Abstract
Objectives:
Medication for the management of chronic obstructive pulmonary disease (COPD) may be delivered by a number of different inhaler devices. This study was undertaken to determine the clinical effectiveness of the Respimat® handheld inhaler device compared with other handheld inhaler devices for the delivery of medication in stable COPD.
Methodology:
A systematic review of high-quality randomized controlled clinical trials comparing Respimat with other inhaler devices using the same medication was performed. Studies were searched for in the Cochrane Central Register of Controlled Trials as well as other relevant electronic databases. Manufacturers of inhaled COPD medication were also contacted for potential trials.
Results:
Seven studies of high methodological quality with 3813 participants were included in the review. Three trials used Handihaler® as the comparator inhaler, three used a chlorofluorocarbon metered-dose inhaler (CFC-MDI), and one trial used a hydroflouroalkane (HFA)-MDI. When Respimat was compared with Handihaler, the following reported outcomes were not significantly different: trough forced expiratory volume in 1 second (FEV1) (weighted mean difference [WMD] 0.01 L; P = 0.14), trough forced vital capacity (FVC) (WMD 0.001 L: P = 0.88), peak FEV1 (WMD 0.01 L: P = 0.08), peak FVC (WMD 0.01 L: P = 0.55), morning peak expiratory flow rate (PEFR) (WMD 5.06 L/min: P = 0.08), and evening PEFR (WMD 4.39 L/min: P = 0.15). Furthermore, there were no differences when Respimat was compared with Handihaler for risk of exacerbations (relative risk [RR] 0.94: P = 0.81), dry mouth (RR 1.57: P = 0.34), or nasopharyngitis (RR 1.42: P = 0.22). For Respimat compared with CFC-MDI, the only outcome for which data were available for meta-analysis was exacerbations, which were not significantly different (RR 1.20: P = 0.12). In addition, five trials with 2136 patients showed that there was no difference in risk of exacerbations or nasopharyngitis when Respimat was compared with all other handheld inhaler devices (RR 1.18: P = 0.13 and RR 1.33: P = 0.19, respectively). None of the clinical outcome measures reported was significantly different when the same, higher, or lower doses of medication were used in the inhaler devices being compared. Unfortunately, none of the included trials reported mortality as an outcome measure.
Conclusions:
Evidence from high-quality trials published to date suggests that the Respimat inhaler does not provide any additional clinical benefit to that provided by other inhaler devices in the management of COPD. Although in vitro studies have reported differences between the Respimat inhaler device and other handheld devices, we found no difference in any clinical outcome measures, including lung function and adverse events. Although recent reports have highlighted concerns of increased mortality with the Respimat inhaler device, none of the included trials reported mortality as an outcome. Only a small number of trials reported data that could be used in this systematic review, and a limited number of studies have been published that compare Respimat with other inhaler devices using the same drug and strength. Therefore, further trials comparing Respimat with other handheld inhaler devices using the same drug and dose are required before firm conclusions can be drawn. The concern with increased mortality with Respimat use should be investigated urgently.
Keywords: Respimat, aerosol cloud, handheld inhaler devices, COPD
Background
Respimat® Soft Mist™ Inhaler (Respimat; Boehringer Ingelheim, Germany) is a handheld inhaler device similar in size to the standard pressurized metered-dose inhaler (MDI). Compared with other inhaler devices currently available, Respimat has a number of benefits from the patient’s perspective.1,2 Respimat is simple to coordinate, and the delivered dose is independent of inspiratory effort. Respimat uses mechanical energy (via means of a spring) to generate a fine, slow-moving mist from an aqueous solution.3 Medication is stored as a solution in the aluminum drug cartridge, with a double-walled plastic collapsible bag that contracts as the solution is used. Actuation of the Respimat dose release utilizes the mechanical energy from the spring to force the metered drug solution through channels, producing two fine jets of liquid at the outlet that converge at a predetermined angle to form the aerosol cloud.4 This cloud contains an aerosol with a fine particle fraction smaller than 5.8 μm, which has been shown in laboratory studies to be at least twice as high as most MDIs and dry powder inhalers.5 This should mean that a higher proportion of the emitted dose is delivered to the lungs and less to the oropharynx with a Respimat inhaler compared with other inhaler devices.6 The “soft mist” moves more slowly and has a more prolonged duration than the aerosol cloud from an MDI. Hochrainer et al have compared Respimat aerosol velocity and spray duration with those of chlorofluorocarbon (CFC)-MDIs and hydroflouroalkane (HFA)-MDIs.5 The aerosol velocity from Respimat was between one-sixth and one-tenth that from the CFC- and HFA-MDIs. The mean velocity of the aerosol cloud measured at a 10 cm distance from the nozzle was 0.8 m/s for Respimat and 2.0–8.4 m/s for MDIs, and the mean duration was 1.5 s and 0.15–0.36 s, respectively.5
In vitro studies using Respimat have shown that in terms of access to the airways, 62% of the delivered dose contains particles that are 5.8 μm, which suggests that this fraction is approximately 2.5 times higher than that for most MDIs,7,8 and the mean velocity of the soft mist is approximately five times lower.5,6 Both of these factors are important in the reduction of oropharyngeal deposition and an increase in lung deposition.7,8 Furthermore, in vitro studies have also suggested that a lower dose of tiotropium (5 μg and 10 μg) is needed in the Respimat device when compared with the Handihaler device (Boehringer Ingelheim, Ingelheim, Germany).6,9
The combination of smaller particle size, lower velocity, and longer duration of the aerosol cloud with the Respimat inhaler implies that there would be improved coordination of inhalation with actuation, higher lung deposition, and lower oropharyngeal deposition compared with MDIs. Studies published to date tend to extrapolate in vitro nonclinical findings to clinical effectiveness.3,5,7,8,10,11 For example, an improvement in aerosol velocity, particle size, or lung deposition (nonclinical measures) may be observed in a laboratory setting, but this does not necessarily lead to improvements in lung function, a decrease in exacerbations, or improvements in quality of life (clinical measures), as these parameters need to be measured directly in a clinical (trial) setting. Many of the studies conducted to date have compared the Respimat inhaler with other handheld inhaler devices using various devices and combinations of drug and strengths. Some studies have included half of the dose of drug in the Respimat inhaler when compared with another device, making the assumption that the Respimat inhaler is twice as efficacious as the comparator.2,12–15 To eliminate the influence of drug and dose, it is important to use the same drug and dose when comparing two inhaler devices. Only a handful of studies have attempted to compare the Respimat inhaler with other inhaler devices using the same drug and dose,16,17 with varying results. Recent studies18,19 have also reported concerns regarding increased mortality with the use of the Respimat inhaler device in patients with chronic obstructive pulmonary disease (COPD), and this could be due to the greater deposition of bronchodilator medication to the lungs of patients with COPD. Therefore, mortality, among other outcomes, will be investigated in the current study.
