Abstract
Few studies have investigated the significance of decreased FEV1 in non-COPD, nonasthmatic healthy subjects. We hypothesized that a lower FEV1 in these subjects is a potential marker of an increased susceptibility to obstructive lung disease such as asthma and COPD. This was a cross-sectional analysis of 1505 Japanese adults. We divided the population of healthy adults with no respiratory diseases whose FEV1/FVC ratio was ≥70% (n = 1369) into 2 groups according to their prebronchodilator FEV1 (% predicted) measurements: <80% (n = 217) and ≥80% (n = 1152). We compared clinical data – including gender, age, smoking habits, total IgE levels, and annual decline of FEV1 – between these 2 groups. In addition, as our group recently found that TSLP variants are associated with asthma and reduced lung function, we assessed whether TSLP single nucleotide polymorphisms (SNPs) were associated with baseline lung function in non-COPD, nonasthmatic healthy subjects (n = 1368). Although about half of the subjects with lower FEV1 had never smoked, smoking was the main risk factor for the decreased FEV1 in non-COPD, nonasthmatic subjects. However, the subjects with lower FEV1 had a significantly higher annual decline in FEV1 independent of smoking status. Airflow obstruction was associated with increased levels of total serum IgE (P = 0.029) and with 2 functional TSLP SNPs (corrected P = 0.027–0.058 for FEV1% predicted, corrected P = 0.015–0.033 for FEV1/FVC). This study highlights the importance of early recognition of a decreased FEV1 in healthy subjects without evident pulmonary diseases because it predicts a rapid decline in FEV1 irrespective of smoking status. Our series of studies identified TSLP variants as a potential susceptibility locus to asthma and to lower lung function in non-COPD, nonasthmatic healthy subjects, which may support the contention that genetic determinants of lung function influence susceptibility to asthma.
Keywords: airflow obstruction, asthma, chronic obstructive pulmonary disease, pulmonary function test, thymic stromal lymphopoietin
Introduction
Pulmonary function is among the most important health indicators, being a strong predictor of long-term morbidity and mortality.1 Impaired pulmonary function is a strong risk factor for the development of asthma and COPD and a marker of disease severity. The diagnosis and assessment of COPD recommended by Global Initiative for Obstructive Lung Disease (GOLD) guidelines are based on the degree of airflow obstruction as assessed by spirometry.2 Any arbitrary classification of a continuous variable, such as FEV1 and the FEV1/FVC ratio, results in a group of patients with borderline phenotypes,3 and classification based on the FEV1 and FEV1/FVC criteria established by the GOLD guidelines may underemphasize the potential significance of the “unclassified” subgroup of individuals who have a decreased FEV1 and a normal FEV1/FVC ratio. Early-life origin of COPD has recently been emphasized,4,5 and there is evidence that pathological abnormalities of COPD may be present even in the absence of airflow obstruction.6 However, the clinical characteristics of individuals with a decreased FEV1 and a normal FEV1/FVC ratio are not well established, and the validity of classifying these individuals as “normal” is uncertain.
Chronic stimulation of the innate immune system by microbes, inhaled allergens, or components of tobacco smoke is involved in the inflammation and remodeling of airways that underlies asthma and COPD,7,8 and certain genetic variants may play a role in the common pathogenesis of both asthma and COPD.9,10 TSLP is an innate cytokine released by primary human epithelial cells in response to certain microbial products, physical injury, cigarette smoke, or inflammatory cytokines,11,12 and TSLP has an important role in the initiation of allergic and adaptive airway inflammation through innate pathways that initially activate airway epithelium.13 Patients with asthma have higher concentrations of TSLP in their lungs.14,15 Moreover, TSLP is overexpressed in the bronchial mucosa in patients with COPD, which suggests that TSLP not only plays a key role in allergic inflammation, but may also play a role in nonallergic airway inflammation.15 Our group has recently identified the TSLP gene as an asthma susceptibility gene.16 Since it has been suggested that genetic determinants of lung function influence susceptibility to asthma as well as COPD,17 our previous finding may indicate a role of TSLP in the development of airflow obstruction that originates in earlier life.
The two aims of the current study are to describe the characteristics of healthy subjects with lower FEV1 using a large dataset from healthy, non-COPD, nonasthmatic subjects which includes valid lung function measurements, chest roentgenograms, and responses about respiratory symptoms, and to investigate the role of functional polymorphisms at the TSLP gene on baseline lung function defined by FEV1% predicted and FEV1/FVC in these healthy subjects.
