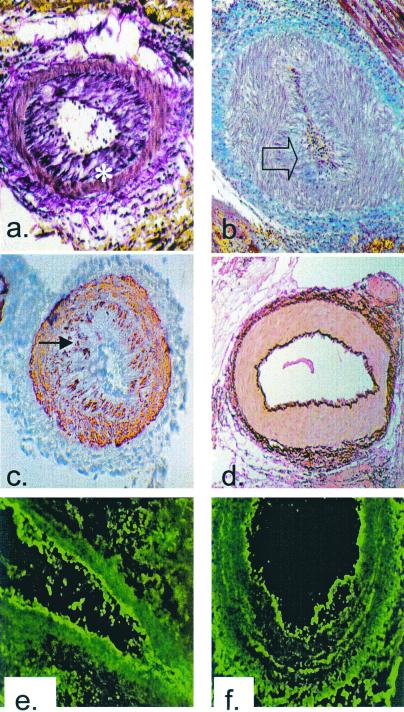
Figure 1.
Histological analysis of cardiac allografts. (a)
Voerhoeff elastin stain of the rejected cardiac allograft from
recipient no. 13511 on POD 5 (×100). Asterisk indicates internal
elastic lamina. (b) Trichrome stain of the rejected
cardiac allograft from recipient no. 13692 on POD 5 (×100). Blue
staining indicates the presence of collagen within occluding neointima,
as indicated by transparent arrow. (c) α-Actin
staining of the rejected cardiac allograft from recipient no. 13692 on
POD 5 showing smooth muscle cell accumulation within the intima,
indicated by filled arrow (×100). (d) Voerhoff elastin
stain of cardiac allograft from DRβ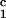 control pig no.
13914 at POD 15 showing no intimal thickening (×100).
(e) Immunofluorescent staining for IgM on the rejected
cardiac allograft from recipient no. 13692 on POD 5 showing antibody
deposition along the arteriolar endothelium (×250).
(f) Immunofluorescent staining for IgG on the
rejected cardiac allograft from recipient no. 13692 on POD 5 showing
antibody deposition along arteriolar endothelium (×250). Naïve
control hearts did not stain for antibody.
control pig no.
13914 at POD 15 showing no intimal thickening (×100).
(e) Immunofluorescent staining for IgM on the rejected
cardiac allograft from recipient no. 13692 on POD 5 showing antibody
deposition along the arteriolar endothelium (×250).
(f) Immunofluorescent staining for IgG on the
rejected cardiac allograft from recipient no. 13692 on POD 5 showing
antibody deposition along arteriolar endothelium (×250). Naïve
control hearts did not stain for antibody.
Proliferative Responses Against Donor Class I Peptides in Immunized Transplant Recipients.
To assess in vitro reactivity to individual class I allopeptides, proliferation assays were performed with splenocytes from the peptide-immunized recipients bearing class I disparate hearts. Table 4 (which is published as supplemental data on the PNAS web site, www.pnas.org) shows that the PC14 peptide-immunized pigs showed strong reactivity to PC14 peptide 3 after immunization but before heart transplantation (SI = 216, 167, 13.7, and 26.4). Of note, all four pigs demonstrated augmented reactivity against donor SLAgg cells after peptide immunization (SI = 191, 205, 48.4, and 71) when compared with naïve controls (SI = 6.3) (supplemental Table 4). Responses to third-party class I disparate SLAhh (class Ia, class IId) stimulator cells were minimal except for pig no. 13692, which generated an isolated heightened third-party response after immunization. This may have been because of assay variability or crossreactive antigens. After rejection of SLAgg hearts, all pigs maintained reactivity to PC14 peptide 3, and two of four recipients gained sensitization to PC14 peptide 1, again confirming self-restricted T-cell allorecognition of donor class I peptides during allograft rejection.
Cytokine Responses in Peptide-Immunized Recipients.