Objectives
The aim of the current study was to conduct a systematic review of high-quality randomized controlled trials to evaluate the effectiveness of the Respimat inhaler when compared with other handheld inhaler devices using the same drug in the management of stable COPD.
Methods
Types of studies
Only randomized controlled trials were considered for inclusion. Trials were laboratory, community, or hospital based and were of any duration involving patients with stable COPD. Cumulative dosing or single dose trials were considered for inclusion, but these were analyzed in a separate subgroup from longer-term studies. Studies had to compare the same medication in both the Respimat inhaler and the comparator inhaler (eg, Respimat containing tiotropium compared with Handihaler containing tiotropium). Devices compared would preferably use the same dose. However, studies that used different doses in devices and compared the closest match between doses used in devices were included in the review. The difference in dose was noted and a subgroup analysis conducted if heterogeneity between the included studies for an outcome was evident. Studies comparing Respimat with an active drug to another device using placebo were excluded from this review.
Types of participants
Studies were considered in patients who met internationally accepted criteria for the definition of COPD (eg, American Thoracic Society [ATS], British Thoracic Society [BTS], European Respiratory Society [ERS], or Global Initiative for Chronic Obstructive Lung Disease [GOLD] guidelines). Studies were also considered if they met a practical definition of chronic airflow obstruction in the appropriate clinical setting of older (>40 years), usually (ex-)smoking patients with a forced expiratory volume in 1 second (FEV1) <70% and FEV1/forced vital capacity (FVC) <70% of predicted or were diagnosed by a respiratory physician as having COPD. Whether or not patients were able to use a particular inhaler prior to study entry did not affect inclusion to that study. It was understood that all patients would likely undergo training in use of the inhaler for the study.
Types of interventions
Studies were considered that described the use of Respimat compared with any other handheld inhaler for the delivery of the same medication. Co-intervention and/or contamination (eg, from crossover design studies) that may have occurred were recorded where data were available, and sensitivity analysis was conducted to test for robustness of treatment effect.
Types of outcome measures
Primary outcomes included mortality, lung function measurements (eg, FEV1, FVC, peak expiratory flow rate [PEFR], functional residual capacity, and total lung capacity) and exacerbations (eg, hospital admissions, unscheduled primary care attendance).
Secondary outcomes included symptom scores, use of additional relief medication, use of inhaled or oral steroid requirements (maintenance, rescue), patient preference, adverse effects, measures of systemic bioavailability, subsidiary physiological measures (eg, 6- or 12-minute walks and arterial blood gases), and validated quality of life measures (eg, St George’s Respiratory Questionnaire).
Search strategy for identification of studies
The Cochrane Central Register of Controlled Trials was searched for studies as well as separate additional searches carried out on PubMed (1966–September 2010), EMBase (1980–September 2010), Medline (1950–Septemebr 2010), CINAHL (1982–September 2010), Current Contents (1998–September 2010), and the Web of Science (1898–September 2010). Citations were reviewed without language restriction. To provide an inclusive search, all database searches were conducted without any limitations, and we used a single search term: “respimat”.
The reference lists of all included studies and review articles were checked in order to identify any further relevant citations not captured by electronic or manual searches. Authors of included trials were contacted for any other unpublished studies and to determine whether more data or clarification were required for their studies. In addition, pharmaceutical companies who manufactured inhaled COPD medication were contacted in order to obtain details of any further published or unpublished studies.