Methods
Subjects
This was a cross-sectional analysis of 1505 Japanese men and women aged 22 to 81 years who visited the Tsukuba Medical Center for annual medical checkup from June 2008 to May 2009. Detailed information on respiratory health, lifestyle, and exposure conditions to environmental irritants such as tobacco smoking, allergens, and air pollutions was collected. Lung function data, chest radiographic findings, and the results of a standard clinical examination were also available for all participants. Based on a detailed questionnaire on pulmonary symptoms, a physical examination including pulmonary auscultation and chest roentgenogram, we carefully excluded the presence of pulmonary diseases such as asthma, COPD, chronic bronchitis, old tuberculosis, and pulmonary fibrosis.
Our diagnosis of asthma basically relied on a positive response to the following questions: “Have you ever been told by a doctor that you have asthma” and “Do you still have asthma?” in addition to the presence of asthmatic symptoms such as wheezing, cough, dyspnea, and chest tightness or the current use of anti-asthmatic medications. Chronic bronchitis was defined as self-report of cough or phlegm during the day or at night on most days for as much as 3 months each year for 2 or more years. Clinical diagnosis of COPD was defined as reporting of any respiratory symptom (exertional breathlessness, chronic cough, regular sputum, frequent winter bronchitis, or wheeze) and evidence of airway obstruction on spirometry (both FEV1/FVC < 0.7 and FEV1 < 80% predicted). Subjects who reported previously doctor-diagnosed chronic bronchitis or emphysema were also excluded. Pulmonary tuberculosis may cause similar respiratory symptoms and chronic airflow limitation to those seen with COPD. Thus, we carefully excluded subjects with scarring on chest radiograph and/or previous history of pulmonary tuberculosis treatment.
Classification of the subjects
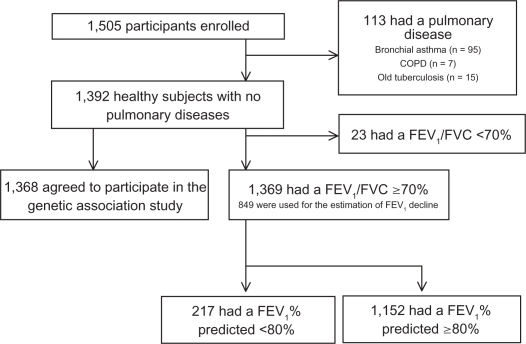
Of the 1505 subjects, 113 subjects with a history of asthma, COPD, chronic bronchitis, interstitial pneumonia, or pulmonary tuberculosis were excluded; 1392 (92.5%) subjects who had no pulmonary diseases were eligible to participate in the study. None of these 1392 subjects were taking bronchodilators such as β-agonists, anticholinergics, antileukotrienes, methylxanthines, or inhaled corticosteroids. Of the 1392 subjects without respiratory diseases, 23 subjects had FEV1/FVC < 70%. To characterize the group of individuals with decreased FEV1 (predicted FEV1 < 80%) and normal ratio of FEV1/FVC, we divided the 1369 healthy subjects into 2 subgroups according to their prebronchodilator FEV1 (% predicted): <80% (lower FEV1, n = 217) and ≥80% (higher FEV1, n = 1152).
Of these subjects, 365 healthy subjects with normal lung function were used as the control group in our previous genetic study of TSLP for adult asthma.16 The flowchart of the current study is shown in Figure 1.
Figure 1.
Recruitment and enrollment of study participants. Of the 1,505 participants, 113 subjects with a history of asthma, COPD, chronic bronchitis, or pulmonary tuberculosis were excluded, and 4 of those excluded were identified as having 2 or 3 pulmonary diseases. Of the 1,392 subjects without respiratory diseases, 23 subjects had FEV1/FVC < 70%. To characterize the group of individuals with decreased FEV1 (predicted FEV1 < 80%) and normal ratio of FEV1/FVC, we divided 1,369 healthy subjects whose FEV1/FVC was ≥70% into 2 subgroups according to their prebronchodilator FEV1 (% predicted): <80% (lower FEV1, n = 217) and ≥80% (higher FEV1, n = 1,152). We also examined the relationship between TSLP single nucleotide polymorphisms and baseline lung function using 1,368 healthy subjects, who agreed to provide a DNA sample for genotyping. Of the 1,392 healthy subjects, the median number of measurements was 9 (range 4–18 measurements) and the median number of years in retrospective follow-up was 11 (range 4–23 years) for the 866 subjects who had an annual checkup for 4 or more years of the retrospective follow-up and had at least 4 separate pulmonary function test measurements. Valid estimates of the annual decline of FEV1 were obtained for 849 healthy subjects with FEV1/FVC > 70% or for 827 healthy subjects with FEV1/FVC > lower limit of normal.