To characterize the nature of the T-cell response generated against class I allopeptides, cytokine profiles of T cells from pigs immunized with the mixture of PC14 peptides were analyzed after restimulation with individual PC14 peptides. An analysis was done of the relative production of IFN-γ and IL-10 by T cells from peptide-immunized swine after restimulation in vitro with PC14 peptides 1, 2, and 3 and before heart transplantation. There was significant production of IFN-γ in response to peptide 3 but no IFN-γ production in response to peptides 1 or 2. IL-10 was not produced in response to any of the PC14 peptides (data not shown).
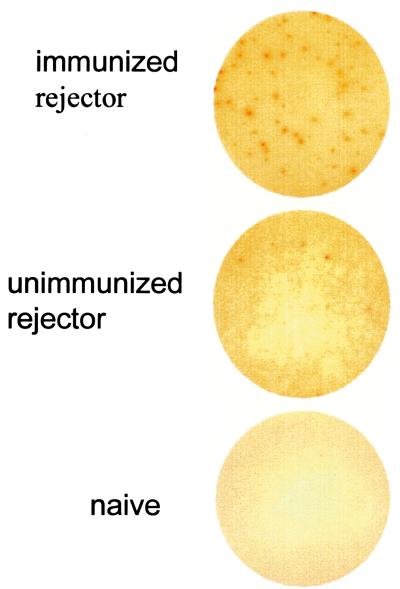
To assess the frequency of T cells responding to allogeneic peptide, swine-specific ELISPOT assays were performed. ELISPOT wells for IFN-γ revealed that after rejection, the peptide-immunized rejecter had approximately seven times as many spots in response to PC 14 peptide 3 as the unimmunized acute rejecter pig (41–68/3 × 105 vs. 6–9/3 × 105) (Fig. 2). Naïve responders produced no spots to allogeneic class I peptide, and less than three spots were detected against the other two peptides in all of the responders. These data are consistent with the observation that only PC14 peptide 3 induced a positive DTH response after immunization and suggest that immunization with the immunogenic PC14 peptide 3 generated a predominantly Th1-type response in vivo.
Figure 2.
ELISPOT detection of swine reactivity to allogeneic PC14 peptide 3. Representative IFN-γ ELISPOT wells by using 3 × 105 pig responder splenocytes per well plus PC14 peptide 3 (50 μg/ml) are shown. Responder splenocytes were harvested from peptide-immunized CyA-treated rejecter pig no. 14071 (Top), unimmunized acute rejecter pig no. 13384 (Middle), and a naïve pig (Bottom).
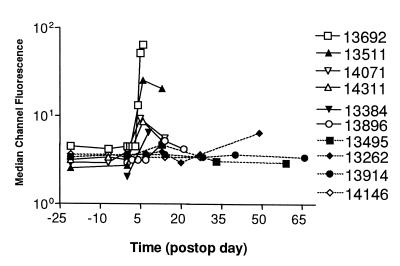
Alloantibody Production in Peptide-Immunized Transplant Recipients.
Sera collected from peptide-immunized animals before and after cardiac
transplantation were analyzed for the presence of donor-specific IgM
and IgG by flow cytometry. Immunization with the PC14 peptide mixture
accelerated the production of antidonor IgM in heart-transplant
recipients as compared with unimmunized CyA-treated and
DRβ -immunized control pigs (Fig.
3). The production of antidonor IgM in
the peptide-immunized pigs was even faster and more robust than that
detected in the unimmunized and nonimmunosuppressed recipients (Fig.
3). In contrast, minimal antidonor IgG was detected in the PC14
peptide-immunized pigs by the time of rejection (data not shown). To
determine whether the absence of antidonor IgG in the sera of
peptide-immunized animals was because of antibody absorption by graft
endothelium, immunofluorescent staining was performed on specimens from
hearts rejected by peptide-immunized animals. Significant amounts of
antidonor IgM (Fig. 1e) and antidonor IgG (Fig.
1f) were present on the arteriolar endothelium,
suggesting that IgM and IgG alloantibodies were both generated in
response to peptide immunization, but that the kinetics of alloantibody
production varied between the two isotypes.
-immunized control pigs (Fig.
