SUMMARY
The Consortium for Anthelmintic Resistance and Susceptibility (CARS) brings together researchers worldwide, with a focus of advancing knowledge of resistance and providing information on detection methods and treatment strategies. Advances in this field suggest mechanisms and features of resistance that are shared among different classes of anthelmintic. Benzimidazole resistance is characterized by specific amino acid substitutions in beta-tubulin. If present, these substitutions increase in frequency upon drug treatment and lead to treatment failure. In the laboratory, sequence substitutions in ion-channels can contribute to macrocyclic lactone resistance, but there is little evidence that they are significant in the field. Changes in gene expression are associated with resistance to several different classes of anthelmintic. Increased P-glycoprotein expression may prevent drug access to its site of action. Decreased expression of ion-channel subunits and the loss of specific receptors may remove the drug target. Tools for the identification and genetic analysis of parasitic nematodes and a new online database will help to coordinate research efforts in this area. Resistance may result from a loss of sensitivity as well as the appearance of resistance. A focus on the presence of anthelmintic susceptibility may be as important as the detection of resistance.
Keywords: anthelmintic resistance, benzimidazole, macrocyclic lactone, levamisole, monepantel, ligand-gated ion-channel, P-glycoprotein, beta-tubulin
INTRODUCTION
The Consortium for Anthelmintic Resistance and Susceptibility (CARS) met for the third time on 8 August 2009 in Calgary, Canada. The primary goal of CARS is to develop genetic markers for the detection and monitoring of anthelmintic resistance and also aims to facilitate interactions among researchers interested in the development of anthelmintic resistance, coordinate research efforts in areas of major interest and provide information to researchers, veterinarians, livestock producers and the public. Our current understanding of resistance to the 3 major classes of anthelmintics, the benzimidazoles, the macrocyclic lactones and cholinergic receptor anthelmintics is progressing rapidly due to significant advances in technologies and awareness among drug companies, veterinarians and farmers that anthelmintic resistance is a major concern for the sustainable management of parasitism in animals.
Parasitic diseases and the emergence of anthelminthic resistance are major problems for animal and human health (Harhay et al. 2010). Such resistance in livestock is a persistent and growing issue in all parts of the world and requires immediate attention (Molento, 2009). Several reviews of anthelmintic resistance have been published in this increasingly active area of research in the last few years. Much research is focused on trying to identify specific genetic differences that either cause resistance or are sufficiently closely associated with resistance to be able to serve as molecular markers (McCavera et al. 2007; von Samson-Himmelstjerna et al. 2007; Beech and Silvestre, 2010). Increasing evidence suggests that resistance is often the result of changes in genes other than the immediate drug target, including transporters and drug metabolism (Cvilink et al. 2009). With the failure of drug treatment, strategies to minimize the spread of resistance with the use of targeted treatment are receiving much attention (Cabaret, 2008; Kenyon et al. 2009).
In this active area of research, there is a critical need for detailed knowledge of the mechanisms of resistance and the discovery of useful diagnostic markers (James et al. 2009). In this review, we take the opportunity to emphasize recent progress in this field, specifically focusing on diagnostic tools being developed to identify anthelmintic resistance and focus on some of the central themes that appear to be shared among mechanisms of resistance to the different classes of drug (Table 1).
Table 1. Genes associated with resistance to four different classes of anthelmintic.
(For each gene, the molecular change associated with the appearance of anthelmintic resistance is indicated, along with the type of diagnostic test typically used to identify mutations associated with resistance: SNP-PCR, PCR based test that identifies specific single nucleotide polymorphism as indicated in the text; QT-PCR, quantitative PCR that can estimate the relative abundance of different RNA transcripts, PCR, indicates a test where the specific size of a PCR product, or the presence or absence of a specific product forms the basis of the test.)
| Drug | Target | Resistance gene | Molecular change | Molecular test |
|---|---|---|---|---|
| BZ | beta-tubulin | beta-tubulin | F200Y | SNP-PCR |
| E198A | SNP-PCR | |||
| F167Y | SNP-PCR | |||
| LEV | AchR | unc-38 | Decreased expression | QT-PCR |
| unc-63 | Altered transcript | PCR | ||
| acr-8 | Altered transcript | PCR | ||
| MPTL | AchR | mptl-1 | Altered transcript/decreased expression | PCR/QT-PCR |
| des-2 | Altered transcript/decreased expression | PCR/QT-PCR | ||
| deg-3 | Altered transcript/decreased expression | PCR/QT-PCR | ||
| IVM/MOX | Glu/GABA Channel | avr-14 | L256F | SNP-PCR |
| lgc-37 | K169R | SNP-PCR | ||
| glc-5 | A169 V | SNP-PCR | ||
| ggr-3 | Decreased expression | QT-PCR | ||
| pgpA | Increased expression | QT-PCR |
SNP DETECTION
Molecular tests for the detection of specific substitutions that cause, or are linked to anthelmintic resistance, have been developed employing a variety of different techniques, such as restriction enzyme digestion, direct sequencing, pyrosequencing and diagnostic PCR.