The Medical Ethics Committees of the University of Tsukuba (Tsukuba, Japan) and the Tsukuba Medical Center (Tsukuba, Japan) both approved the study, and each subject provided written informed consent.
Pulmonary function test (PFT)
Spirometry performance was required to meet the criteria by the Japanese Respiratory Society (JRS).18 Data for FEV1, FVC, and FEV1/FVC ratio were available for all participants. Lung function was measured in the standing position using an electronic spirometer (Autospiro series; Minato Medical Science Co., Ltd, Osaka, Japan). No bronchodilation was applied. Well-trained spirometry technicians continuously monitored the procedure and reviewed flow-volume curves to ensure adherence to standards. Participants performed up to 3 forced expiratory maneuvers to obtain acceptable maneuvers; FEV1 and FVC values taken to characterize each participant were the maximum results obtained from acceptable maneuvers. FEV1 and FVC were expressed as percentage of predicted values according to the prediction equations of the JRS. FEV1/FVC ratio was calculated using actual values. Calibration was checked weekly with a 2-L syringe. Calibration checks were also undertaken as specified by the manufacturer. All available longitudinal data of FEV1 were collected retrospectively to evaluate the annual decline of FEV1 for every participant.
Genotyping
A total of 23 polymorphisms in the TSLP gene have been identified in the Japanese population.19 Three tag SNPs in TSLP, rs3806933, rs2289276, and rs2289278, were genotyped using the TaqMan allele-specific amplification method (Applied Biosystems, Foster City, CA) as described previously.16
Statistics
Descriptive statistics were expressed as mean ± standard deviation (SD). We compared clinical data – gender, age, body mass index (BMI), smoking habits (never smoker, ex-smoker, and current smoker), smoking index, total serum IgE levels, atopy (defined as at least 1 positive response to 14 inhaled antigens), and annual decline of FEV1 – between 2 groups (the lower FEV1 group and the higher FEV1 group) using a 2-tailed Student’s t-test or Pearson’s chi-square test as appropriate. Smoking index was calculated by multiplying smoking dose (cigarettes per day) and duration (years smoked), and was categorized into 3 groups: 0, 0 to 200, and >200 cigarette-years.
The use of a fixed threshold to define airway obstruction is associated with some extent of misclassification.21 Therefore, in addition to the fixed values of FEV1/FVC (70%) and FEV1 (80% of predicted), we also used the age- and sex-specific lower limit of normal (LLN) as an alternative threshold for the FEV1/FVC ratio and FEV1 to categorize 1392 healthy subjects without pulmonary diseases. We used spirometry reference equations for FEV1/FVC and FEV1 for men and women from a large Japanese population-based study (1863 never-smoking adults from ages 18 to 95 years).22 The LLNs for FEV1/FVC and FEV1 were calculated by subtracting 1.645 × residual standard deviation (RSD) from the predicted means assuming a Gaussian distribution of the residuals.
Values of the annual decline of FEV1 were computed for each individual across the repeated measurements using a linear mixed-effect model. We included only those subjects who took the annual checkup for at least 4 years of retrospective follow-up and who had at least 4 separate PFT measurements to obtain an accurate estimate; among 1392 healthy subjects, 866 subjects had valid estimates of the annual decline of FEV1. The median number of measurements was 9 (range 4–18 measurements) and the median number of years in retrospective follow-up was 11 (range 4–23 years) for the 866 subjects. We used a random intercept to take into account the heterogeneity across subjects and the correlation induced by having repeated observations on the same subjects. We chose 25 years of age as the starting point for the analyses because maximum lung function would be achieved before that age and lung function is thought to be in decline by that age.20 Estimation of an annual decline of FEV1 was performed using SYSTAT software, version 13 (Systat Software, Inc., Chicago, IL). Valid estimates of the annual decline of FEV1 were obtained for 849 subjects with FEV1/FVC > 70% or for 827 subjects with FEV1/FVC > LLN.
General linear models were used to assess associations between TSLP polymorphisms and pulmonary function measurements after adjusting for gender, age, BMI, FVC% predicted, smoking status, smoking index, and total serum IgE levels (log-transformed). We used the Hardy-Weinberg equilibrium (HWE) program23 to compare observed numbers of genotypes with the numbers of genotypes expected under HWE. In the association study, we applied a Bonferroni correction on the P-values. As we tested 3 polymorphisms, we used P = 0.05/3 = 0.0167 as a threshold for significance.
For haplotype analyses, we used the Haplo.score program, which adjusted for covariates and calculated simulation P values for each haplotype.24 P values < 0.05 were considered to indicate statistical significance.