Selection of trials
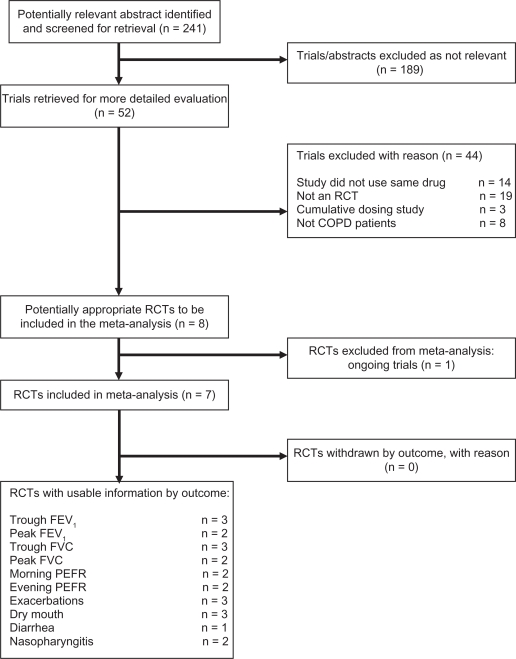
Two reviewers independently reviewed the results of a computerized search based on study title, abstract, and key words/MeSH headings, and any potentially relevant articles were obtained in full. The full texts of all potentially relevant articles were reviewed independently by the same two reviewers to assess each study according to previously written criteria. Written criteria included: 1) trials of a single or combination drug delivered by Respimat versus any other handheld inhaler device in stable COPD, 2) randomized controlled trial, 3) patients with COPD diagnosed according to established internationally accepted guidelines (eg, GOLD, BTS, ATS, ERS) or by using a practical and objective definition of COPD, and 4) laboratory- or community-based study of any duration using the same drug in the devices being compared. Agreement between the two reviewers on inclusion of studies was complete. In all cases, disagreements about inclusion of a study were resolved by consensus. Details of trial selection and reasons for exclusion are shown in Figure 1.
Figure 1.
Results of the search for trials and reasons for excluding studies.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PEFR, peak expiratory flow rate; RCT, randomized controlled trial.
Trial quality assessment
A methodological quality assessment of included trials was performed using the following criteria. Firstly, using the Cochrane Collaboration20 approach for the assessment of allocation concealment, all trials were scored according to the following grades: Grade A: adequate concealment, Grade B: uncertain, Grade C: clearly inadequate concealment, and Grade D: not used.
In addition, each study was assessed using the 0- to 5-point scale described by Jadad et al21 and summarized as follows:
Was the study described as randomized (1 = yes; 0 = no)?
Was the study described as double blind (1 = yes; 0 = no)?
Was there a description of withdrawals and dropouts (1 = yes; 0 = no)?
Was the method of randomization well described and appropriate (1 = yes; 0 = no)?
Was the method of double blinding well described and appropriate (1 = yes; 0 = no)?
Deduct one point if methods for randomization or blinding were inappropriate.
Data extraction
Details of each included trial characteristic were extracted and are shown in Table 1. All standard errors (SE) were converted to standard deviations (SDs) using the following equation: SE × square root (SQRT) of N. For trials that only reported 95% confidence intervals (95% CI) instead of SD or SE, the SD was estimated using the following equation: [(higher 95% CI − mean)/1.96] × (SQRT of N).
Table 1.
Characteristics of included trials
| Study reference | Participants’ characteristics at baseline | Intervention |
|---|---|---|
| Brand et al16 | Laboratory-based study in Germany. Four-day, four-way crossover study with 13 patients included (9M/4F), 5 ex-smokers, and 8 smokers. Mean values: pack-years 46.9, age 61.6 years, FEV1 1.37 L, FEV1% 46.4, FEV1/FVC ratio 0.44 | Respimat (FEN 50 μg and IB 20 μg) vs HFA-MDI (FEN 50 μg and IB 20 μg). Study in patients with poor MDI technique receiving radio-labelled Berodual and measuring lung deposition by gamma scintigraphy |
| Caillaud et al24 | Multicenter, parallel-group, dose-ranging study across 15 centers in France. COPD patients (n = 202; 86% males, mean age 61 years | Respimat (TTB 1.25 μg vs 2.5 μg vs 5 μg vs 10 μg vs 20 μg) vs Handihaler 18 μg vs Respimat (placebo) vs Handihaler (placebo) for 3 weeks. Study drugs were administered once daily (two puffs via Respimat or one capsule via Handihaler). Doses chosen for inclusion in review were Respimat TTB 10 μg two puffs daily vs Handihaler TTB 18 μg one capsule daily |
| Iacono et al17 | Three-period crossover trial involving 36 COPD patients. Mean values: age 52 years, duration of COPD 10 months, FEV1% 52.6, FVC% 72.1, FEV1/FVC% 59.4, FEV1 1.8 L, FVC 3.0 L, and FEV1 reversibility 30.7% | Cumulative doses of IB for 1+1+2+4+8 puffs inhaled at 50-min intervals from one of the following devices: Respimat 10 μg/puff, Respimat 20 μg/puff, and CFC-MDI 20 μg/puff. Doses chosen for inclusion in the review were Respimat 20 μg/puff vs CFC-MDI 20 μg/puff (320 μg/device, over 250 min) |
| Ichinose et al25 | Two-way/two-period crossover design conducted in 27 outpatient centers in Japan. 134 COPD patients completed the trial, 98% were males, 77.1% were ex-smokers, and 66.9% had severe or very severe disease. Mean values: age 70.2 years, FEV1% 43.1, FEV1/FVC% 41.9, smoking pack-years 60.4, and time since diagnosis 5.8 years | Respimat (TTB 5 μg, two puffs of 2.5 μg) plus Handihaler placebo capsule vs Handihaler (TTB 18 μg one capsule daily) plus Respimat placebo inhaler. All treatments were administered for 4 weeks then all patients entered a 4-week wash-out period and then restarted study treatment for a further 4 weeks receiving different (whichever) combination treatment not received in the first period |
