INTRODUCTION
Taylor [1] has proposed that surgical robots be classified as either (a) surgical-assist devices, which modulate a surgeon’s motions, or (b) autonomous robots (which he calls “Surgical CAD/CAM” systems to illustrate the analogy to industrial computer-aided design and manufacturing), which are programmed to replace a portion of the surgical task. Perhaps the most widely known example of a surgical-assist system is the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA), which mimics and modifies the surgeon’s motions. These modifications can include elimination of tremor and scaling of motions such that large motions by the surgeon can be duplicated in a much smaller surgical field. This system has been most heavily used in urologic surgery [2]. Within the field of otolaryngology, it has been implemented for tongue-based resections to avoid splitting the mandible for access [3].
Of autonomous robots, the ROBODOC® System (Integrated Surgical Systems, Davis, CA, USA) is the most widely used and referenced. This system was designed to bore a receiving lumen for orthopedic reconstructive joint surgery and has been used in more than 20,000 cases. In practice, the device is rigidly affixed to the long bone for the procedure. While it was shown that this system can accomplish the task better than a human operator, a class-action law suit was brought in Europe claiming that the wider surgical exposure necessary for the rigid linkage of the robot to the patient led to various complications including muscle and nerve damage, bone dislocation, and chronic pain. Because of these legal troubles, the company ceased operations in 2005 and was acquired by Curexo Medical Technologies (Freemont, CA), who received FDA approval for the ROBODOC® in 2008. The clinical status of the ROBODOC® is in question at the time of this writing.
Because surgical-assist devices are controlled directly by human manipulation, regulatory approval is more straightforward, and they have been first to achieve widespread clinical adoption. Since autonomous robots are not widely commercially available for surgical applications, few surgeons have any experience with them whatsoever. The surgical-assist device has the advantage of continuous human control, but the disadvantage that it cannot function appropriately unless the controlling human maintains continuous visual access both to the anatomy being resected and to those critical structures in the vicinity of the resection that are to be spared. The autonomous robot by contrast (which is also usually subject to continuous monitoring by the surgeon), relies on the human operator only as a backup while it follows the path prescribed by pre-operative planning. That planning, which can be performed in the surgeon’s office before the patient is brought to the operating room, is based on three-dimensional tomographic images, and as a result, the autonomous robot can resect tissue that remains invisible to the human controller, whose role is primarily to stop the process if a technical problem develops.
This scenario, in which preplanning determines the path followed by an autonomous robot, is well-suited for procedures involving rigid tissues such as bone. It is much more challenging to implement any degree of autonomy in robotic procedures involving soft tissues because of their deformation between imaging and intervention, but strategies for doing so are the topic of active research in the engineering community. As these results mature and are brought to clinical use, it is likely that we will see a blurring of the line between surgical-assist devices and autonomous robots, as limited autonomy is introduced to the former, while increasing levels of human control are incorporated into the latter. Regardless of the type or classification of robot used, the intent of incorporating robotic technology into a surgical procedure is to produce a better outcome, which can take the form of many metrics including decreased operative time, improved accuracy, smaller incision, decreased recovery time, or overall decreased cost.
In the field of otology, a core component of otological cases is the mastoidectomy, in which bone is milled away exposing but not damaging vital anatomy. Mastoidectomy lends itself to an autonomous robotic approach for two reasons: (a) the tissue to be resected is encased in rigid bone, and (b) critical anatomical features remain hidden until they are revealed by ablation. The first of these two reasons makes surgery with the autonomous robot feasible; the second makes it useful. The rigidity of bone is essential because it ensures that the three-dimensional structure of the target anatomy is the same during pre-operative imaging and planning as it is during subsequent intervention. The presence of critical hidden anatomy (nerves and other structures that must be spared but are embedded in the bone), exploits the utility of the autonomous robot, because three-dimensional imaging pinpoints the positions of subsurface structures whose general locations can only be estimated during incremental, manual human intervention. As a result, the robot, guided by images that see beneath the surface, can safely ablate bone to which the human operator would be blind.