Results
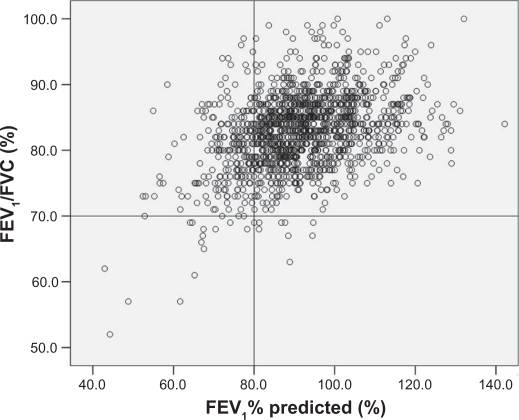
The characteristics of the population studied are shown in Table 1. The individual values of FEV1 and FEV1/FVC for all healthy subjects (n = 1392) are shown in Figure 2. Based on GOLD guidelines, 217 subjects who had a reduced FEV1 (<80% of predicted) could not be classified as having COPD because they had a FEV1/FVC ratio ≥70%. In cross-sectional analyses, the percentage of males in the lower FEV1 group was significantly higher than the percentage of males in the higher FEV1 group. No significant differences in the following factors were observed between the 2 groups – lower FEV1 and higher FEV1: age (range), 51.1 (25–78) vs 50.1 (22–78); BMI, 23.1 vs 23.0; and atopy, 57.6% vs 58.0% for the lower FEV1 group and the higher FEV1 group, respectively. Among subjects who had a normal FEV1/FVC ratio and a decreased FEV1% (the lower FEV1 group), 48.8% had never smoked. Nevertheless, smokers were more common in the lower FEV1 group than in the higher FEV1 group (P < 0.0001). The smoking index was also significantly higher in the lower FEV1 group than in the higher FEV1 group (P < 0.0001). Levels of serum total IgE were higher in the lower FEV1 group (P = 0.029). Annual decline of FEV1 calculated by a linear mixed regression averaged 32.3 mL/year for the lower FEV1 group and 20.8 mL/year for the higher FEV1 group (P < 0.0001). When FEV1 decline was compared within the lower FEV1 group (n = 138) between never smokers and ex- or current smokers, no difference was found according to smoking status (32.4 [SD 25.2] mL/year for 65 never smokers and 32.3 [28.6] mL/year for 73 current or ex-smokers, P > 0.5). In contrast, in the higher FEV1 group (n = 711), a significant difference in the annual decline of FEV1 was found between never smokers and ex- or current smokers (18.8 [SD 23.1] mL/year for 473 never smokers and 24.8 [28.2] mL/year for 238 current or ex-smokers, P = 0.0026).
Table 1.
Characteristics of the participants at baselinea
| Variables | FEV1% predicted <80% (N = 217) | FEV1% predicted ≥80% (N = 1152) | P value |
|---|---|---|---|
| Age range (years) | 51.1 ± 10.5 (25–78) | 50.1 ± 9.3 (22–78) | 0.20 |
| Male sex (%) | 125 (57.6) | 503 (43.7) | 0.0002 |
| BMI | 23.1 ± 3.2 | 23.0 ± 3.1 | 0.72 |
| FVC (L) | 2.73 ± 0.66 | 3.25 ± 0.77 | <0.0001 |
| FVC% predicted (%) | 84.0 ± 9.2 | 104.0 ± 11.5 | <0.0001 |
| FEV1 (L) | 2.18 ± 0.53 | 2.72 ± 0.64 | <0.0001 |
| FEV1/FVC (%) | 80.1 ± 5.5 | 83.8 ± 5.0 | <0.0001 |
| Smoking habitsb (N, %) | <0.0001 | ||
| Never smokers | 106 (48.8) | 759 (65.9) | |
| Ex-smokers | 59 (27.2) | 245 (21.3) | |
| Current smokers | 52 (24.0) | 148 (12.8) | |
| Smoking indexc (N, %) | <0.0001 | ||
| 0 | 106 (48.8) | 759 (65.9) | |
| 0–200 | 32 (14.8) | 139 (12.1) | |
| >200 | 79 (36.4) | 254 (22.0) | |
| Serum IgE (log IU/mL) | 1.86 ± 0.60 | 1.76 ± 0.58 | 0.029 |
| Atopy (%) | 125 (57.6) | 668 (58.0) | 0.94 |
| FEV1 decline (mL/year)d | 32.3 ± 27.0 | 20.8 ± 25.0 | <0.0001 |
| Never smokers (N = 538) | 32.4 ± 25.2 (N = 65)e | 18.8 ± 23.1 (N = 473)f | <0.0001 |
| Ex- and current smokers (N = 311) | 32.3 ± 28.6 (N = 73) | 24.8 ± 28.2 (N = 238) | 0.048 |
Notes:
Data are presented as mean ± SD unless otherwise indicated;
Subjects were classified as never smokers, ex-smokers, or current smokers based on questionnaire responses;
Smoking index was calculated for current and past smokers by multiplying smoking dose (cigarettes per day) and duration (years smoked);
To obtain an accurate estimate of the annual decline of FEV1, we selected only subjects who took the annual checkup for at least 4 years of retrospective follow-up and who had at least 4 separate pulmonary function test measurements (n = 849);
When FEV1 decline was compared within the lower FEV1 group (n = 138) between never smokers and ex- or current smokers, no difference was found (32.4 [SD 25.2] mL/year for 65 never smokers and 32.3 [28.6] mL/year for 73 current or ex-smokers, P > 0.5);
In the higher FEV1 group (n = 711), a significant difference in the annual decline of FEV1 was found between never smokers and ex- or current smokers (18.8 [SD 23.1] mL/year for 473 never smokers and 24.8 [28.2] mL/year for 238 current or ex-smokers, P = 0.0026).