| Kilfeather et al12 | Multicenter, parallel-group trial conducted in 92 centers in Europe. During a 2-week run-in, all patients received Berodual CFC-MDI (IB 20 μg/FEN 50 μg per puff) two puffs four times daily, and patients who had an exacerbation were excluded from the study. 224 patients were in the Respimat group and the CFC-MDI group had 220. Mean values: age 62 years, pack-years 36–37, FEV1 1.15–1.17, FEV1% 40–41, FEV1/FVC% 55–56, and % of patients with FEV1 reversibility >15% 42–51 | Patients remaining after the 2-week run-in period were randomized into one of five treatments: Respimat IB 10 μg/FEN 25 μg one puff, Respimat IB 20 μg/FEN 50 μg one puff, CFC-MDI IB 20 μg/FEN 50 μg two puffs, Respimat placebo one puff, or CFC-MDI placebo two puffs. All treatments were administered four times daily. Doses chosen for inclusion in the review were Respimat IB 20 μg/FEN 50 μg one puff (total dose/day IB 80 μg/FEN 200 μg) vs CFC-MDI IB 20 μg/FEN 50 μg two puffs (total dose/day IB 160 μg/FEN 400 μg) |
| van Noord et al26 | Multicenter, double-blind, crossover study. 205 COPD patients with 147 males, 128 ex-smokers, and COPD diagnosis of 10 years. Mean values: age 64 years, pack-years 51, FEV1 1.05 L, FEV1% 37, FVC 2.54 L, FEV1/FVC% 42, FEV1 reversibility 19.9% | Respimat (TTB 5 μg, two puffs of 2.5 μg) plus Handihaler placebo capsule vs Respimat (TTB 10 μg, two puffs of 5 μg) plus Handihaler placebo vs Handihaler (TTB 18 μg one capsule daily) plus Respimat placebo inhaler vs Respimat placebo and Handihaler placebo. All treatments were administered for 4 weeks then all patients entered a 4-week wash-out period. This was a 4-week treatment period study with a 4-week washout between each treatment. Doses chosen for inclusion in review were Respimat TTB 10 μg vs Handihaler 18 μg |
| Zuwallack et al15 | Multinational (13 countries) multicenter (179) parallel trial. Following a 2-week run-in, 1480 patients were recruited and 1460 were evaluable. Mean age was 64.1 years; 65.4% of patients were male and 89.0% were white. The mean duration of COPD was 8.4 years. Current smokers (n = 600) or ex-smokers (n = 860) with a mean FEV1% of 41.4, FVC 2.6 L, mean FEV1/FVC% of 44.8, smoking pack-years 51–52 years, FEV1 reversibility 0.217–0.216 L | Respimat (IB 20 μg and SAL 100 μg) vs CFC-MDI (IB 36 μg and SAL 206 μg) vs Respimat (IB 20 μg). All treatments were administered four times daily. All treatments were administered four times daily for 12 weeks. Doses chosen for inclusion in review were Respimat IB 20 μg and SAL 100 μg vs CFC-MDI IB 36 μg and SAL 206 μg |
Abbreviations: CFC, chlorofluorocarbon; COPD, chronic obstructive pulmonary disease; FEN, fenoterol hydrobromide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HFA, hydrofluoroalkane; IB, ipratropium bromide; MDI, metered-dose inhaler; SAL, salbutamol; TTB, tiotropium bromide.
Statistical considerations
Trials were combined for meta-analysis using the Cochrane Collaboration systematic review software (Review Manager 4.3.2) from the Nordic Cochrane Centre, Rigshospitalet, Denmark.22 Two types of comparisons were conducted. Firstly, all studies comparing Respimat with another inhaler device were compared. Secondly, data from all devices compared with Respimat were combined to provide an overall comparison of all devices with Respimat. Mantel and Haenszel’s fixed effect model23 was used in the meta-analysis. Dichotomous outcomes such as exacerbations were assessed using relative risk (RR) (with 95% CI). Data from each of the continuous outcomes were analyzed as weighted mean differences (WMD with 95% CI).
If there was a sufficient number of included trials, subgroup analysis would have been carried out using medication dose, age, and disease severity. For each outcome measure, the null hypothesis that there is no heterogeneity between included randomized controlled trials was tested. Sensitivity tests were used to investigate any possible heterogeneity in the size of the measured response attributable to the subgroups identified previously and due to study quality. If sufficient studies were included, funnel plots were constructed for the outcome measures in order to test for possible publication bias.
Description of included trials
All seven included trials were either funded or supported by Boehringer Ingelheim, the manufacturer of Respimat inhaler device and producer of tiotropium and ipratropium. Contact with included study authors on repeated occasions to obtain additional data or clarification of trial results failed to produce any responses. Therefore, all data presented in this systematic review were obtained from those published in the manuscripts and available in the public domain.
Trial design and patient demographics
There were two laboratory-based trials,16,17 and the remaining five trials12,15,24–26 were based in either the community or hospital setting. There were four crossover-designed trials16,17,25,26 and three parallel-designed trials.12,15,24 Five of the trials were multicenter and/or multinational.12,15,24–26 Patients included in the seven trials had the following values (range): age 46–70 years, FEV1 1.05–1.8 L, FVC 2.54–3.0 L, FEV1% reversibility 20%–51%, FEV1% predicted 37–52, FEV1/FVC% 42–55, and smoking pack-years 36–60 years.
