Abstract
Northern grasshopper mice (Onychomys leucogaster) are among the most highly carnivorous rodents in North America. Because predatory mammals may have specialization of senses used to detect prey, we investigated the organization of sensory areas within grasshopper mouse neocortex and quantified the number of myelinated axons in grasshopper mouse trigeminal, cochlear, and optic nerves. Multiunit electrophysiological recordings combined with analysis of flattened sections of neocortex processed for cytochrome oxidase were used to determine the topography of primary somatosensory cortex (S1) and the location and size of both the visual and auditory cortex in adult animals. These findings were then related to the distinctive chemoarchitecture of layer IV visible in flattened cortical sections of juvenile grasshopper mice labeled with the serotonin transporter (SERT) antibody, revealing a striking correspondence between electrophysiological maps and cortical anatomy.
Keywords: S1, trigeminal, somatosensory, visual, forepaw, evolution, predator
Introduction
The northern grasshopper mouse (Onychomys leucogaster) is a small nocturnal mammal that inhabits short-grass prairies and semi-desert regions primarily in the western United States (Fig. 1A). Grasshopper mice differ from other rodents in being the most highly carnivorous genus of rodent in North America, with animal matter composing up to 89% of their diet (Horner et al., 1964; Landry, 1970). They occasionally prey on other small mammals such as pocket mice, deer mice, voles, and even cotton rats three times their weight (Horner et al., 1964; Ruffer, 1968; McCarty, 1978; Timberlake and Washburne, 1989). As might be expected, grasshopper mice exhibit behavioral and morphological adaptations commensurate with a predatory lifestyle including low population densities and large home ranges (Bailey and Sperry, 1929; Blair, 1953; Egoscue, 1960; Ruffer, 1968).
Figure 1.
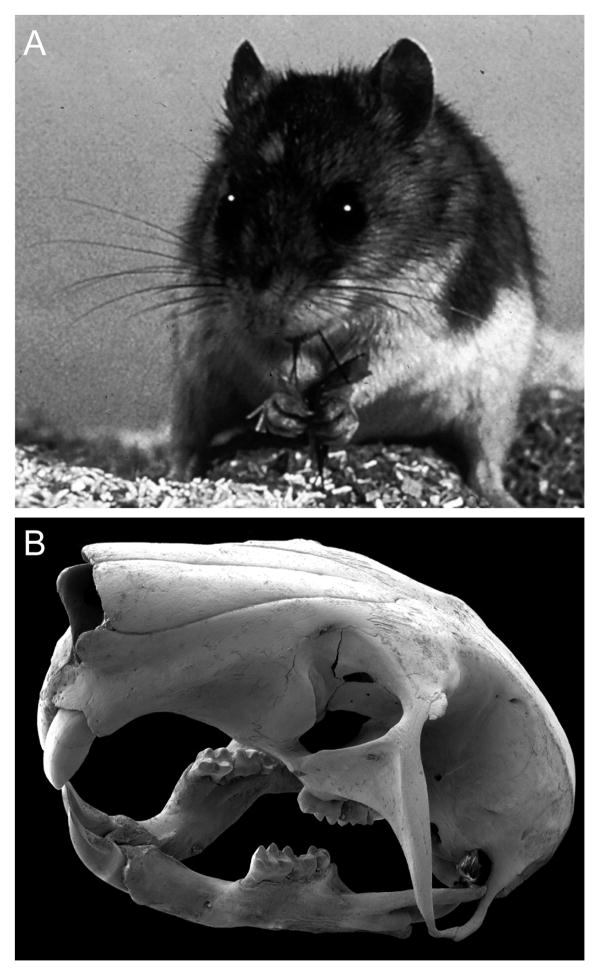
The grasshopper mouse (A) is a carnivorous rodent with prominent vibrissae and long claws. B) A grasshopper mouse skull imaged using a scanning electron microscope illustrates dentition modified for carnivory. Image for A provided by Jan Decher. The black background was digitally shaded in B.
Studies of grasshopper mice also indicate that they are comparatively aggressive and resistant to the inhibitory effects of novel or aversive stimuli from prey (Timberlake and Washburne, 1989; Langley, 1994). Grasshopper mice have developed a resistance to the toxins from certain prey items such as Arizona bark scorpion venom (Rowe and Rowe, 2008) and are reported to be tenacious predators (Timberlake and Washburne, 1989; Langley, 1994). Whereas most animals learn to avoid noxious prey, grasshopper mice persistently attack insects with formidable defenses, enabling them to exploit prey that are avoided by other species. These behaviors include an impressive repertoire of tactics specific to the prey's defenses. For instance, the southern grasshopper mouse species (Onychomys torridus), subdues chemically-toxic stink beetles by forcing the caudal end of the beetle into the dirt and quickly biting off the head, thus avoiding their chemical spray (Eisner and Meinwald, 1966). Scorpions are disarmed by first immobilizing the stinger and then consuming the cephalothorax (Langley, 1981). Preying on lubber grasshoppers requires an initial attack aimed at the powerful legs that could otherwise injure the grasshopper mouse (Whitman et al., 1986). In the case of vertebrate prey, such as the horned lizard, vulnerable points such as the eyes are attacked to avoid the spiny scales (Frank, 1989). Small mammalian prey are often killed by an incisor bite aimed at the base of the skull (Bailey and Sperry, 1929) and occasionally grasshopper mice even strangle prey (Egoscue, 1960).