Figure 2.
Plot of individual values of FEV1% predicted and FEV1/FVC. Data from 1392 healthy adults with no respiratory diseases.
Among 1392 healthy subjects, 866 subjects had a valid estimate of the annual decline in FEV1. When these 866 subjects were studied, the annual decline of FEV1 between lower FEV1 group and higher FEV1 group was also significantly different irrespective of how we define the lower FEV1 groups (FEV1% < 80% predicted or FEV1 < LLN). In addition, when 61 healthy subjects with FEV1/FVC < LLN were excluded from the analysis, the annual decline in FEV1 consistently differed between higher and lower groups, as the lower FEV1 group was defined using the cut-off of FEV1 < LLN (Table 2).
Table 2.
Association between FEV1 and a decline of FEV1 in healthy subjects
| Criteria for defining normal FEV1/FVC ratio | Criteria for defining the higher FEV1 group | Annual FEV1 declined (lower FEV1 group) | Annual FEV1 declined (higher FEV1 group) | P |
|---|---|---|---|---|
| FEV1/FVC ≥ 70% (n = 1369)a | FEV1% ≥ 80% | 32.3 ± 27.0 (n = 138) | 20.8 ± 25.0 (n = 711) | <0.0001 |
| FEV1/FVC ≥ LLN (n = 1331)b | FEV1 ≥ LLN | 31.1 ± 29.8 (n = 84) | 21.1 ± 24.3 (n = 743) | 0.0005 |
| None (n = 1392)c | FEV1% ≥ 80% | 34.2 ± 29.3 (n = 147) | 21.0 ± 25.1 (n = 719) | <0.0001 |
| None (n = 1392)c | FEV1 ≥ LLN | 36.1 ± 34.3 (n = 106) | 21.5 ± 24.5 (n = 760) | <0.0001 |
Notes: Valid estimates of the annual decline of FEV1 were obtained for 849,
827,
and 866
healthy subjects, respectively;
mL/year.
Abbreviation: LLN, lower limit of normal.
DNA for a genetic association study was available for 1368 of the 1392 healthy subjects with no pulmonary diseases. All SNPs investigated were within HWE (P > 0.05). The overall success rate for genotyping was 99.3%. The genotypic frequency of TSLP gene polymorphisms among the healthy Japanese participants (n = 1368) are shown in Table 3. SNP rs3806933 was associated with a lower FEV1% predicted (corrected P = 0.036) and with a lower FEV1/FVC ratio (corrected P = 0.033). SNP rs2289276 was also associated with a lower FEV1/FVC (corrected P = 0.029). Both associations were driven by carriers of 2 minor alleles (recessive models); significant associations were noted with a lower FEV1% predicted and with a lower FEV1/FVC at rs3806933 (corrected P = 0.027 and corrected P = 0.015, respectively) and at rs2289276 (corrected P = 0.042 and corrected P = 0.015, respectively). In contrast, there was no association between the genotype at rs2289278 and the FEV1% predicted or the FEV1/FVC phenotype. We did not observe any significant association between any one of these SNPs and the annual decline in FEV1.
Table 3.