Trial interventions, devices, and drug doses
Two trials were of single day duration. Brand et al16 compared fenoterol 50 μg plus ipratropium 20 μg in both the Respimat and Handihaler in patients with poor MDI technique. Iacono et al17 used cumulative doses of ipratropium (one to eight puffs) inhaled at 50-minute intervals from one of the following devices: Respimat 10 μg/puff, Respimat 20 μg/puff, or CFC-MDI 20 μg/puff. No usable data were reported in these two trials.
Five trials that provided all of the data for the review included three trials that used Handihaler and two that used CFC-MDI. Caillaud et al24 used the Respimat inhaler with a range of doses of tiotropium (1.25 μg, 2.5 μg, 5 μg, 10 μg, and 20 μg, each dose given once daily using two puffs) compared with Handihaler 18 μg one capsule once daily for 21 days. A trial by van Noord et al26 also compared the Respimat containing tiotropium 10 μg with the Handihaler with 18 μg of tiotropium for 4 weeks. Ichinose et al25 used Respimat with tiotropium 5 μg compared with Handihaler with tiotropium 18 μg one capsule daily for 4 weeks.
Kilfeather et al12 compared Respimat with CFC-MDI with both devices containing ipratropium 160 μg plus fenoterol 400 μg daily for 12 weeks, and Zuwallack et al15 compared Respimat with CFC-MDI using varying doses, with the dose included in the review being Respimat ipratropium 20 μg plus salbutamol 100 μg and for the CFC-MDI ipratropium 36 μg plus salbutamol 206 μg for 12 weeks.
Methodological quality of included studies
The seven included trials were of high-quality design, as the majority of the trials went to great lengths to double blind the patients and researchers of the inhaler devices by using placebo devices. This double-dummy design ensured that patients handled study inhalers equally and prevented both the investigators and patients from differentiating active drug from placebo, despite the different physical appearance of the devices. Except for the Iacono et al17 trial scoring an ‘A’ for Cochrane study quality grading, all other trials scored a ‘B’, and when using the Jadad quality scale all trials scored 5, indicating that all included trials were of high methodological quality.
Results
A search of several databases identified 241 abstracts for possible inclusion in the review. On closer screening, 52 abstracts were selected and full-text articles retrieved. Closer examination of the 52 full-text articles excluded 45 as not being appropriate, and the remaining seven studies12,15–17,24–26 with 3813 participants were included in the review.
Three trials24–26 compared Respimat with Handihaler, three trials12,15,17 compared Respimat with CFC-MDI, and one trial16 compared Respimat with HFA-MDI. However, only five trials12,15,24–26 reported data that could be used in the review, as the remaining two trials were either single-day16 or single-dose17 studies. Outcome measures for which two or more trials contributed data are discussed here, and details of all outcomes measures in the review are listed in Table 2. Unfortunately, none of the included studies reported mortality as an outcome measure.
Table 2.
Summary of effect sizes from outcomes reported in the included trials
| Comparison/outcome | WMD*/RR** | 95% CI | P value | No. of studies in outcome (participants) |
|---|---|---|---|---|
| Respimat versus Handihaler | ||||
| Trough FEV1 (L) | 0.01* | 0.00 to 0.03 | 0.14 | 3 (693) |
| Trough FVC (L) | 0.001* | −0.04 to 0.03 | 0.88 | 3 (693) |
| Peak FEV1 (L) | 0.01* | 0.00 to 0.03 | 0.08 | 2 (642) |
| Peak FVC (L) | 0.01* | −0.02 to 0.04 | 0.55 | 2 (642) |
| Morning PEFR (L/min) | 5.06* | −0.69 to 10.72 | 0.08 | 2 (425) |
| Evening PEFR (L/min) | 4.39* | −1.54 to 10.31 | 0.15 | 2 (425) |
| Adverse events | ||||
| Exacerbations | 0.94* | 0.58 to 1.54 | 0.81 | 3 (715) |
| Dry mouth | 1.57* | 0.62 to 3.97 | 0.34 | 3 (715) |
| Diarrhea | 0.33* | 0.04 to 3.17 | 0.34 | 1 (294) |
| Nasophryngitis | 1.42* | 0.81 to 2.46 | 0.22 | 2 (664) |
| Respimat versus CFC-MDI | ||||
| Adverse events | ||||
| Exacerbations | 1.20 | 0.95 to 1.51 | 0.12 | 2 (1421) |
| Nasophryngitis | 1.21 | 0.62 to 2.38 | 0.58 | 1 (977) |
Notes:
WMD;
RR.
Abbreviations: CFC-MDI, chlorofluorocarbon metered-dose inhaler; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PEFR, peak expiratory flow rate; RR, relative risk; WMD, weighted mean difference.
Respimat versus Handihaler
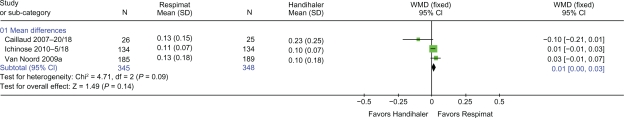
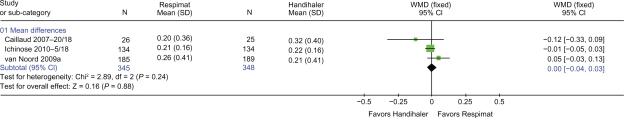
Three trials24–26 with 693 patients reported trough FEV1 (Figure 2) and trough FVC (Figure 3). There was no difference in either trough FEV1 or trough FVC when Respimat was compared with Handihaler (WMD 0.01 L: 95% CI 0.00 to 0.03, P = 0.14 and WMD 0.001 L: 95% CI −0.04 to 0.03, P = 0.88, respectively). Two trials25,26 with 642 patients provided data on peak FEV1 and peak FVC. There was no difference in peak FEV1 or peak FVC when Respimat was compared with Handihaler (WMD 0.01 L: 95% CI 0.00 to 0.03, P = 0.08 and WMD 0.01 L: 95% CI −0.02 to 0.04, P = 0.55, respectively).