Once we have chosen a surgical situation, like the mastoidectomy, in which rigid bone comprises the target and critical anatomy is hidden from view, the central issue in autonomous robotics becomes the accurate determination of the single point in the three-dimensional pre-operative image that corresponds to a point in the surgical field, and vice versa. This determination of corresponding points in two spaces is termed “registration”, and the success of the robotic approach hinges on the accuracy of registration from the space of the pre-operative tomographic image to the space of the intra-operative patient. This problem is not new. It has, for example, been faced for decades by stereotactic surgeons and others who have provided geometrical guidance intra-operatively based on pre-operative images [4]. To achieve it, an autonomous robot must register these two spaces either through rigid fixation, as is done by the stereotactic frame and by ROBODOC®, or via optical or magnetic tracking, such as that achieved with image-guided surgical (IGS) systems via the alignment of skin surfaces or fiducial markers.
In this paper, we describe the use of an autonomous robot to perform a mastoidectomy using infrared tracking to monitor the motion of both the specimen and the robot. The method is applied to cadaveric temporal bone specimens. To accomplish this task, fiducial markers were implanted in the specimens, which were subsequently CT scanned. A surgeon identified the boundaries of the mastoid on the CT scan, and the robot was then programmed to mill out the mastoid according to those boundaries. With both the robot and the specimen being tracked, any movement of the specimen during ablation was continuously compensated for by corrective motions of the robot. While the robot is subject to human control via a manual emergency stop button, it otherwise operates completely autonomously. To the best of our knowledge, this is first report of an autonomous robot performing a mastoidectomy.
MATERIALS AND METHODS
Our goal is to successfully develop an autonomous robot system that can mill the regions identified by the surgeon in a CT scan. To achieve this goal, we developed the OTOBOT™ system (Figure 1), which incorporates an industrial robot, the Mitsubishi RV-3S (Mitsubishi Electric & Electronics USA, Inc., Cyprus, CA), controlled by custom software written in Matlab® and Simulink (The MathWorks Inc., Natick, MA) with real-time feedback of the robot’s movement provided via a Polaris Spectra® optical tracking system (NDI, Waterloo, ON, Canada) [5]. Figure 1 shows the experimental setup of the system. We custom-built the end effector of the robot to hold a surgical drill. To enable continuous, accurate tracking of both the patient (a temporal bone specimen in the current study) and the robot, three separate coordinate reference frames each of which comprises four reflective spherical markers are attached rigidly to the robot’s end effector, to the robot base, and to the patient. This arrangement enables real-time tracking of the robot movement, and hence the drill tip location, relative to the patient, whether or not the patient moves. That tracking is performed by the NDI Polaris, which is not shown but which faces the robot from approximately the position of the camera that acquired the picture shown in Figure 1—about 1-1/2 meters away.
Figure 1.
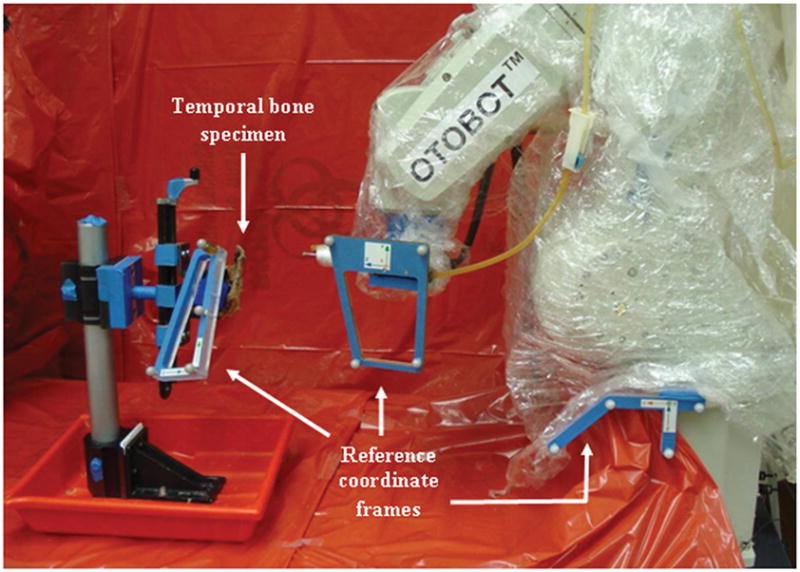
OTOBOT™ robotic system set up to perform mastoidectomy on patient (temporal bone specimen in this current study). The robotic system consists of a Mitsubishi RV-3S industrial robot controlled by custom software. Coordinate reference frames with markers are attached rigidly to the robot end effector, robot base, and patient to allow tracking of the movements of the robot, drill, and patient during milling. An NDI Polaris optical tracking system (not shown here) is used to track the markers.