Grasshopper mice first diverged from Peromyscus (deer mice) in the late Miocene, approximately 6 million years ago, appearing in their present form in the middle Pliocene (Hibbard, 1968; McCarty, 1978). Fossil records of dentition suggest that grasshopper mice retained an omnivorous lifestyle until the radiation of deer mice and concurrent onset of competition for resources, at which point transition to a carnivorous lifestyle became evident in the Pleistocene (Carleton and Eshelman, 1979). Grasshopper mice appear to have developed numerous specializations for predation including: long claws that aid in seizure of prey with the forepaws; well-developed jaw muscles allowing strong bite force and a wide gape for consuming larger prey; modified dentition with shortened incisors and molars that are less adapted for grinding plant matter (Fig. 1B); and a modified stomach optimized for increased digestion of insects (Bailey and Sperry, 1929; Horner et al., 1964; Landry, 1970; Satoh and Iwaku, 2006). Here we investigate their central nervous system to explore potential neural correlates of the transition to carnivory. An additional goal is to provide data for comparative studies aimed at determining features of cortical organization common to mammals and features that may be unique to specific lineages or lifestyles. Using multiunit electrophysiological recordings we delineated the topography and orientation of neocortical sensory areas in the grasshopper mouse with a focus on primary somatosensory cortex. We relate these findings to modules and barrels in S1 and provide evidence for a least one additional somatosensory area in lateral cortex. In addition, we identify an auditory area and primary visual cortex (V1). Finally, we counted myelinated axons within the trigeminal, optic, and cochlear cranial nerves in order to assess the relative importance of somatosensation, vision, and audition in grasshopper mice.
Materials and Methods
Animals
Adult northern grasshopper mice (Onychomys leucogaster; n=9) from laboratory colonies at the University of Arkansas, Little Rock and the University of Wisconsin, Stevens Point were pair-housed and provided free access to food (rodent chow supplemented with mealworms and wax worms) and water in a 14/10-hour light/dark cycle at 68-77°F. Additionally, 3 pups were obtained from an adult breeding pair and sacrificed at P7, P9 and P13 (weights of 6.5, 6.9, and 9.0 g, respectively) to examine the timeline of serotonin transporter (SERT) expression in the neocortex. The skull of one additional adult grasshopper mouse was cleaned, sputter-coated with gold, and imaged using a Tescan Vega-II scanning electron microscope (Tescan USA Inc.) to illustrate cranial and dental features (Fig. 1B). All research procedures were approved by the Institutional Animal Care and Use Committee at Vanderbilt University.
Electrophysiology
A surgical plane of anesthesia was induced with an i.p. injection of 15% urethane in distilled water (1 g/kg) in adult grasshopper mice. Additional injections of 10% ketamine (15 mg/kg, i.p.) were given as needed. Body temperature was maintained with a heating pad and hot water bottles. Animals were secured by a head post with dental cement, and the left hemisphere of the cerebral cortex was exposed by craniotomy with the dura removed. The brain was protected with liquid silicon and a digital photograph of the cortical surface was taken. Tungsten microelectrodes (1.0 MΩ at 1 kHz) placed perpendicular to the cortical surface were used to perform multiunit electrode recordings in layer IV of the cortex. Neuronal responses were amplified and delivered to an oscilloscope and speaker. Selected electrode penetration sites were marked with electrolytic lesions (10 μA while withdrawing electrode at 50 μm/sec) to serve as anatomical landmarks.
Receptive fields of neurons at each penetration site were mapped by stimulating the teeth, vibrissae, and body surface. Mapping of receptive fields focused on cutaneous stimulation of the animal's body using calibrated monofilaments (von Frey hairs - synthetic hairs for quantitative mechanical stimulation of skin receptors). Responses to periodontal receptors of the teeth were evoked by light touch (using von Frey hairs) or light taps. Moving beams of light were used to identify visual responses. A series of clicks was used to evaluate auditory responses, although specific frequencies were not defined. Specific retinotopy and tonotopy of visual and auditory cortex respectively were not explored.
After each recording procedure was complete, grasshopper mice were given an overdose of sodium pentobarbital (at least 120 mg/kg, i.p.) and perfused transcardially with 0.01 M phosphate-buffered saline (PBS, pH 7.2) followed by 4% paraformaldehyde in 0.01 M PBS (pH 7.2). For each specimen the brain was removed and postfixed overnight. The cortex was separated and flattened, then cryoprotected in 30% sucrose/PBS, and a freezing microtome was used to cut sections at 50 μm parallel to the cortical surface.
Cortical immunohistochemistry and histochemistry
For each specimen, following sectioning, the left hemisphere was processed for the metabolic enzyme cytochrome oxidase (Wong-Riley, 1979) to reveal sensory areas. The right hemisphere of each juvenile specimen was processed using the anti-5-HT transporter antibody (SERT; rabbit polyclonal, 1:1000; Calbiochem/EMD Biosciences, La Jolla, CA, USA; isotype IgG; catalog number PC177L). The immunogen was a synthetic peptide corresponding to amino acids 602-622 of rat 5-HT transporter, and the specificity of this antibody has been determined by immunoblotting analysis using rat brain extracts of the cortex, hypothalamus, midbrain, and hindbrain, which specifically detected a single band (Calbiochem/EMD Biosciences, La Jolla, CA, USA). This protein has been shown to recognize the serotonin (5-HT) transporter in the cortex, raphe nuclei, hypothalamus, and spinal cord of rats (Coccaro and Murphy, 1990; Blakely et al., 1994; Zhou et al., 1996; Boylan et al., 2000) and staining specificity has been previously characterized in rats (Coccaro and Murphy, 1990; Blakely et al., 1994; Zhou et al., 1996; Boylan et al., 2000), mice (Eagleson et al., 2007; Hoerder-Suabedissen et al., 2008), humans (Verney et al., 2002), vervet monkeys (Way et al., 2007), chimpanzees and rhesus macaques (Raghanti et al., 2008). Staining is completely eliminated by pretreatment of antibody with Serotonin (5-HT) Transporter Control Peptide (Calbiochem/EMD Biosciences, La Jolla, CA, USA; catalog number PP87) at a concentration of 5 μg/ml. Controls for the specificity of SERT labeling were provided by the use of preadsorption controls as well as the demonstration that labeling was characteristic of thalamocortical projection zones producing a pattern of immunoreactivity that appeared identical to that of other rodents (e.g., Eagleson et al., 2007). Negative controls omitting the primary antibody controlled for the specificity of the secondary antiserum. Sections were initially collected in tissue freezing medium and stored at -20°C for approximately 3 weeks. Briefly, as described elsewhere (Boylan et al., 2000; Eagleson et al., 2007), free-floating sections were incubated in primary antibody (anti-SERT) for 72 hours at 4°C followed by secondary incubations in biotin-SP-conjugated donkey anti-rabbit IgG (1:1000, Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour at room temperature and processed by using the Vectastain ABC histochemical method (Vector Labs, Burlingame, CA, USA). Sections were treated for 4 minutes at room temperature in 0.5% 3′3′-diaminobenzidine (DAB) with 0.05% H2O2. The sections were washed, mounted onto gelatin-subbed slides, dehydrated with alcohols, cleared with CitriSolve (Fisher), and coverslipped in DPX (Fisher).