Associations between TSLP genotype at 3 single nucleotide polymorphisms (SNP) and lung function
| SNP | N | FEV1% predicted (%) (95%CI) | Nominal P value | FEV1/FVC (%) (95%CI) | Nominal P value | |
|---|---|---|---|---|---|---|
| rs3806933 | CC | 721 | 92.04 (91.57–92.51) | 0.012a | 83.29 (82.89–83.68) | 0.011a |
| CT | 526 | 91.15 (90.60–91.71) | 82.46 (81.99–82.92) | |||
| TT | 112 | 90.95 (89.75–92.15) | 82.49 (81.48–83.49) | |||
| rs3806933 | CC | 721 | 92.04 (91.57–92.51) | 0.0089a | 83.29 (82.89–83.68) | 0.0050a |
| CT + TT | 638 | 91.12 (90.62–91.62) | 82.46 (82.04–82.88) | |||
| rs2289276 | CC | 777 | 91.97 (91.52–92.43) | 83.26 (82.88–83.65) | ||
| CT | 488 | 91.13 (90.55–91.70) | 0.019 | 82.42 (81.94–82.90) | 0.0096a | |
| TT | 91 | 90.95 (89.61–92.29) | 82.44 (81.32–83.56) | |||
| rs2289276 | CC | 777 | 91.97 (91.52–92.43) | 83.26 (82.88–83.65) | ||
| CT + TT | 579 | 91.10 (90.57–91.63) | 0.014a | 82.43 (81.98–82.87) | 0.0049a | |
| rs2289278 | CC | 844 | 91.38 (90.94–91.82) | 82.73 (82.36–83.10) | ||
| CG | 466 | 91.97 (91.38–92.56) | NSb | 83.21 (82.72–83.71) | NS | |
| GG | 52 | 92.22 (90.44–93.99) | 82.80 (81.32–84.28) | |||
| rs2289278 | CC | 844 | 91.38 (90.94–91,82) | NS | 82.73 (82.36–83.10) | NS |
| CG + GG | 518 | 91.99 (91.43–92.56) | 83.17 (82.70–83.64) |
Notes:
P < 0.05 after Bonferroni correction;
NS, not significant; some subjects could not be genotyped for technical reasons.
As recent studies have shown sex-specific associations between variants of the TSLP gene and asthma,25 levels of total serum IgE and specific IgE antibody toward cockroach,26 we further conducted a sex-stratified analysis; however, we could not determine the sex-specific effect on these associations (data not shown). We also did not find convincing evidence that the effect of TSLP genetic variants in FEV1% predicted or FEV1/FVC was modified by environmental exposure to tobacco smoke in the study population (data not shown).
The haplotype composed of these 3 variants was significantly associated with FEV1/FVC, with a P value of 0.018 from 1000 simulations of a global score test, as implemented in Haplo.score.24 The haplotype most strongly associated with lower FEV1/FVC was rs3806933C/rs2289276C/rs2289278C (haplotype-specific scores = −2.79, P = 0.0018) on the basis of 10,000 simulations; this haplotype showed significantly increased promoter activity in a previous study.16 For FEV1% predicted, haplotype analysis did not further improve the significance compared with the single SNP analysis.
Discussion
We studied prebronchodilator lung function results from a large number of Japanese adults without pulmonary disease. This study showed that the group of individuals who have normal FEV1/FVC ratio and decreased FEV1 mainly consists of smokers with higher cumulative tobacco consumption. In addition, this group was characterized by higher levels of total serum IgE and an increased annual decline in FEV1. Furthermore, this study provided preliminary evidence that functional SNPs in the TSLP gene influence FEV1 and FEV1/FVC in healthy subjects independently of the impact of tobacco smoke, emphasizing a distinct role for TSLP in airway obstruction that is a potentially important shared risk factor for asthma and COPD.
Although smoking is a major, modifiable risk factor, nonsmokers are similarly at risk for lung function decline; there was no difference in the annual FEV1 decline between smokers (32.4 mL/year) and nonsmokers (32.3 mL/year) in the lower FEV1 group. In a study with 20-year follow-up, average decline in FEV1 of 2496 healthy adults without airflow obstruction was 18 mL/year,27 which is compatible with that of 1152 healthy adults with normal FEV1 in the current study. An analysis of the Framingham Offspring Cohort also showed that the mean FEV1 decline was 19.6 mL for 666 males and 17.6 mL for 912 females in healthy never smokers.28 In contrast, in a study of 3-year follow-up, the mean yearly absolute decline of FEV1 in adult patients with mild asthma without severe asthma-related events was from 27 mL to 34 mL,29 which was similar to that found in 217 healthy adults with lower FEV1 in the current study. Never smokers comprise a substantial proportion of individuals with COPD, which has been associated with a prior diagnosis of asthma, a family history of asthma, and respiratory infections in childhood.4,5,30 Therefore, our findings indicate that the group of healthy subjects with lower FEV1 is a mixture of individuals who are susceptible to 1 or more irritants including microbial infections, allergens, or tobacco smoking. The lower FEV1 in otherwise healthy adults may be a marker for an increased FEV1 decline and an increased risk for chronic inflammatory lung diseases such as asthma and COPD, indicating the importance of recognizing decreased FEV1 in targeting intervention efforts among smokers and nonsmokers alike.