While the three coordinate reference frames provide the means to determine the instantaneous positions and poses of the robot and patient relative to the physical space in which the actual milling will take place, they provide no means to register that physical space to CT image space, in which the boundaries of the regions to be milled are specified. For that purpose, we employ bone-implanted fiducial markers. This choice was made because bone-implanted markers are the most accurate available option, consistently enabling accuracies of 1.5 millimeters or better [6]. Three markers are screwed into bone in an arrangement surrounding the mastoid region, as shown in Figure 2. These markers, which are made of titanium, show up clearly in a CT scan and can be localized using image processing techniques. Their locations can also be acquired in physical space via a calibrated probe that is tracked by the NDI Polaris system.
Figure 2.
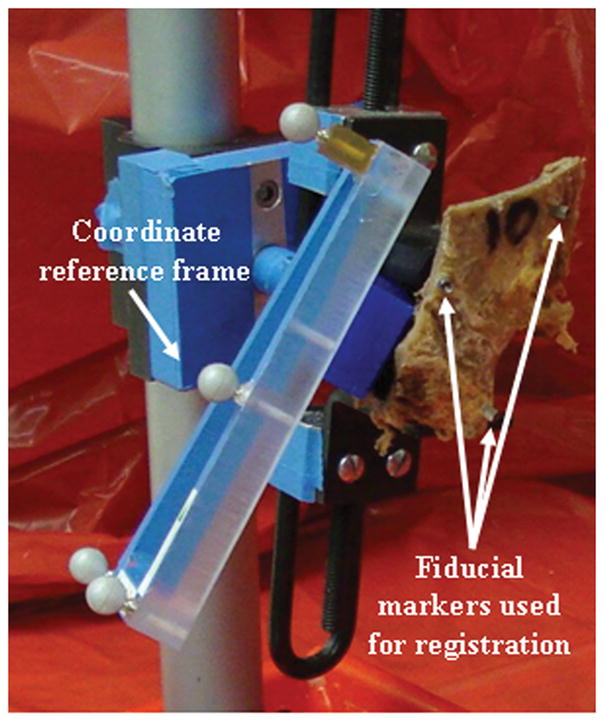
Temporal bone specimen with coordinate reference frame attached. Bone-implanted fiducial markers are used for registration of image and physical space.
Prior to the start of the milling procedure, three fiducial markers are bone-implanted into the temporal bone specimen, and a clinically-applicable temporal bone CT scan is subsequently acquired. The boundaries of the desired region to be milled are contoured on this same CT scan by the surgeon using custom segmentation software (Figure 3). The surgeon outlines the boundaries on axial slices of the image while simultaneously viewing intersecting coronal and sagittal slices. The boundaries are chosen to encompass the region to be ablated while maintaining a safe distance from the critical structures to be spared, including the facial nerve, the tegmen, the sigmoid sinus, the external auditory canal, and the labyrinth. At the conclusion of the contouring phase, a three-dimensional rendering of the region that is to be milled is displayed. At this point the surgeon can make modifications to the contours if required. When the surgeon is satisfied with the segmentation, the region is transformed into a set of points that determine the trajectory of the drill tip to be executed by the robot. The fiducial markers are also localized in the CT image (may be done before or after segmentation is done). A coordinate reference frame is then rigidly attached to the specimen, and the locations of the fiducial markers are acquired in physical space using the calibrated probe. The CT image and physical space are registered by applying point-based rigid-body registration [7] to the two sets of fiducial points—one in each space. Using the registration thus found, the trajectory points for the robot are transformed from the CT space to physical space, and the transformed points are given as input to the software that controls robot movement.