Cortical area measurement
Grayscale images of histological sections were acquired with a Zeiss AxioCam HRc camera (Zeiss, Jena, Germany) mounted onto a Zeiss Axioskop microscope and using Zeiss Axiovision 4.5 software. Images were imported into the public domain program ImageJ, version 1.33, for morphometric analysis. Figures were prepared using Adobe Photoshop CS3 (Adobe Systems Incorporated, San Jose, CA, USA) and adjusted to optimize contrast. Neocortical areas were measured using optimal tangential sections reacted for SERT or CO. Only those sections with the entire posteromedial barrel subfield (PMBSF) or primary visual area (V1) present were analyzed. The PMBSF and forelimb areas of S1, as well as area V1, were measured in representative sections with the clearest outline in flattened cortical sections from juvenile specimens (P7 and P9) reacted for SERT. Forelimb area is reported as a percentage of S1 both including and excluding oral/intraoral modules because these latter areas may have been excluded from previous analyses. Flattened cortical sections from adult specimens processed for cytochrome oxidase were also measured for PMBSF and V1 area.
Nerve processing and axon quantification
Following perfusion portions of the optic (n=4), trigeminal (n=4) and cochlear nerves (n=3) (∼1 mm for each) were excised and placed in 2.5% glutaraldehyde in 0.1 M PBS, pH 7.4, for at least one hour. Each sample was washed twice with 0.2 M PBS, pH 7.4, for 10 minutes and postfixed for 2 hours with OsO4 in 0.1 M PBS, pH 7.4. Samples were then washed twice in 0.1M PBS followed by dehydration in a graded series of ethanol washes culminating in three changes of 100% ethanol. Individual samples were then placed on a rotator overnight in a 1:1 mixture of EMBed812 (EM Sciences) to 100% propylene oxide. This was followed by placement in 100% EMBed812 resin for 2 hours. Finally, samples were polymerized in an oven at 70°C overnight. Samples were sectioned with a diamond knife (Diatome US, Hatfield, PA, USA) on a Reichert Ultracut E ultramicrotome at 0.5 μm. Sections were transferred to glass slides, stained with 1% toluidine blue, and coverslipped.
Optimal cross-sections of each nerve were imaged at 100× as described above. Images were imported into Adobe Photoshop CS3 (Adobe Systems Incorporated, San Jose, CA, USA) and montaged to create a composite image of the entire nerve. Axons were then counted manually (see Figure 4).
Figure 4.
Semi-thin light microscopic preparations and quantification of myelinated axons in trigeminal, cochlear and optic nerves. Cross-sections of representative optic (A) and trigeminal (B) nerves from the grasshopper mouse, shown at the same scale, illustrate their relative sizes and the myelinated axons (inset).
Results
Histochemical and immunohistochemical characterization
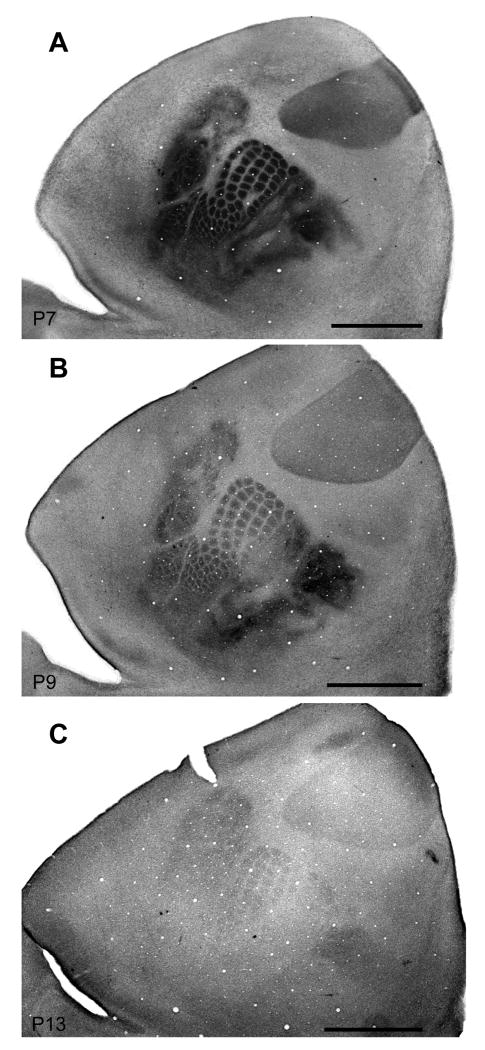
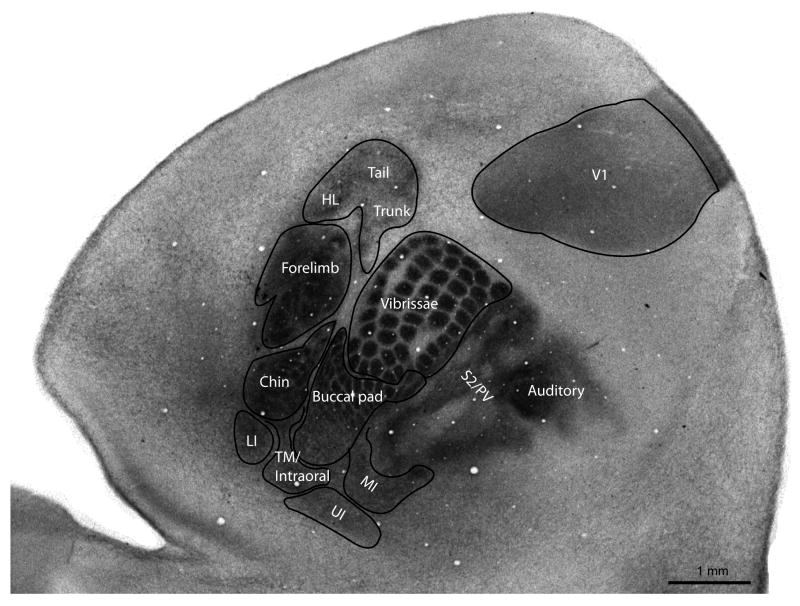
Because serotonin immunoreactivity presents a transient postnatal pattern in the primary sensory cortical areas of rats, mice, and hamsters that matches the distribution of thalamocortical axon terminals (Fujimiya et al., 1986; D'Amato et al., 1987; Rhoades et al., 1990; Bennett-Clarke et al., 1993; Boylan et al., 2000) we first used immunohistochemistry to determine the developmental time course of expression of the serotonin transporter (SERT) on thalamocortical afferents in the primary sensory areas of the cortex. SERT expression was present at P7 and P9 but became barely discernible at P13 (Fig. 2), a time course intermediate between that of mice (Fujimiya et al., 1986) and rats (D'Amato et al., 1987; Boylan et al., 2000). The labeling of thalamocortical afferents allowed us to clearly delineate the boundaries of primary sensory areas in flattened preparations of neocortex. Subsequent electrophysiological recordings revealed how the different modules visible in S1 corresponded to representations of various body parts (Fig. 3). SERT labeling also corresponded well with flattened cortical preparations sectioned through layer IV and processed for cytochrome oxidase, differentiating regions of chronically high metabolic activity and thus distinguishing primary sensory areas (Wong-Riley et al., 1979). Characteristic CO-densely regions were present for the head, trunk, and limb representations within S1, including prominent barrels within the head representation and a large forelimb representation relative to the hindlimb. Far lateral cortex also contained multiple modular representations of oral structures including the tongue, lower and upper incisor, and intraoral regions.