3). The production of antidonor IgM in
the peptide-immunized pigs was even faster and more robust than that
detected in the unimmunized and nonimmunosuppressed recipients (Fig.
3). In contrast, minimal antidonor IgG was detected in the PC14
peptide-immunized pigs by the time of rejection (data not shown). To
determine whether the absence of antidonor IgG in the sera of
peptide-immunized animals was because of antibody absorption by graft
endothelium, immunofluorescent staining was performed on specimens from
hearts rejected by peptide-immunized animals. Significant amounts of
antidonor IgM (Fig. 1e) and antidonor IgG (Fig.
1f) were present on the arteriolar endothelium,
suggesting that IgM and IgG alloantibodies were both generated in
response to peptide immunization, but that the kinetics of alloantibody
production varied between the two isotypes.
Figure 3.
Immunization with allogeneic donor class Ic peptides
accelerated the generation of anti-donor IgM in host sera. Flow
cytometric analysis was performed to evaluate the levels of antidonor
IgM in sera from unimmunized acute rejecters (nos. 13384 and 13896),
unimmunized, CyA-treated pigs (nos. 13495 and 13262),
PC14-peptide-immunized, CyA-treated pigs (nos. 13692, 13511, 14071, and
14311), and DRβ -peptide-immunized, CyA-treated pig
(nos. 13914 and 14146).
-peptide-immunized, CyA-treated pig
(nos. 13914 and 14146).
Discussion
Chronic rejection is the primary limitation to long-term success in organ transplantation; therefore, understanding the pathogenesis of this process is of major clinical importance. This study was undertaken to establish the role of indirect allorecognition in a clinically relevant model of cardiac allograft vasculopathy. We used MHC-inbred miniature swine as recipients of allogeneic cardiac allografts because the porcine MHC (SLA) is well characterized, allowing us to synthesize donor SLA peptides; in addition, miniature swine provide the only reproducible model of chronic cardiac allograft rejection in large animals (19). Furthermore, this preclinical system circumvents some of the important limitations inherent in rodent models, including the known differences that exist in immunity and atherogenesis between large animals (including humans) and rodent species. For instance, rodents do not constitutively express class II MHC antigens on their coronary vascular endothelium, whereas larger animals, including humans, do express these important transplant antigens (20). Likewise, the pig is more similar to humans in its cardiovascular morphology and physiology (21) and its susceptibility to atherosclerosis (22, 23).
Our data clearly indicate that indirect allorecognition occurs during acute (24, ‖) and chronic rejection but most importantly, that indirect allorecognition of donor antigen promotes development of allograft vasculopathy, the sine qua non of chronic organ transplant rejection. We show that indirect allorecognition of donor MHC class I peptides not only induced but greatly accelerated the development of the fibroproliferative intimal lesions associated with CAV. As early as POD 5, severe intimal lesions had developed in the cardiac allografts of peptide-immunized recipients. We have transplanted over 18 class I mismatched hearts with CyA alone and, in each case, the hearts survived more than 35 days, and vascular lesions were never observed before POD 28 (15). Given this high degree of reproducibility, differences from these results obtained in small numbers of MHC inbred animals provide significant information. Histologically, the arterial lesions exhibited both collagen and smooth muscle cell accumulation and, in some cases, resulted in complete luminal occlusion. These lesions reproduced with fidelity the vascular lesions observed in human heart-transplant recipients undergoing chronic rejection. The rapidity with which peptide immunization induced intimal proliferation was surprising. However, a recent detailed electron microscopic analysis of transplant arteriopathy demonstrated that smooth muscle cells can migrate from media to intima within a week of transplantation (25). This finding suggests that the chronicity of CAV in human transplantation relates more to the lingering tempo of the inciting immune response than to the time needed for the formation of atheromatous vascular lesions. Of note, our model is limited in its ability to distinguish whether the early lesions induced in the peptide-sensitized pigs were generated by the same mechanisms as the lesions observed in grafts surviving over 2 months, although the vascular lesions were histologically identical.