Figure 3.
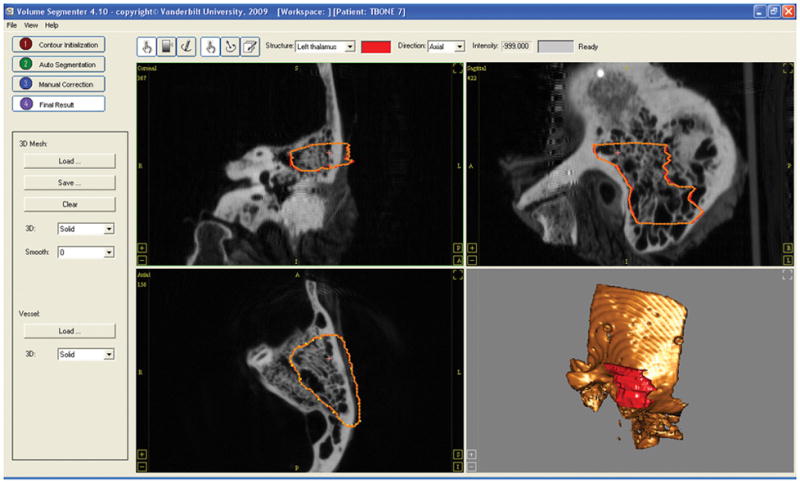
Screenshot of the software used for the segmentation. The surgeon contours the region to drill in the axial view of the image (bottom left). A three-dimensional shape of the region chosen for milling is displayed to the surgeon at the end of the segmentation.
The specimen with the coordinate reference frame is then placed within the workspace of the robot. Care is taken to make sure that the markers in all three coordinate reference frames are within the field of view of the optical tracking system. The milling is then initiated by starting the robot-control software application. The movements of the robot and patient are continuously tracked, and the control software compensates for any movement of the patient during milling. A manual emergency stop button is available for the robot if the robot moves in an undesired fashion. A continuous computer screen update is provided showing the progress of the milling as percentage of the total points covered.
Robotic mastoidectomy was performed on three temporal bone specimens using the procedure described above. A 5 mm diameter surgical drill tip was used for the milling, and the drill was powered to run at 80,000 rpm throughout the milling. We included a 1 mm safety region during milling to compensate for possible small penetrations of the drill tip beyond the region of interest (arising from small inaccuracies in registration, real-time tracking, or robot control). For this initial study, the robot was set to move at the constant speed of 1 millimeter per second (mm/s) during milling. We observed no undesired movement of the robot during milling, and hence we did not use the emergency stop button at any time.
RESULTS
Figure 4 shows the results of the milling of a temporal bone specimen. All three milled temporal bone specimens were CT scanned after the procedure for the purpose of analyzing the results of the milling. On analysis of the scan, no damage to any critical structure was identified. To determine whether the targeted bone was in fact removed, the pre-operative and post-operative CT scans of each bone were registered using the three fiducial markers, and the originally delineated region was superposed from the pre-operative CT scan onto the post-operative CT scan. For the three bones, we calculated the percentage of each targeted volume that was removed. For the three bones, these percentages were 97.70%, 99.99%, and 96.05%. Maximum error was identified as 0.6 mm.
Figure 4.