Figure 2.
Flattened cortical sections of juvenile grasshopper processed for the serotonin transporter (SERT). A-C show the time course of expression of SERT for thalamocortical afferents in the primary sensory areas. In postnatal grasshopper mice SERT expression persisted until P13 (C) when it became barely discernible, a time course similar to that of other rodents. SERT labeling also corresponded well with flattened cortex sections through layer IV that were processed for cytochrome oxidase and distinguished primary sensory areas. Scale bar = 2 mm.
Figure 3.
Flattened cortical section from a juvenile grasshopper mouse (P7) labeled for primary sensory areas and for functional subdivisions within somatosensory cortex. The labeling of SERT-positive thalamocortical afferents delineated the boundaries of primary sensory areas, allowing assignment of preliminary functional representations that were subsequently confirmed through cortical recordings. HL = hindlimb, LI = lower incisor, MI = mixed incisor, PV = parietal ventral cortex, S2 = secondary somatosensory cortex, UI = upper incisor, V1 = primary visual cortex.
We quantified the area of V1 along with the area of a well-defined portion of S1, the posteromedial barrel subfied (PMBSF), to allow comparisons with other species (e.g., see Kaskan et al., 2005). Total neocortical area in juvenile grasshopper mice (n = 2) was 36.70 ± 0.32 mm2, with V1 occupying 4.26 ± 0.54 mm2 (approximately 12%) and PMBSF of S1 occupying 0.90 ± 0.08 mm2 (approximately 5%). Total neocortical area in adult grasshopper mice (n = 4) was 58.31 ± 1.44 mm2, with V1 occupying 8.47 ± 0.46 mm2 (approximately 15%) and PMBSF occupying 2.98 ± 0.10 mm2 (approximately 5%). To further assess the dedication of neural resources across the primary sensory modalities the optic, trigeminal, and cochlear nerves were sectioned and the number of axons within each was quantified (see Fig. 4). The trigeminal nerve was by far the largest in absolute size, but contained only one third the number of myelinated axons (24,105) found in the thinner optic nerve (78,646). The cochlear nerve was the smallest, containing only 5,887 myelinated axons.
Multiunit electrophysiological recordings: primary somatosensory cortex
Recordings were made from nine adult grasshopper mice for a total of 564 electrode penetration sites with strong responses to light stimulation of the skin, fur, whiskers and periodontium. Primary somatosensory cortex (S1) was identified as a complete and systematic representation of the contralateral body surface. The somatotopic map for S1 was orderly and continuous in each case with a single representation of each body part present.
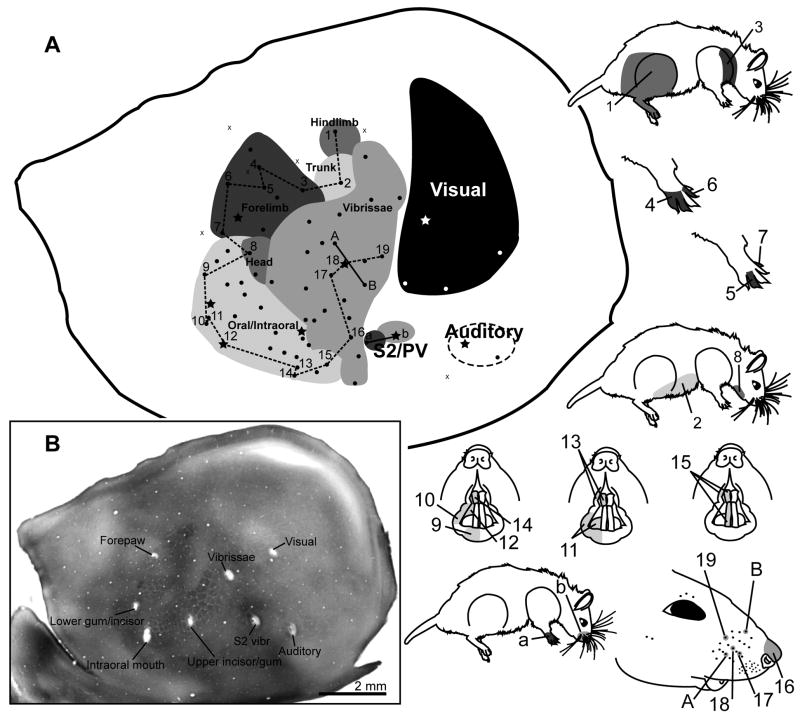
Figure 5 shows the recording data from a representative case with 68 electrode penetrations from which responses were recorded while stimulating the body, face, and periodontium (additional cases can be seen in supplemental Figures 1-4). These recordings delineated a complete map of the contralateral face and body surface with responses to stimulation of the skin surface and pelage fur, whiskers, incisors, tongue, and intraoral region. Within S1 the orientation of the body representation was inverted such that the hindlimb and tail were located medially and the face and oral structures were located laterally. The somatotopic organization of the body representation is shown with receptive fields illustrated for a progression of selected penetration sites (Fig. 5; note that numerical and alphabetical order does not necessarily reflect temporal progression during recordings). Penetration sites 1-3 delineate the location of the hindlimb representation (site 1, located medially) relative to the trunk (site 2) and the forelimb (site 3). The digits were located rostrally within the forelimb representation and there was a rostral-to-caudal progression from the thumb to more distal digit representations (Fig 5, sites 4-7), respectively, as has been shown in other rodents (Dawson and Killackey, 1987; Henry et al., 2006).