The mechanism that mediates the association of the TSLP gene with the lower levels of FEV1 in healthy subjects is not defined. The level of FEV1 at a given time point in an individual depends on 2 potentially independent processes: the maximum lung function obtained during development, and the rate of decline of lung function with age. Because we failed to see genetic effects of TSLP on the annual decline of FEV1, the association between basal lung function and TSLP genotype may be the result of abnormalities in lung development, rather than accelerated decline of lung function. TSLP is released predominantly by bronchial epithelial and smooth muscle cells in response to certain microbial products, allergen-associated molecules, tobacco smoke, physical injury, or inflammatory cytokines.11,31 TSLP integrates environmental stresses with an initial activation of resident innate immune cells, followed by activation of the adaptive immune system. Having a predisposition toward exaggerated TSLP responses against innocuous environmental antigens, individuals with particular TSLP genotypes would be expected to have impaired lung development which could lead to increased susceptibility to altered lung functions as adults.
Significant associations of an altered lung function in healthy subjects were obtained with the same TSLP SNPs, rs3806933 (−847C/T) and rs2289276 (−82C/T), which were associated with asthma susceptibility in our previous study;16 the same alleles were associated with both asthma and lower FEV1 in non-COPD nonasthmatic subjects. The rs3806933 site is in the promoter region of the TSLP gene, and the minor allele has been shown to create a binding site for the transcription factor activating protein (AP)-1. The variant enhances AP-1 binding to the regulatory element and increases promoter-reporter activity of TSLP in response to poly(I:C) stimulation in normal human bronchial epithelium.19 AP-2α protein, a possible transcriptional repressor, binds to sequences containing the rs2289276 (−82C/T) SNP with a higher affinity to −82C (on the protective allele).16 Our previous and current findings are consistent with a detrimental role of increased TSLP expression in pulmonary function and the pathogenesis of chronic inflammatory lung diseases. However, there remains a possibility that functional SNPs, other than rs3806933 and rs2289276, which are in linkage disequilibrium with either or both rs3806933 and rs2289276, may be truly responsible for our findings.
Recently, 2 studies have reported genome-wide association studies for lung function, using cross-sectional spirometric measurements in healthy individuals.32,33 They identified 6 genetic loci newly associated with natural variation in lung function, and it has been suggested that genetic determinants of lung function influence susceptibility to asthma and COPD. However, the list of the top 2000 SNPs associated with FEV1 and FEV1/FVC in these studies did not include any SNPs that are located in and around the TSLP gene including 3 SNPs examined in the current study. Failure to observe a SNP-for-SNP replication in ethnically diverse populations is not uncommon, and can result from a variation in allele frequencies, population admixture, heterogeneity of the phenotype, and environmental factors.
Our study has several limitations that require discussion. Importantly, we do not have information about postbronchodilator FEV1 for these subjects. For the clinical identification of early stages of COPD, the GOLD provided an international standard for diagnosis based on FEV1/FVC ratio <0.70 measured by postbronchodilator spirometry,2 and the use of prebronchodilator measurements prevents the identification of reversible obstruction. Reversible obstructions, however, are mostly undiagnosed asthma, and in this study, we carefully excluded asthma by a detailed questionnaire on pulmonary symptoms (cough, sputum, exertional dyspnea, wheeze), and physical examinations including pulmonary auscultation. In addition, prebronchodilator lung function has been used in many epidemiological studies and has been shown to have value in predicting health outcomes on a population level.5,21,34
Another limitation of our study is the retrospective nature of the FEV1 follow-up. The follow-up periods and numbers of spirometry measurements varied among subjects. This variation may have contributed to a biased estimation of the annual decline in FEV1 among subjects; however, we used a linear mixed-effects model to control for correlations among repeated measures within each subject, and we limited the analysis to include only subjects who had at least 4 separate FEV1 measurements during 4 or more years follow-up. Nonetheless, our results will need to be replicated in other, ideally prospective, studies with large cohorts.
Third, it is unclear whether the excess decline in FEV1 found in this study led to significantly more asthma or COPD development. However, it is important to recognize that excess decline of 10 to 15 mL/year in the lower FEV1 group compared with the higher FEV1 group would lead to an excess loss of 200 to 300 mL in 20 years, which would be expected to affect COPD incidence, particularly in subjects whose baseline lung function is lower. Serial measurements of FEV1 can presumably identify COPD at a point in time where the impact of the disease is generally limited. Our study suggests that prebronchodilator FEV1 may be a good marker of disease susceptibility even when the subjects have a normal FEV1/FVC ratio, allowing much earlier detection of COPD. Further prospective studies will determine whether lower FEV1 in healthy subjects without pulmonary diseases is associated with the future development of asthma or COPD.