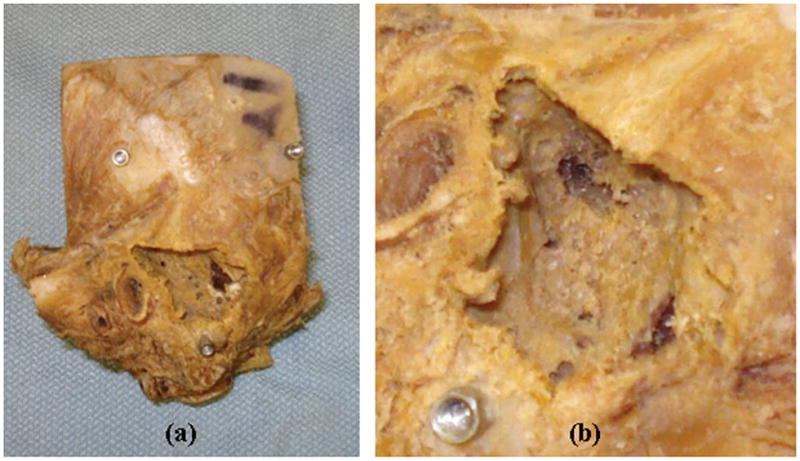
(a) Temporal bone specimen after completion of robot milling. (b) Close-up of the milled region. All three fiducials are visible in (a); one is visible in (b).
DISCUSSION
Herein, we have described a first step along what will likely be a lengthy road towards clinical testing and implementation of mastoidectomy via an autonomous robot. This first step involved the modification of an industrial robot to perform complex milling on a cadaveric specimen under infrared tracking of both robot and specimen. In our testing on three specimens, we found that the surgeon’s pre-operative plan was successfully executed by the robot with at least 96% of the targeted bone removed without damage to critical structures. Excitement at this success is tempered, however, by the realization that a great deal of work remains before this concept can be tested in the operating room. While the fundamental engineering concepts behind the robotic technique are well developed, less well studied is the translation of such concepts to clinical applications. Issues such as maintenance of sterility, logistics regarding transportation and set-up of the robot, and redundant safety constraints will need to be incorporated, as have been done with both the da Vinci® and ROBODOC® systems, both of which took decades to go from bench top to clinical use.
We acknowledge limitations of the proposed system, most notably the lack of soft-tissue work, which comprises at least a substantial portion of any ear surgery. Working with existing technology, we designed our system to aid the surgeon by automating the most predictable component of the surgery, mastoid milling, based on CT scan of the specimen. The potential advantages of this approach include (a) reliability and positional accuracy of the robot, (b) “X-ray vision” afforded by the registration of the pre-operative CT to the intraoperative patient, and (c) possible economic benefits (e.g. reduced time of intervention, improved productivity, or other, as yet to be identified, metrics).
Regarding (a), robots are highly reliable and accurate. The Mitsubishi RV-3S robot used in this study has a repeatability of 0.02 mm. In addition to high accuracy, robots are highly reliable in repetitive tasks, with error rates lower than humans, and no risk of performance degradation due to fatigue [8]. In-short, properly-calibrated robots can perform specified tasks with high accuracy and efficiency. Regarding (b), coupling IGS with an autonomous robot leads to “X-ray vision” which allows such systems to see subsurface features before they may be injured. In the bone component of mastoid surgery, this “X-ray vision” offers a potentially large benefit in that the robot could be used to perform a highly accurate three-dimensional mastoidectomy—leaving a 1–2 mm margin of safety over vital anatomy—freeing the human operator to perform the more high-level fine dissection where human judgment is paramount.
Despite benefits (a) and (b), we recognize that catastrophic failure is a possibility as, for example, if the registration between the CT and the target tissue was performed incorrectly, thereby placing the robot at the wrong starting point. Given the consequences of such damage, we believe that—particularly in initial cases—there is no substitute for continuous monitoring by a trained surgeon. Put another way, the robot may be trusted to carry out the surgeon’s plan only so long as a human is there to verify that it is doing what it was told. As such we envision, and are working on, human oversight systems such as having the surgeon depress the foot pedal to keep the robot moving and the drill spinning, a hand-held pause button, and the possibility of slow-motion control to reduce robot speed when in close proximity to vital anatomy.