Figure 5.
Topography and chemoarchitecture of the grasshopper mouse neocortex with mapping of S1. A) Schematic of the microelectrode-derived map of cutaneous inputs to the neocortex showing representative receptive field sequences to the left. B) Locations of microlesions, labeled according to corresponding receptive fields, in a flattened cortical section processed for CO. Rostral is left, medial is up for the cortical schematic and CO section.
The forepaw representation appeared to be relatively large in grasshopper mouse S1. This was primarily evident during electrophysiological mapping. Of the 564 recording sites across 9 animals, 91 responded to forelimb stimulation and 69 were specific to the forepaw. Also, despite the otherwise broad receptive fields characteristic of S2/PV, recording sites responsive to the forepaw alone were found in several instances (see below). In the flattened cortex preparations labeled for SERT, the forelimb representation occupied 17.5% of S1 in the P7 grasshopper mouse (19% with oral/intraoral modules excluded) and 15.5% of total S1 in a P9 grasshopper mouse (17.2% with oral/intraoral included).
Electrode penetrations further lateral in cortex responded to stimulation of the head beginning with the chin, followed by the lower lip and tongue, the intraoral region, and the upper lip and upper incisor as the electrode was moved more laterally (Fig 5, sites 8-15). Site 15 responded to stimulation of both the upper and lower incisor and might correspond to the mixed incisor representation found in rat S2 (Remple et al., 2003). This sequence continued to a penetration site responsive to stimulation of the nose (site 16) and a rostrocaudal sequence of individual whiskers (sites 17-19). A separate pair of penetrations (sites A-B) illustrates the orientation of the vibrissae representation, with a ventrally located vibrissa on the mystacial pad (site A) represented medial to a dorsally located vibrissa (site B) of the mystacial pad. Two additional sites in this case responded more broadly and weakly to tactile stimulation of the forepaw (site a) and whiskers (site b) and likely represent S2 or PV based on response properties, location, and histochemical staining characteristics (see next section). Finally, a relatively large cortical area responded strongly to visual stimuli and a more lateral area of the cortex responded to auditory stimuli. The overall somatotopy of the body representation in S1 was investigated in 8 additional mapping cases. Four of these cases are illustrated in supplemental Figures 1-4.
Additional somatosensory areas
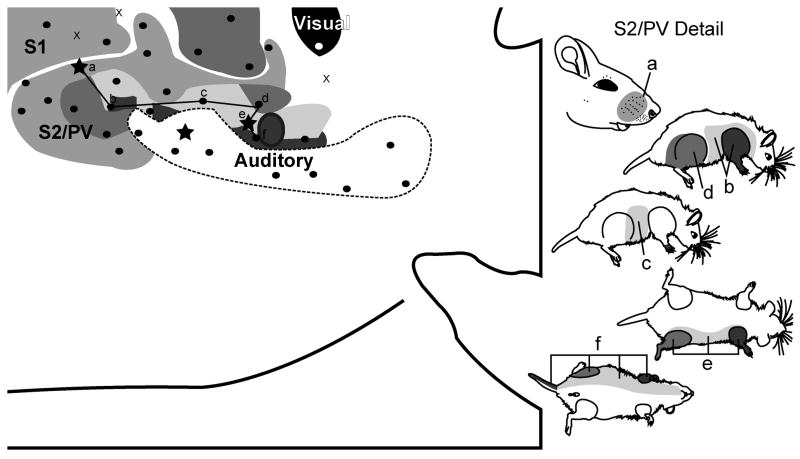
Along the caudolateral extent of S1, responses to stimulation of the body surface were weaker and associated with larger receptive fields. Recordings identified at least one additional representation of the contralateral body surface composed of a smaller, mirror-image of S1 and sharing a common border at the midline representation of the face and snout. Within this area, the face representation was located rostromedially and the limbs and trunk were located caudolaterally. Four cases (Figs. 5; supplemental Figs. 1, 3 and 4) provided evidence for additional somatosensory areas lateral to S1, however the most complete map is shown in Fig. 6 (showing S2/PV; for the complete case see supplemental Fig. 4). Receptive fields for the whiskers generally encompassed the entire mystacial field (e.g., Fig. 5 site b) and receptive fields for the body often included limbs and trunk (Fig. 6 site b) or almost the entire contralateral body surface (Fig. 6 sites e-f). Although this area was darkly labled in SERT-processed sections from juveniles, it did not contain barrels typical of the S1 representation of whiskers and forepaw pads.
Figure 6.
Topography of the grasshopper mouse neocortex with mapping of S2/PV. The schematic of the microelectrode-derived map of cutaneous inputs to the neocortex shows a representative receptive field sequence (see supplementary Fig. 4 for complete mapping schematic). Rostral is left, medial is up for the cortical schematic.
The overall somatotopy in this region suggests that many of the electrode penetrations were located in S2 (e.g., Sur et al., 1981; Krubitzer et al., 1986). The facial representation was organized such that rostral surfaces of the periphery were represented more rostrally (e.g. supplemental Fig. 3, sites a-b) whereas the forelimb representation was located lateral to the face representation (Fig. 5 sites a-b; Fig. 6 sites a-b) and at some sites the receptive fields were restricted to the forepaw (e.g. Fig. 5 site a; also see supplemental Fig. 3, site c). As the electrode was moved more caudally, the trunk, hindlimb, and tail representations were identified (Fig. 6 sites b-f).