Together, these data provide evidence that indirect allorecognition of a limited number of antigenic determinants plays a major role in the pathogenesis of chronic CAV. These findings are supported by rodent studies that have suggested that CAV may be initiated by CD4+ T cells that recognize MHC antigens via the indirect pathway of allorecognition (26–30) and by recent human studies that have demonstrated the persistence of donor specific MHC allopeptide T-cell reactivity in patients with chronic rejection of cardiac (6, 9) kidney (10), and lung (11) allografts.
Obviously, transplant recipients do not get primed by immunization with donor peptides in adjuvant. However, our data and data from rodents and humans indicate that CD4+ T cells from transplant recipients are primed to donor alloantigen presented by recipient APCs during the course of rejecting a graft, albeit with a low precursor frequency (27). Thus, indirectly primed CD4+ T cells may promote chronic rejection by effecting a low-grade smoldering alloimmune response, as has been suggested in several studies in human recipients of cardiac and kidney grafts (2, 6, 10). Interestingly, these studies also showed, as do we in our model, that because of epitope shifting or spreading, there is continuous activation of naive CD4+ T cells by new epitopes. The exact mechanisms of epitope spreading are unclear, but it is a phenomenon that has been established in autoimmune diseases, such as diabetes, multiple sclerosis, and arthritis, where CD4+ T cells recognize and respond to new peptide determinants of specific autoantigens. These diseases are clinically and morphologically characterized by the same progressive course as chronic rejection.
The immune effector mechanism that actually activates the endothelium and initiates atherogenesis is not known. We show that IFN-γ production is significantly up-regulated in peptide-immunized recipients that developed early vascular lesions. IFN-γ has been shown to play a role in the initiation of the cascade of events that lead to CAV in rodent models (31). Furthermore, IFN-γ has recently been shown to induce atherosclerosis in the absence of leukocytes (32). Perhaps a low-level DTH-like response, mediated by IFN-γ-producing CD4+ T cells that have been primed by donor peptides, activates macrophages and endothelial cells, leading to the proliferation of smooth muscle cells and ultimately CAV (27). Alternatively, CD4+ T cells reactive via the indirect pathway could initiate chronic rejection by providing help for alloantibody production (33) or CD8+ T cell effector functions (34).
Effectively suppressing or eliminating T cells with indirect allospecificity represents a significant clinical challenge because, (i) donor allopeptides are continuous shed from an allograft, (ii) CD4+ T cells are able to recognize new allodeterminants (donor peptides) and thereby expand the host's T-cell repertoire over time through epitope spreading, and (iii) there is evidence that current clinically available immunosuppressive agents do not effectively prevent indirect allorecognition (35). Thus, devising novel strategies for the induction of tolerance in T cells with indirect allospecificity may be the most effective strategy to prevent chronic rejection and prolong the lifespan of organ allografts (15). We are attempting to achieve this goal by using protocols to, (i) induce mixed hematopoietic chimerism (36), (ii) cotransplant vascularized and functional donor thymus (37), and (iii) block T-cell costimulation (38, 39).
Supplementary Material
Acknowledgments
We thank Dr. David H. Sachs for supplying the inbred MGH miniature swine and for helpful advice, Mr. J. Scott Arn for herd management and quality control typing, Dr. Yuchiao Chang for statistical analysis, and Dr. Henry Winn for his critical review of the manuscript. This work is supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health (2RO1-HL54211–04). R.S.L. is a recipient of the American College of Surgeon's Resident Research Fellowship and of a Research Fellowship Award from the International Society of Heart and Lung Transplantation.
Abbreviations
- SLA
swine leukocyte antigen
- CAV
cardiac allograft vasculopathy
- CyA
cyclosporine A
- SI
stimulation index
- POD
postoperative day
- DTH
delayed-type hypersensitivity
- APC
antigen-presenting cell
Footnotes
Vella, J. P., Magee, C., Vos, L., Carpenter, C. B. & Sayegh, M. H. (1997) J. Am. Soc. Nephrol. 8, 668 (abstr.).