Regarding (c), there are many possible ways in which a robotic mastoidectomy may be economically beneficial. This may include reduced time of intervention. For this first demonstration of the technology over 90 minutes were required. This extended time occurred because (i) the robot was programmed to move at a very slow speed of 1 mm/sec and (ii) a primitive path planning strategy where the robot often “backtracked” through areas previously drilled to reach the next coordinate to be ablated, leading to wasted robot motion. Future work will involve the automatic planning of optimal or near-optimal drill paths through the surgeon-specified volume using generalizations of existing two dimensional planning algorithms such as in [9]. Regarding (i), the 1 mm/sec was chosen as an initial speed based on our desire to carefully monitor the progress of the robot. We are confident that we can increase this speed to 5 mm/sec, as has been reported in the milling of cranial bone by others [10]. This increase in speed alone would reduce the 90 minute intervention to 18 minutes.
Furthermore, regarding (ii), we feel that we can optimize path planning, eliminating about 50% of redundant movements, which were a consequence of our primitive planning strategy. This improvement would further reduce the time of intervention to approximately 9 minutes. From other clinical studies in which we are involved, we know that it takes approximately 10–12 minutes to place fiducial markers, acquire the CT scan, and set up comparable technology. Thus, we feel it reasonable that the bone excavation of a mastoidectomy using a robot could be performed routinely in approximately 20 minutes. While this is not dramatically quicker than a highly experienced otologic surgeon, it may find utility in freeing the surgeon to concentrate on the more high-level dissection in close proximity to vital anatomy.
Future economic assessments will be necessary to justify clinical use of such a system, as has been underway for the da Vinci® system, which retails for $1.4 million. Use of the da Vinci® robot for prostatectomy adds over $2,500 to the cost of each surgical intervention [11]. To date, the offsetting benefits include decreased recovery time and decreased complications [12]. Because our system is far from routine clinical use, we can report, in terms of economics, only the cost of the system, which is approximately $40,000 (robot $19,900, infrared tracking system $15,000, and control computer $5,000). Estimating the cost of development and experimentation required to obtain FDA approval and commercialize the robot, we feel the machine may retail for $500,000. As wear on the robot, whose movements are far smaller and slower than those required for routine industrial applications, will allow it to be used on thousands of cases with minimal maintenance, we predict its cost per procedure will be below $100. Other costs accruing from this technology will be associated with markers and drapes, which per case should be below $100.
Noting that the typical outcomes associated with mastoid surgery (e.g. post operative hearing, success of tympanic membrane grafting, recurrence of disease, acquisition of speech after cochlear implant) are independent of whether the drill is held by a human or a robot, the major potential benefit offered by this technology may be reduced operative time. However, until the robot can completely replace the surgeon, its benefit will be bound by the need for the surgeon or an assistant to monitor the procedure thus tying up operating room time. At this point, we predict that the savings from reduced operating room time can be expected to more than offset the additional costs (predicted above to be $200 or less) associated with this technology. There may be other benefits that we are as yet unaware of (e.g. fewer drill bits per case, fewer complications) that may make the economic argument for this technology more compelling.
Before such a clinical system is deployed, many intermediate steps need to be taken. Our near-term future work will be focused on improving the efficiency of the path plan so that the milling time can be reduced. Medium-term future work will include redundant safety checks including monitoring force feedback at the point where the robot grips the drill, potentiometers on robot joints (in addition to the optical encoders used in industrial robots), improvements to the user interface for the surgeon, and the development of techniques for bagging and sterilization of robot components. Only after these steps have been accomplished will our system be ready for clinical testing.
CONCLUSION
The preliminary work we have presented here, given the relatively simple and controlled conditions of a cadaveric specimen in a laboratory, shows that an autonomous robotic system is capable both of determining a trajectory and of directing a drill along that same trajectory so as to perform a prescribed mastoidectomy with 96% removal of desired bone volume and with no damage to critical structures. There is much to do, however, before such a system can be considered for clinical use. The major issue is to establish a level of patient safety that is at least at the level of current clinical practice. This safety level will require that a surgeon remain in charge of the procedure including sitting at the surgical bed, monitoring the robot’s progress, and stopping the system if a problem develops. This work is only a start, but it provides encouragement that, with a plan based on pre-operative tomographic images that give information about what is below the bone surface, an accurate registration to transfer that information to the patient in the operating room, and an obedient robot continuously monitored by the surgeon, robotic mastoidectomy is technologically feasible.