Visual and auditory areas
Although receptive fields were not mapped, strong visual and auditory responses were obtained from a number of cortical areas. Large areas of neocortex were devoted to vision and a smaller area of cortex responded to auditory stimuli (e.g., Figs. 5). In one case (supplemental Fig. 3), cortical areas rostral and caudolateral to visually-responsive cortex responded to both auditory and visual stimuli, although auditory responses rapidly habituated.
Discussion
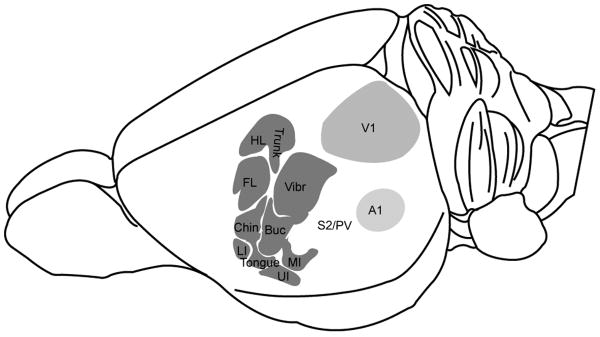
Using multiunit electrophysiological recordings, combined with sections of flattened neocortex, we investigated the organization of S1 and found evidence for at least one additional somatosensory area in the lateral cortex of grasshopper mice. Primary visual cortex and an auditory area were also identified based on their distinctive appearances in sections processed for cytochrome oxidase or SERT-immunolabeling and their responses to visual and auditory stimuli. In addition, myelinated axons were quantified from trigeminal, optic, and cochlear nerves. A composite showing the relative sizes of sensory areas within the neocortex as well as the topographical organization of primary somatosensory cortex is shown in Figure 7.
Figure 7.
Composite illustration showing the functional representations within S1 of the grasshopper mouse.
Primary somatosensory cortex
The neocortex of the grasshopper mouse contained a large and distinctive primary somatosensory cortex (S1) consisting of a complete somatotopic map of the contralateral body surface, as found in other rodents (Welker, 1971, 1976; Dawson and Killackey, 1987; Waters et al., 1995; Henry et al., 2006; Campi et al., 2007). S1 was easily identified in grasshopper mice based on relative location, orientation, histological characteristics, and response properties. In particular, the cortex from juveniles processed for SERT provided a striking correlation between histologically visible modules, including barrels, and the representations of different body parts (Figs. 2-3).
As in most other rodent species, the large mystacial vibrissae dominated the somatosensory representation and corresponded to a prominent barrel field visible in both CO and SERT processed sections. Smaller barrels were located more rostrally and laterally, corresponding to the microvibrissae (buccal pad) around the oral region. At the far lateral and rostral extreme of S1, responses were primarily obtained from oral structures including the lips, tongue, and teeth. This region of cortex is not always investigated during electrophysiological experiments, perhaps because of its far lateral position in the neocortex, and the difficulty localizing oral receptive fields. However recent investigations suggest that most mammals have a relatively large representation of oral structures in this location (Manger, 1996; Jain et al., 2001; Remple et al., 2003; Kaas et al., 2006; Iyengar et al., 2007).
The forepaw representation also appeared to be a relatively large component of grasshopper mouse S1. Behavioral observations indicate that grasshopper mice initially use their forepaws to seize and manipulate fast-moving prey such as crickets. In contrast, deer mice (rodents of similar size and overlapping habitat) and hamsters seize prey with their mouth (Langley, 1994). This suggests a more specialized attack by grasshopper mice (Eisenberg and Leyhausen, 1972) that relies on the forelimb, and perhaps corresponds to a magnification of this representation relative to other rodents (Dawson and Killackey, 1987).
Additional Somatosensory Areas
Evidence for at least one additional somatosensory area was found in lateral cortex. In the most extensive case, a complete representation of the contralateral body surface (e.g. Fig. 6) was found, with the face and whiskers represented more rostromedially whereas the limbs and trunk of the body were represented caudolaterally. This area was a mirror image of S1 with a common border along the midline of the snout and vibrissae fields. As the electrode was moved laterally, across the S1 border, receptive fields became markedly larger as has been reported for both S2 and PV (Nelson et al., 1979; Carvell and Simons, 1986; Krubitzer et al., 1986; Krubitzer and Kaas, 1990; Krubitzer and Calford, 1992; Krubitzer et al., 1995; Remple et al., 2003). The larger receptive fields were consistent with the small overall size of S2 and PV compared to S1, following the principle that receptive field size is inversely proportional to area of neocortical representation (Sur et al., 1980). Because two lateral somatosensory areas (S2 and PV) are found in most small mammals, it seems likely that we have recorded from both S2 and PV in different cases in the present investigation. Thus we have labeled the area S2/PV to reflect this interpretation.
Visual and auditory cortex
A distinctive primary visual area (V1) was identified in flattened cortical sections (e.g., Fig. 3) and electrophysiological mapping (e.g., Fig. 5) of grasshopper mouse neocortex. Additional visual responses were obtained lateral to V1, some of which almost certainly correspond to V2 as identified in a wide range of mammals and other rodents (Rosa and Krubitzer, 1999). V2 typically occupies a band of cortex just lateral to V1 and contains a retinotopic map in a mirror image of V1. In one case areas between V1 and the auditory area responded to both visual and auditory stimuli (supplementary figure 3) and in another case there was interdigitation of somatosensory and visual stimuli (supplementary figure 4), suggesting that cortex lateral to V2 may be involved in multimodal processing and perhaps some parts of V2 and auditory cortex process multiple modalities (e.g. Campi et al., 2007). Further studies would be necessary to evaluate whether visual structures (V1 and the optic nerve) are larger than would be predicted allometrically for grasshopper mice (see Kaskan et al., 2005 and Finlay et al., 2008), and if so, whether that might be an adaptation to predation. Deer mice would be a particularly intriguing comparison as a closely related herbivorous species.