In conclusion, although the FEV1/FVC ratio is a relatively sensitive index of mild airflow obstruction, it seems that lower FEV1 in non-COPD, nonasthmatic subjects may be of some help in identifying subjects at risk for these chronic inflammatory pulmonary diseases. Given the increasing societal burden of airflow obstruction, early detection of an altered lung function in asymptomatic adults is important. The demonstration of increased annual decline of FEV1, even in healthy adults with lower FEV1, should alert clinicians to the importance of case detection in subjects at risk, irrespective of their smoking status. This study may suggest the need for a definition of COPD that is not exclusively based on spirometry. In addition, we identified TSLP as associated with natural variation in lung function, bringing new insight into our understanding of the genetic basis of lung development and related airway disorders, such as asthma and COPD.
Acknowledgments
We thank all the subjects of this study for their participation which made this study possible. The study was partly supported by a Grant-in-Aid for Scientific Research (B), No. 21390254, from the Japan Society for the Promotion of Science.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2009). http://www.goldcopd.com/.
- 3.Köhler D, Fischer J, Raschke F, Schönhofer B. Usefulness of GOLD classification of COPD severity. Thorax. 2003;58(9):825. doi: 10.1136/thorax.58.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 5.De Marco R, Accordini S, Marcon A, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. [Epub 2010 Oct 8]. [DOI] [PubMed]
- 6.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 7.Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181(12):1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 8.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadeghnejad A, Ohar JA, Zheng SL, et al. Adam33 polymorphisms are associated with COPD and lung function in long term tobacco smokers. Respir Res. 2009;10:21. doi: 10.1186/1465-9921-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunninghake GM, Cho MH, Tesfaigzi Y, et al. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361(27):2599–2608. doi: 10.1056/NEJMoa0904006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura Y, Miyata M, Ohba T, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122(6):1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11(4):289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174(12):8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 15.Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 16.Harada M, Hirota T, Jodo AI, et al. TSLP promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. [Epub 2010 Jul 23]. [DOI] [PMC free article] [PubMed]
- 17.Weiss ST. Lung function and airway diseases. Nat Genet. 2010;42(1):14–16. doi: 10.1038/ng0110-14. [DOI] [PubMed] [Google Scholar]
- 18.The Committee of Pulmonary Physiology, Japanese Respiratory Society: Guideline of respiratory function tests. Spirometry, flow-volume curve, diffusion capacity of the lung. Nihon Kokyuki Gakkai Zasshi. 2004;(Suppl):1–56. [in Japanese]. [PubMed] [Google Scholar]
- 19.Harada M, Hirota T, Jodo AI, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40(3):368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 20.Rijcken B, Weiss ST. Longitudinal analyses of airway responsiveness and pulmonary function decline. Am J Respir Crit Care Med. 1996;154:S246–S249. doi: 10.1164/ajrccm/154.6_Pt_2.S246. [DOI] [PubMed] [Google Scholar]
- 21.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of FEV1 to FVC as a basis for establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(5):446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Committee of Pulmonary Physiology, Japanese Respiratory Society: The normal ranges of spirogram and arterial blood gas analysis in neversmoking Japanese. Nihon Kokyuki Gakkai Zasshi. 2001;39(5):S1–S17. [in Japanese]. [Google Scholar]
- 23.Terwillinger JD, Ott J. Handbook of Human Genetic Linkage. Baltimore, MD: Johns Hopkins University Press; 1994. [Google Scholar]
- 24.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J. Hum Genet. 2002;70(2):425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunninghake GM, Soto-Quirós ME, Avila L, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65(12):1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunninghake GM, Lasky-Su J, Soto-Quirós ME, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177(8):830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 28.Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med. 2010;123(5):468.e1–e7. doi: 10.1016/j.amjmed.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 30.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in Never-Smokers: Results from the population-based BOLD Study. Chest. [Epub 2010 Sep 30]. [DOI] [PMC free article] [PubMed]
- 31.Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smokeexposed human airway smooth muscle. J Immunol. 2010;185(5):3035–3040. doi: 10.4049/jimmunol.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42(1):36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hancock DB, Eijgelsheim M, Wilk JB, et al. Meta-analyses of genomewide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42(1):45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst-Hensch NM, Curjuric I, Pierre-Olivier B, et al. Longitudinal change of prebronchodilator spirometric obstruction and health outcomes: results from the SAPALDIA cohort. Thorax. 2010;65(2):150–156. doi: 10.1136/thx.2009.115063. [DOI] [PubMed] [Google Scholar]