Figure 2.
Trough FEV1 L at end of intervention reported by three trials. WMD calculated using fixed-effect model with 95% CI. A square box indicates the WMD for each trial with the line through it representing 95% CI. WMD values left of the no effect line ‘0’ favor Handihaler, and values on the right favor Respimat. The solid diamond indicates the overall effect size each inhaler device has on FEV1. The Chi-square value (4.17) and the degrees of freedom value (df = 2) provide a measure of heterogeneity with a P value (P = 0.09) of the combined overall result that contributed to the effect size for FEV1. The z-statistic (1.49) with its P value (P = 0.14) indicates the level of significance of the overall effect size.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; SD, standard deviation; WMD, weighted mean difference.
Figure 3.
Details of trials reporting trough FVC (L).
Abbreviations: CI, confidence interval; FVC, forced vital capacity; SD, standard deviation; WMD, weighted mean difference.
Two trials24,26 with 425 patients contributed data toward morning and evening PEFR. There was no difference in morning or evening PEFR when Respimat was compared with Handihaler (WMD 5.06 L/min: 95% CI −0.69 to 10.72, P = 0.08 and WMD 4.39 L/min: 95% CI −1.54 to 10.31, P = 0.15, respectively).
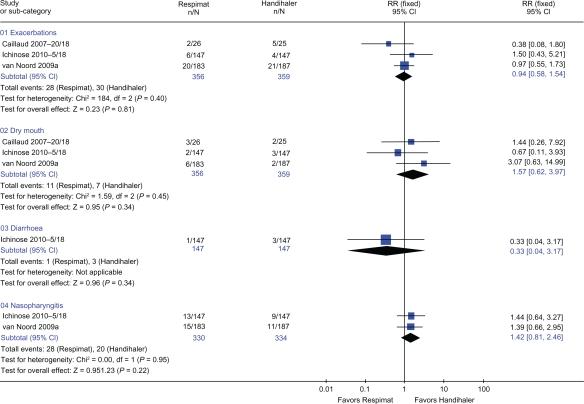
Three trials24–26 with 715 patients provided data for rates of exacerbations and dry mouth (Figure 4). There was no difference in risk of exacerbations or dry mouth when Respimat was compared with Handihaler (RR 0.94: 95% CI 0.58 to 1.54, P = 0.81 and RR 1.57: 95% CI 0.62 to 3.97, P = 0.34, respectively). Two trials25,26 with 664 patients provided data for nasopharyngitis (Figure 4). There was no difference in risk of nasopharyngitis when Respimat was compared with Handihaler (RR 1.42: 95% CI 0.81 to 2.46, P = 0.22).
Figure 4.
Details of trials included for exacerbations, dry mouth, diarrhea, and nasopharyngitis. Relative risk (RR) calculated using fixed-effect model with 95% confidence intervals (CI). A square box indicates the RR for each trial with the line through it representing 95% CI. RR values left of the no effect line ‘1’ favor Respimat, and values on the right favor Handihaler. The solid diamond indicates the overall mean effect each inhaler devices has on adverse events. The Chi-square value (eg, for exacerbations = 0.14) and the degrees of freedom value (df = 2) beside the Chi-square value in graph give a measure of heterogeneity with a P value (P = 0.40 for exacerbations) of the combined results that contributed to the effect size for exacerbations. The z-statistic (0.23) with its P value (P = 0.81) indicates the level of significance of the overall effect size.
Respimat versus CFC-MDI
Two trials12,15 with 1421 patients showed no difference in risk of exacerbations when Respimat was compared with CFC-MDI (RR 1.20: 95% CI 0.95 to 1.51, P = 0.12).
Respimat versus all other handheld inhaler devices
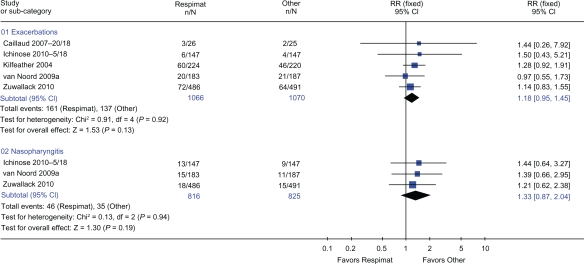
Five trials,12,15,24–26 three12,24,25 comparing the Handihaler with Respimat and two15,26 comparing the CFC-MDI with Respimat, could be combined to provide data for rates of exacerbations and nasopharyngitis. These five trials12,15,24–26 with 2136 patients (Figure 5) showed that there was no difference in risk of exacerbations when Respimat was compared with all other handheld inhaler devices (RR 1.18: 95% CI 0.95 to 1.45, P = 0.13). Three trials15,25,26 with 1641 patients provided data towards nasopharyngitis (Figure 5), which showed that there was no difference in risk of nasopharyngitis when Respimat was compared with all other handheld inhaler devices (RR 1.33: 95% CI 0.87 to 2.04, P = 0.19).
Figure 5.
Details of adverse events from trials that compared the Respimat inhaler with other inhaler devices.
Abbreviations: CI, confidence interval; RR, relative risk.