A distinctive and uniformly dark area in CO and SERT processed sections corresponded to auditory cortex, as is often reported for other species. The histochemically apparent region responded to auditory stimuli and was relatively small compared to primary somatosensory and visual areas. However auditory responses were found beyond the boundaries of this module in some cases (see supplementary data), suggesting a larger extent of cortex may be involved in auditory processing than indicated by the borders of the CO dense region.
Behavioral studies of grasshopper mouse auditory acuity have produced contradictory results. Although several studies suggest that audition is well-developed in the grasshopper mouse and important for capturing prey (Bailey and Sperry, 1929; Egoscue, 1960; Langley, 1983), sound localization tests revealed a poor discrimination threshold similar to that of other rodents (Heffner and Heffner, 1988). Despite poor discrimination thresholds, grasshopper mice appeared to make more efficient use of binaural cues than their herbivorous counterparts (Heffner and Heffner, 1988) but otherwise did not appear to have specialized levels of auditory sensitivity for predation (Heffner and Heffner, 1985). However, the repertoire of vocalizations and use of calling behaviors – particularly alarm calls – in the grasshopper mouse is thought to be extensive and critical for survival. Many of these calls exist in the ultrasonic range, which was not explored in the present study and might account for a larger area of auditory cortex than was delineated electrophysiologically.
In summary, grasshopper mouse neocortex is dominated by large visual and somatosensory areas and a smaller auditory region in lateral cortex. This is generally consistent with the volumes of afferent input quantified for selected cranial nerves, with approximately 78,600, 24,100, and 5,800 myelinated afferents found in the optic, trigeminal, and cochlear nerves respectively. Flattened sections of juvenile cortex processed for SERT reveal the precise borders of primary visual cortex, the detailed representations of individual body parts in primary somatosensory cortex, and the approximate size of an auditory area that likely includes A1 and perhaps surrounding auditory areas.
Supplementary Material
Acknowledgments
We thank Danielle Gauthier for excellent technical assistance and Dr. Kathie Eagleson for optimization of SERT staining in this species. Thanks also to Jan Decher for the image shown in Fig. 1A and to the Vanderbilt Department of Animal Care for their attentive oversight of the grasshopper mice.
Grant sponsor: NIH grant # DE016061 and an NSF grant# 0844743 to K.C.C.
Literature Cited
- Bailey V, Sperry CC. Life history and habits of grasshopper mice, Onychomys. Tech Bull US Dept Agric. 1929;145:1–19. [Google Scholar]
- Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J Comp Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci USA. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair WF. Population dynamics of rodents and other small mammals. Adv Genet. 1953;5:1–41. doi: 10.1016/s0065-2660(08)60404-6. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonine transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Bennett-Clarke CA, Chiaia NL, Rhoades RW. Time course of expression and function of the serotonin transporter in the neonatal rat's primary somatosensory cortex. Somatosens Mot Res. 2000;17:52–60. doi: 10.1080/08990220070292. [DOI] [PubMed] [Google Scholar]
- Campi KL, Karlen SJ, Bales KL, Krubitzer L. Organization of sensory neocortex in prairie voles (Microtus ochrogaster) J Comp Neurol. 2007;502:414–426. doi: 10.1002/cne.21314. [DOI] [PubMed] [Google Scholar]
- Carleton MD, Eshelman RE. A synopsis of fossil grasshopper mice, genus Onychomys, and their relationship to recent species (Papers on Paleontology, No 21) Ann Arbor: Museum of Paleontology, University of Michigan; 1979. pp. 45–47. [Google Scholar]
- Carvell GE, Simons DJ. Somatotopic organization of the secondary somatosensory area (SII) in the cerebral cortex of the mouse. Somatosens Res. 1986;3:213–237. doi: 10.3109/07367228609144585. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Murphy DL. Serotonin in major disorders (progress in psychiatry series) Amer Psych P; Washington D.C.: 1990. [Google Scholar]
- D'Amato RJ, Blue ME, Largent BL, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci USA. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Schlueter McFadyen-Ketchum LJ, Ahrens ET, Mills PH, Does MD, Nickols J, Levitt P. Disruption of Foxg1 expression by knock-in of Cre recombinase: effects on the development of the mouse telencephalon. Neuroscience. 2007;148:385–399. doi: 10.1016/j.neuroscience.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egoscue HJ. Laboratory and field studies of the northern grasshopper mouse. J Mammal. 1960;41:99–110. [Google Scholar]
- Eisenberg J, Leyhausen P. The phylogenesis of predatory behavior in mammals. Zeitschrift fur Tierpsychologie. 1972;30:59–93. doi: 10.1111/j.1439-0310.1972.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Eisner T, Meinwald J. Defensive secretions of arthropods. Science. 1966;153:1341–1350. doi: 10.1126/science.153.3742.1341. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Franco EC, Yamada ES, Crowley JC, Parsons M, Muniz JA, Silveira LC. Number and topography of cones, rods and optic nerve axons in New and Old World primates: implications for the evolution of retinal development. Vis Neurosci. 2008;25:289–299. doi: 10.1017/S0952523808080371. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Kimura H, Maeda T. Postnatal development of serotonin nerve fibers in the somatosensory cortex of mice studied by immunohistochemistry. J Comp Neurol. 1986;246:191–201. doi: 10.1002/cne.902460205. [DOI] [PubMed] [Google Scholar]
- Frank DH. Diss. Cornell U; 1989. Spatial organization, social behavior, and mating strategies of the southern grasshopper mouse (Onychomys torridus) in southeastern Arizona. [Google Scholar]
- Heffner HE, Heffner RS. Hearing in two cricetid rodents: wood rat (Neotoma floridana) and grasshopper mouse (Onychomys leucogaster) J Comp Psychol. 1985;99:275–288. [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Sound localization in a predatory rodent, the northern grasshopper mouse (Onychomys leucogaster) J Comp Psychol. 1988;102:66–71. doi: 10.1037/0735-7036.102.1.66. [DOI] [PubMed] [Google Scholar]