Acknowledgments
The project described was supported by Award Number R21EB006044-01A1 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Contributor Information
Andrei Danilchenko, Graduate Student, Dept. of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN
Ramya Balachandran, Dept. of Otolaryngology, Vanderbilt University Medical Center, Nashville, TN
Jenna L Toennies, Graduate Student, Dept. of Mechanical Engineering, Vanderbilt University, Nashville, TN
Stephan Baron, Institute of Mechatronic Systems, Leibniz Universitat Hannover, Hanover, Germany.
Benjamin Munske, Institute of Mechatronic Systems, Leibniz Universitat Hannover, Hanover, Germany.
J. Michael Fitzpatrick, Dept. of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN
Thomas J. Withrow, Dept. of Mechanical Engineering, Vanderbilt University, Nashville, TN
Robert J Webster, III, Dept. of Mechanical Engineering, Vanderbilt University, Nashville, TN
Robert F Labadie, Associate Professor, Dept. of Otolaryngology, Vanderbilt University Medical Center, Nashville, TN.
References
- 1.Taylor R. A perspective on medical robotics. Proc IEEE. 2006 September;94(9):1652–1664. [Google Scholar]
- 2.Guru KA, Hussain A, Chandrasekhar R, Piacente P, Bienko M, Glasgow M, Underwood W, Wilding G, Mohler JL, Menon M, Peabody JO. Current status of robot-assisted surgery in urology: a multi-national survey of 297 urologic surgeons. Can J Urol. 2009;16(4):4736–41. [PubMed] [Google Scholar]
- 3.Hockstein NG, Nolan JP, O’Malley BW, Jr, Woo YJ. Robot-assisted pharyngeal and laryngeal microsurgery: results of robotic cadaver dissections. Laryngoscope. 2005;115(6):1003–8. doi: 10.1212/01.WNL.0000164714.90354.7D. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick JM. The role of registration in accurate surgical guidance. Journal of Engineering in Medicine. 2010 May;224(5):607–22. doi: 10.1243/09544119JEIM589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron H Eilers, Munske B, Toennies JL, Balachandran R, Labadie RF, Ortmaier T, Webster RJ., III . Journal of Engineering in Medicine. 5. Vol. 224. Percutaneous Inner-Ear Access Via an Image-Guided Industrial Robot System. Need pages (May 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer CR, Jr, Fitzpatrick JM, Wang MY, Galloway RL, Jr, Maciunas RJ, Allen GS. Registration of head volume images using implantable fiducial markers. IEEE Transactions on Medical Imaging. 1997 Aug;16:447–462. doi: 10.1109/42.611354. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick JM, Hill DLG, Maurer CR. “Registration”. Medical Image Processing. In: Sonka M, Fitzpatrick JM, editors. Volume II of the Handbook of Medical Imaging. SPIE Press; 2000. pp. 447–513. [Google Scholar]
- 8.Paul Richard P, Nof Shimon Y. Work methods measurement--a comparison between robot and human task performance. International Journal of Production Research. May79;17(3):277. 27p, 3 Diagrams, 20 Charts; (AN 5550948) [Google Scholar]
- 9.Gabriely Yoav, Rimon Elon. Spanning-tree based coverage of continuous areas by a mobile robot. Annals of Mathematics and Artificial Intelligence. 2001;31:77–98. [Google Scholar]
- 10.Engelhardt M, Bast P, Lauer W, Rohde V, Schmieder K, Radermacher K. Manual vs. robotic milling parameters for development of a new robotic system in cranial surgery. International Congress Series; 2004. pp. 533–538. [Google Scholar]
- 11.Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy: cost comparison of open, laparoscopic, and robot assisted techniques. J Urology. 2004;172(4):1431–1435. doi: 10.1097/01.ju.0000139714.09832.47. [DOI] [PubMed] [Google Scholar]
- 12.Menon M. Robotic radical retropubic prostatectomy. BJU Int. 2003;91(3):175–180. doi: 10.1046/j.1464-410x.2003.04070.x. [DOI] [PubMed] [Google Scholar]