Data were not available for the following outcome measures: mortality, symptom scores, use of additional relief medication, use of inhaled or oral steroid requirement (maintenance, rescue), patient preference, measures of systemic bioavailability, subsidiary physiological measures (eg, 6- or 12-minute walks and arterial blood gases), and validated quality of life measures. In addition, there was an insufficient number of included trials for any subgroup analysis.
None of the outcome measures reported was significantly different when the same, higher, or lower doses of medication were used in the inhaler devices being compared.
Discussion
Evidence from seven high-quality randomized controlled trials with 3813 patients comparing Respimat with other inhaler devices produced similar outcomes. No differences were found in the following reported outcome measures: trough and peak FEV1, trough and peak FVC, morning and evening PEFR, or risk of exacerbations, drug mouth, or nasopharyngitis. No differences between devices were evident when the Respimat was compared with either the Handihaler or CFC-MDI or when a composite comparison of both devices was compared with Respimat (Figure 5). None of the included trials reported mortality as an outcome measure.
Only five of the trials reported data that could be used in the review. This and the limited number of studies comparing Respimat with other inhaler devices using the same drug and the small number of included patients in trials do not permit firm conclusions to be drawn. Nevertheless, to date, evidence from these high-quality trials suggests that the Respimat inhaler device does not provide any additional clinical benefit to that provided by other inhaler devices, namely Handihaler and CFC-MDI, in the management of COPD. Contrary to comparative in vitro studies published by the manufacturer (sponsor) of Respimat inhaler device claiming superiority over other devices, to date, there is no clinical evidence to suggest that the Respimat inhaler device provides any greater benefit than that afforded by other inhaler devices. We have no reason to believe that the Respimat inhaler device would provide greater benefit than any other handheld inhaler device. An extensive Cochrane systematic review published in 200227,28 strongly suggested that all handheld inhaler devices were clinically equivalent and that patients should be provided with devices that suit their purpose best. Since the publication of this key document back in 2002, evidence from the current systematic review comparing Respimat with other inhalers confirms previous findings.
A comprehensive search strategy was developed for this review. Every effort was made to identify all of the relevant studies. No study was excluded due to language. Although several attempts were made to identify unpublished work, it is still possible that some studies have been missed. However, the small number of eligible studies was due not to restricted selection criteria but rather to the absence of identified trials evaluating the Respimat inhaler device compared with other handheld inhaler devices using the same medication in COPD.
A common problem with conducting such a systematic review is that the included studies were designed as tests of the superiority of one device over another in which the null hypothesis was of equal efficacy. Such studies require fewer patients than those designed to test equivalence (null hypothesis that one device is superior to the other), and they also require predetermined limits of equivalence as in the Iacono et al trial,17 although this study was powered for superiority to test equivalence. Thus, the studies may have been underpowered. Failure to detect a difference should not necessarily imply equivalence, as trials designed to compare efficacy increase the chances of a type II error.
Another problem with such trials is that patients are usually selected on the basis of being able to use a particular inhaler device as an inclusion criterion; therefore, the results would favour that particular device. Anecdotally, inhaler device familiarity may increase medication adherence, but COPD is among the conditions with the lowest levels of adherence.29 Nevertheless, inhaled medication adherence in COPD is a complex issue that involves a number of factors, which include the medication itself, the delivery device, the patient, and the healthcare professional.30 However, in the seven randomized controlled trials included in this review, previous patient familiarization with devices being compared was not mentioned as a requirement for study entry. Therefore, the limited results presented from these seven trials can be applied to most patients with stable COPD. Although adequate device use was not a requirement for entry into the study, we should assume that all patients recruited into these trials were taught to use the devices being compared.
Conclusion
There is currently no evidence to suggest that the Respimat inhaler device provides any additional clinical benefit to that provided by other handheld inhaler devices. Although the Respimat inhaler appears to be a more efficient device in vitro when compared with other devices, findings from such laboratory-based studies have failed to translate into any meaningful clinical outcomes. No differences were found in reported lung function or adverse events, and none of the included trials reported mortality as an outcome. However, only a small number of trials reported data that could be used in this systematic review, and a limited number of studies have been published that compare Respimat with other inhaler devices using the same drug. Therefore, further trials comparing Respimat with other inhaler devices (including the Turbuhaler® [AstraZeneca, London, UK], HFA-MDI, Accuhaler® [GlaxoSmithKline, Uxbridge, UK], Handihaler®, and Autohaler® [3M Pharmaceuticals, St Paul, MN]) using the same drug and dose are required before firm conclusions can be drawn. Until such evidence becomes available, all hand-held inhaler devices should be considered clinically equivalent, and patients should be provided with inhaler devices that best suit their needs and maximize inhaler medication adherence. Furthermore, concerns reported in recently published studies regarding increased COPD mortality risk with the use of Respimat inhaler device require urgent investigation.
Acknowledgments
The authors acknowledge Elissa McDonald (Department of Ophthalmology, Faculty of Medicine and Health Sciences, University of Auckland, New Zealand) for screening studies and double-data entry check into RevMan, and Anja Merten (Medical Affairs Associate, Boehringer Ingelheim, North Ryde, Australia) for providing database search results of potential studies and provision of full-text articles.
Footnotes
Disclosure
No funding or support from any organization was received for the conduct of this study. There are no other known conflicts of interest.