- Henry EC, Remple MS, O'Riain MJ, Catania KC. Organization of somatosensory cortical areas in the naked mole-rat (Heterocephalus glaber) J Comp Neurol. 2006;495:434–452. doi: 10.1002/cne.20883. [DOI] [PubMed] [Google Scholar]
- Hibbard CW. Paleontology. In: King JA, editor. Biology of Peromyscus. Special publication no 2. Amer Soc Mammal; Stillwater, Oklahoma: 1968. pp. 6–26. [Google Scholar]
- Hoerder-Suabedissen A, Paulsen O, Molnár Z. Thalamocortical maturation in mice is influenced by body weight. J Comp Neurol. 2008;511:415–420. doi: 10.1002/cne.21853. [DOI] [PubMed] [Google Scholar]
- Horner BE, Taylor JM, Padykula HA. Food habits and gastric morphology of the grasshopper mouse. J Mammal. 1964;45:513–535. [Google Scholar]
- Iyengar S, Qi H, Jain N, Kaas JH. Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of new world monkeys. J Comp Neurol. 2007;501:95–120. doi: 10.1002/cne.21232. [DOI] [PubMed] [Google Scholar]
- Jain N, Qi HX, Catania KC, Kaas JH. Anatomic correlates of the face and oral cavity representations in the somatosensory cortical area 3b of monkeys. J Comp Neurol. 2001;429:455–68. doi: 10.1002/1096-9861(20010115)429:3<455::aid-cne7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Iyengar S. Cortical network for representing the teeth and tongue in primates. Anat Rec. 2006;288:182–190. doi: 10.1002/ar.a.20267. [DOI] [PubMed] [Google Scholar]
- Kaskan PM, Franco EC, Yamada ES, Silveira LC, Darlington RB, Finlay BL. Peripheral variability and central constancy in mammalian visual system evolution. Proc Biol Sci. 2005;272:91–100. doi: 10.1098/rspb.2004.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Calford MB. Five topographically organized fields in the somatosensory cortex of the flying fox: microelectrode maps, myeloarchitecture, and cortical modules. J Comp Neurol. 1992;317:1–30. doi: 10.1002/cne.903170102. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Manger P, Pettigrew J, Calford M. Organization of somatosensory cortex in monotremes: in search of the prototypical plan. J Comp Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture, and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. J Comp Neurol. 1986;250:403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- Landry SO., Jr The rodents as omnivores. Quart Rev Biol. 1970;45:351–372. doi: 10.1086/406647. [DOI] [PubMed] [Google Scholar]
- Langley W. Effect of prey defenses on attack behavior of Onychomys torridus. Zeitschrift fur Tierpsych. 1981;56:115–127. [Google Scholar]
- Langley WM. Relative importance of the distance senses in grasshopper mouse predatory behavior. Anim Behav. 1983;31:199–205. doi: 10.1016/0376-6357(85)90070-1. [DOI] [PubMed] [Google Scholar]
- Langley WM. Comparison of predatory behaviors of deer mice (Peromyscus maniculatus) and grasshopper mice (Onychomys leucogaster) J Comp Psychol. 1994;108:394–400. [Google Scholar]
- Manger PR, Woods TM, Jones EG. Representation of face and intra-oral structures in area 3b of macaque monkey somatosensory cortex. J Comp Neurol. 1996;371:513–521. doi: 10.1002/(SICI)1096-9861(19960805)371:4<513::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- McCarty R. Mammalian Species, Onchymys leucogaster. Amer Soc Mammal. 1978;87:1–6. [Google Scholar]
- Nelson RJ, Sur M, Kaas JH. The organization of the secondary somatosensory area (SmII) of the grey squirrel. J Comp Neurol. 1979;184:473–490. doi: 10.1002/cne.901840304. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008;18:584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- Remple MS, Henry EC, Catania KC. Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol. 2003;467:105–118. doi: 10.1002/cne.10909. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Chiaia NL, Bennett-Clarke CA. Development and plasticity of the serotoninergic projection to the hamster's superior colliculus. J Comp Neurol. 1990;299:151–166. doi: 10.1002/cne.902990203. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? Trends Neurosci. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- Rowe AH, Rowe MP. Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon. 2008;52:597–605. doi: 10.1016/j.toxicon.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Ruffer DG. Agonistic behavior of the northern grasshopper mouse (Onychomys leucogaster breviauritus) J Mamm. 1968;49:481–487. [PubMed] [Google Scholar]
- Satoh K, Iwaku F. Jaw muscle functional anatomy in northern grasshopper mouse, Onychomys leucogaster, a carnivorous murid. J Morph. 2006;267:987–999. doi: 10.1002/jmor.10443. [DOI] [PubMed] [Google Scholar]
- Sur M, Merzenich MM, Kaas JH. Magnification, receptive-field area, and “hypercolumn” size in areas 3b and 1 of somatosensory cortex in owl monkeys. J Neurophysiol. 1980;44:295–311. doi: 10.1152/jn.1980.44.2.295. [DOI] [PubMed] [Google Scholar]
- Sur M, Weller RE, Kaas JH. The organization of somatosensory area II in tree shrews. J Comp Neurol. 1981;201:121–133. doi: 10.1002/cne.902010109. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Washburne DL. Feeding ecology and laboratory predatory behavior toward live and artificial moving prey in seven rodent species. Animal Learning & Behavior. 1989;17(1):2–11. [Google Scholar]
- Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- Waters RS, Li CX, McCandlish CA. Relationship between the organization of the forepaw barrel subfield and the representation of the forepaw in layer IV of rat somatosensory cortex. Exp Brain Res. 1995;103:183–197. doi: 10.1007/BF00231705. [DOI] [PubMed] [Google Scholar]
- Way BM, Laćan G, Fairbanks LA, Melega WP. Architectonic distribution of the serotonin transporter within the orbitofrontal cortex of the vervet monkey. Neuroscience. 2002;148:937–948. doi: 10.1016/j.neuroscience.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971;26:259–275. [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol. 1976;166:173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- Whitman DW, Blum MS, Jones CG. Prey specific attack behavior in the southern grasshopper mouse, Onychomys torridus. Anim Behav. 1986;34:295–297. [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochromes oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Xu Y, Bledsoe S, Lin R, Kelley MR. Serotonin transporter antibodies: production, characterization, and localization in the brain. Brain Res Mol Brain Res. 1996;43:267–278. doi: 10.1016/s0169-328x(96)00209-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.