References
- 1.Sayegh M H, Turka L A. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Sun Y K, Xi Y P, Maffei A, Reed E, Harris P, Suciu-Foca N. J Exp Med. 1993;177:1643–1650. doi: 10.1084/jem.177.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechler R I, Lombardi G, Batchelor J R, Reinsmoen N L, Bach F H. Immunol Today. 1990;11:83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- 4.Braun M Y, McCormack A, Webb G, Batchelor J R. Transplantation. 1993;55:177–182. doi: 10.1097/00007890-199301000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. N Engl J Med. 1999;340:115–121. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Ciubotariu R, Liu Z, Colovai A I, Ho E, Itescu S, Ravalli S, Hardy M A, Cortesini R, Rose E A, Suciu-Foca N. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornick P I, Mason P D, Yacoub M H, Rose M L, Batchelor R, Lechler R I. Circulation. 1997;97:1257–1263. doi: 10.1161/01.cir.97.13.1257. [DOI] [PubMed] [Google Scholar]
- 8.Vella J P, Knoflach A, Waaga A M, Sayegh M H. Graft. 1998;1 (Suppl. II):11–17. [Google Scholar]
- 9.Hornick P I, Mason P D, Baker R J, Hernandez-Fuentes M, Frasca L, Lombardi G, Taylor K, Weng L, Rose M L, Yacoub M H, et al. Circulation. 2000;101:2405–2410. doi: 10.1161/01.cir.101.20.2405. [DOI] [PubMed] [Google Scholar]
- 10.Vella J P, Spadafora-Ferreira M, Murphy B, Alexander S I, Harmon W, Carpenter C B, Sayegh M H. Transplantation. 1997;64:795–800. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 11.SivaSai K S, Smith M A, Poindexter N J, Sundaresan S R, Trulock E P, Lynch J P, Cooper J D, Patterson G A, Mohanakumar T. Transplantation. 1999;67:1094–1098. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pennington L R, Lunney J K, Sachs D H. Transplantation. 1981;31:66–75. [PubMed] [Google Scholar]
- 13.Sullivan J A, Oettinger H F, Sachs D H, Edge A S. J Immunol. 1997;159:2318–2326. [PubMed] [Google Scholar]
- 14.Madsen J C, Sachs D H, Fallon J T, Weissman N J. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 15.Madsen J C, Yamada K, Allan J S, Choo J K, Erhorn A E, Pins M R, Vesga L, Slisz J V, Sachs D H. Transplantation. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Lee R S, Yamada K, Womer K L, Pillsbury E P, Allison K S, Marolewski A L, Geng D, Thall A D, Arn J S, Sachs D H, et al. J Immunol. 2000;164:3434–3444. doi: 10.4049/jimmunol.164.6.3434. [DOI] [PubMed] [Google Scholar]
- 17.Billingham M E, Cary N R, Hammond M E, Kemnitz J, Marboe C, McCallister H A, Snovar D C, Winters G L, Zerbe A. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 18.Liu Z, Colovai A I, Tugulea D, Reed E F, Fisher P E, Mancini D, Rose E A, Cortesini R, Michler R E, Suciu-Foca N. J Clin Invest. 1996;98:1150–1157. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen J C. Graft. 1998;1 (Suppl. II):41–44. [Google Scholar]
- 20.Choo J K, Seebach J D, Nickeleit V, Shimizu A, Lei H, Sachs D H, Madsen J C. Transplantation. 1997;64:1315–1322. doi: 10.1097/00007890-199711150-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kirkman R L. In: Of Swine and Men: Organ Physiology in Different Species. Hardy M A, editor. Vol. 1. Oxford, U.K.: Elsevier; 1989. pp. 125–139. [Google Scholar]