References
- 1.Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:381–390. doi: 10.2147/copd.s3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schurmann W, Schmidtmann S, Moroni P, et al. Respimat Soft Mist inhaler versus hydrofluoroalkane metered dose inhaler: patient preference and satisfaction. Treat Respir Med. 2005;4(1):53–61. doi: 10.2165/00151829-200504010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Dalby R, Spallek M, Voshaar T. A review of the development of Respimat® Soft Mist™ Inhaler. Int J Pharm. 2004;283(1–2):1–9. doi: 10.1016/j.ijpharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Spallek M, Hochrainer D, Wachtel H. Optimizing nozzles for soft mist inhalers. Respiratory Drug Delivery. 2002;VIII(2):375–378. [Google Scholar]
- 5.Hochrainer D, Holz H, Kreher C, et al. Comparison of the aerosol velocity and spray duration of Respimat® Soft Mist™ inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–282. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- 6.Zierenberg B. Optimizing the in vitro performance of Respimat. J Aerosol Med. 1999;12:S19–S24. doi: 10.1089/jam.1999.12.suppl_1.s-19. [DOI] [PubMed] [Google Scholar]
- 7.Newman SP, Brown J, Steed KP, et al. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of Respimat with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113(4):957–963. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- 8.Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhaler. J Aerosol Med. 2005;18(3):264–272. doi: 10.1089/jam.2005.18.264. [DOI] [PubMed] [Google Scholar]
- 9.Worth Longest P, Hindle M. Evaluation of the Respimat Soft Mist Inhaler using a concurrent CFD and in vitro approach. J Aerosol Med. 2009;22(2):99–112. doi: 10.1089/jamp.2008.0708. [DOI] [PubMed] [Google Scholar]
- 10.Steed KP, Towse LJ, Freund B, Newman SP. Lung and oropharyngeal depositions of fenoterol hydrobromide delivered from the prototype III hand-held multidose Respimat nebuliser. Eur J Pharm Sci. 1997;5(2):55–61. [Google Scholar]
- 11.Wood CC, van Toor B, Hasselbarth V, et al. Improved efficiency of pulmonary delivery of budesonide with Respimat® Soft Mist™ inhaler compared with turbohaler or pMDI. J Allergy Clin Immun. 2006;117(2):S82–S82. [Google Scholar]
- 12.Kilfeather SA, Ponitz HH, Beck E, et al. Improved delivery of ipratropium bromide/fenoterol from Respimat® Soft Mist™ inhaler in patients with COPD. Resp Med. 2004;98(5):387–397. doi: 10.1016/j.rmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Kunkel G, Magnussen H, Bergmann KC, et al. Respimat® (a new soft mist inhaler) delivering fenoterol plus ipratropium bromide provides equivalent bronchodilation at half the cumulative dose compared with a conventional metered dose inhaler in asthmatic patients. Respiration. 2000;67(3):306–314. doi: 10.1159/000029515. [DOI] [PubMed] [Google Scholar]
- 14.Pavia D, Moonen D. Preliminary data from phase II studies with Respimat, a propellant-free soft mist inhaler. J Aerosol Med. 1999;12:S33–S39. doi: 10.1089/jam.1999.12.suppl_1.s-33. [DOI] [PubMed] [Google Scholar]
- 15.Zuwallack R, De Salvo MC, Kaelin T, et al. Efficacy and safety of ipratropium bromide/albuterol delivered via Respimat inhaler versus MDI. Respir Med. 2010;104(8):1179–1188. doi: 10.1016/j.rmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Brand P, Hederer B, Austen G, et al. Higher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis. 2008;3(4):763–770. [PMC free article] [PubMed] [Google Scholar]
- 17.Iacono P, Velicitat P, Guemas E, et al. Improved delivery of ipratropium bromide using Respimat® (a new soft mist inhaler) compared with a conventional metered dose inhaler: cumulative dose response study in patients with COPD. Resp Med. 2000;94(5):490–495. doi: 10.1053/rmed.1999.0770. [DOI] [PubMed] [Google Scholar]
- 18.Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460–1472. doi: 10.1016/j.rmed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Bateman E, Singh D, Smith D, et al. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208. [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. Chichester: John Wiley & Sons Ltd; 2005. [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Review Manager (RevMan) Copenhagen: The Cochrane Collaboration; 2006. Version 43 for Windows [program] [Google Scholar]
- 23.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 24.Caillaud D, Le Merre C, Martinat Y, et al. A dose-ranging study of tiotropium delivered via Respimat Soft Mist inhaler or HandiHaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(4):559–565. [PMC free article] [PubMed] [Google Scholar]
- 25.Ichinose M, Fujimoto T, Fukuchi Y. Tiotropium 5 microg via Respimat and 18 microg via HandiHaler; efficacy and safety in Japanese COPD patients. Resp Med. 2010;104(2):228–236. doi: 10.1016/j.rmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Van Noord JA, Cornelissen PJG, Aumann JL, et al. The efficacy of tiotropium administered via Respimat® Soft Mist™ inhaler or HandiHaler® in COPD patients. Resp Med. 2009;103(1):22–29. doi: 10.1016/j.rmed.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Ram FS, Brocklebank DM, Muers M, et al. Pressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;1:CD002170. doi: 10.1002/14651858.CD002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright J, Brocklebank D, Ram F. Inhaler devices for the treatment of asthma and chronic obstructive airways disease (COPD) Qual Saf Health Care. 2002;11(4):376–382. doi: 10.1136/qhc.11.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 30.Lareau S, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:401–406. doi: 10.2147/COPD.S14715. [DOI] [PMC free article] [PubMed] [Google Scholar]