- 22.Muller D, Ellis S, Topol E. J Am Coll Cardiol. 1992;19:418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- 23.Fuster V, Fass D N, Kaye M P, Josa M, Zinsmeister A R, Bowie E J W. Circ Res. 1982;51:587–593. doi: 10.1161/01.res.51.5.587. [DOI] [PubMed] [Google Scholar]
- 24.Auchincloss H, Lee R S, Shea S, Markowitz J S, Grusby M J, Glimcher L H. Proc Natl Acad Sci USA. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bojakowski K, Religa P, Bojakowski M, Hedin U, Gaciong Z, Thyberg J. Transplantation. 2000;70:65–72. [PubMed] [Google Scholar]
- 26.Benham A M, Sawyer G J, Fabre J W. Transplantation. 1995;59:1028–1032. doi: 10.1097/00007890-199504150-00019. [DOI] [PubMed] [Google Scholar]
- 27.Vella J P, Magee C, Vos L, Womer K, Rennke H, Carpenter C B, Hancock W, Sayegh M H. Transplantation. 1999;67:1523–1532. doi: 10.1097/00007890-199906270-00005. [DOI] [PubMed] [Google Scholar]
- 28.Adams D H, Russell M E, Hancock W W, Sayegh M H, Wyner L R, Karnovsky M J. Immunol Rev. 1993;134:5–19. doi: 10.1111/j.1600-065x.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 29.Krasinskas A M, Eiref S D, McLean A D, Szeto W Y, Kreisel D, Moore J S, Rosengard B R. Transplantation. 2000;70:514–521. doi: 10.1097/00007890-200008150-00020. [DOI] [PubMed] [Google Scholar]
- 30.Mandelbrot D, Furukawa Y, McAdam A, Alexander S I, Libby P, Mitchell R N, Sharpe A H. J Immunol. 2000;163:3753–3757. [PubMed] [Google Scholar]
- 31.Nagano H, Libby P, Taylor M K, Hasegawa S, Stinn J L, Becker G, Tilney N L, Mitchell R N. Am J Pathol. 1998;152:1187–1197. [PMC free article] [PubMed] [Google Scholar]
- 32.Tellides G, Tereb D A, Kirkiles-Smith N C, Kim R W, Wilson J H, Schechner J S, Lorber M I, Pober J S. Nature (London) 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 33.Russell P S, Chase C M, Winn H J, Colvin R B. J Immunol. 1994;152:5135–5141. [PubMed] [Google Scholar]
- 34.Allan J S, Choo J K, Vesga L, Arn J S, Pins M R, Sachs D H, Madsen J C. Ann Thorac Surg. 1997;64:1019–1025. doi: 10.1016/s0003-4975(97)00796-0. [DOI] [PubMed] [Google Scholar]
- 35.Sawyer G J, Dalchau R, Fabre J W. Transplant Immunol. 1993;1:77–81. doi: 10.1016/0966-3274(93)90063-e. [DOI] [PubMed] [Google Scholar]
- 36.Schwarze M L, Menard M T, Fuchimoto Y, Huang C A, Houser S, Mawulawde K, Allison K S, Sachs D H, Madsen J C. Ann Thorac Surg. 2000;70:131–138. doi: 10.1016/s0003-4975(00)01564-2. [DOI] [PubMed] [Google Scholar]
- 37.Lambrigts D, Menard M T, Alexandre G P J, Franssen C, Meurisse M, Van Calster P, Coignoul F, Mawulawde K, Choo J K, Yamada K, et al. Transplantation. 1998;66:810–814. doi: 10.1097/00007890-199809270-00019. [DOI] [PubMed] [Google Scholar]
- 38.Lee R S, Rusche J R, Maloney M, Sachs D H, Sayegh M H, Madsen J C. J Immunol. 2001;166:1572–1582. doi: 10.4049/jimmunol.166.3.1572. [DOI] [PubMed] [Google Scholar]
- 39.Chandraker A, Azuma H, Nadeau K, Carpenter C B, Tilney N L, Hancock W W, Sayegh M H. J Clin Invest. 1998;101:2309–2318. doi: 10.1172/JCI2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox J G, Cohen B J, Loew F M, editors. Laboratory Animal Medicine. Orlando, FL: Academic; 1984. [Google Scholar]
- 41.Grossblatt N, editor. Guide to the Care and Use of Laboratory Animals. Natl. Acad. Press, Washington, DC: Institute of Laboratory Resources Commission of Life Sciences Natural Research Council; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.