Restriction enzymes recognize specific nucleotide sequences (often 4 or 6 bases) at which they cleave DNA. With a degree of serendipity, it is possible for a single nucleotide polymorphism (SNP) of interest to create or destroy a specific restriction site, although it is very unusual to be able to survey >5–10% of any given sequence in this way. PCR primers can be engineered that include a specific nucleotide mismatch that creates a polymorphic restriction site in a PCR product which is absent from the original sequence (Webster et al. 2008). This greatly increases the number of sites that can be surveyed using this approach.
Direct sequencing of PCR amplified DNA containing regions of interest from individual diploid parasites can reveal all polymorphism within a defined region (de Lourdes Mottier and Prichard, 2008; Palcy et al. 2008). Variants can be identified by comparing sequence chromatograms with a reference sequence using, for example, SeqDoC (Crowe, 2005). A limitation of this technique is that genotypes can be identified but not specific haplotypes, although they can be inferred if certain assumptions are made about the population sampled. Pyro-sequencing (Ronaghi et al. 1998) using PCR primers designed to detect specific polymorphisms can be used to screen relatively large numbers of individual parasites and can also quantify the presence of different alleles in bulk DNA samples (Hodgkinson et al. 2008; Diawara et al. 2009; Hoglund et al. 2009; von Samson-Himmelstjerna et al. 2009). In the past, this technique was expensive relative to other methods, but is now less costly to perform. The technique is intended to identify SNPs within only a very short stretch of DNA but, again, recent advances in technology are increasing this range.
A simple and cost effective approach is the use of diagnostic PCR primers that bind only to specific sequence variants, with the 3′-end of the primer overlapping a SNP of interest (Schwenkenbecher et al. 2007; Garg and Yadav, 2008; Palcy et al. 2008; Chen et al. 2009; Rufener et al. 2009a; von Samson-Himmelstjerna et al. 2009; Schwenkenbecher and Kaplan, 2009). Primers for which the most 3′ nucleotide lies on the SNP of interest are often difficult to optimize for use in PCR, but the introduction of a deliberate mismatch in the penultimate nucleotide of the primer has been shown to achieve specific detection (Chen et al. 2009; Rufener et al. 2009a). Tests for the specific detection of each allelic variant must be conducted to verify that heterozygotes are not misidentified as homozygotes due to the presence of null alleles (Beech et al. 1994). Detection can be simplified if primers are designed such that both alleles of a particular variant can be detected in the same PCR reaction, a technique termed Amplification Refractory Mutation System-PCR (ARMS-PCR) (Ye et al. 2001; Munoz et al. 2009). These tests can quantify allele frequencies if combined with real-time PCR of bulk DNA or RNA samples (Schwenkenbecher et al. 2007; Palcy et al. 2008; Chen et al. 2009; Schwenkenbecher and Kaplan, 2009; von Samson-Himmelstjerna et al. 2009). Modifications of the basic PCR protocol, such as SmartAmp2, to include proteins that prevent non-specific amplification can significantly improve the quality of the results (Mitani et al. 2007). Incorporation of chemically modified nucleotide residues, so-called ‘locked nucleotides’, into the detection primer increase the stability of double-stranded molecules containing them. This Locked Nucleotide Amplification (LNA) allows sensitive and specific detection of known SNPs by real-time PCR (Walsh et al. 2007).
BENZIMIDAZOLES
Resistance to the benzimidazole (BZ) class of anthelmintics has been observed in parasitic nematodes of livestock animals since the early 1960 s (Drudge et al. 1964). The beta-tubulin protein, BEN-1, confers BZ sensitivity in Caenorhabditis elegans (see Driscoll et al. 1989) and clear evidence exists that 3 different single amino acid substitutions (i.e., F167Y, E198A and F200Y) in the beta-tubulin protein of different nematode species can be responsible, each leading separately to resistance (Kwa et al. 1994; Silvestre and Cabaret, 2002; Ghisi et al. 2007).
These techniques have revealed a pattern of substitutions associated with BZ resistance, several features of which are particularly interesting in light of what might be expected from an evolutionary standpoint (Gilleard and Beech, 2007). Appearance of the substitutions F167Y, E198A and F200Y following BZ treatment does not occur in every case. Both the F167Y and F200Y substitutions occur in 4 species of cyathostomins of horses resistant to BZs (Hodgkinson et al. 2008). In Trichostrongylus axei of sheep, BZ resistance is associated with the substitution at position F200Y. Thus far, neither F167Y nor E198A have been observed (Palcy et al. 2008). A survey of BZ resistance in Haemonchus contortus from sheep in the Himalayan and sub-Himalayan region of north west India found that the substitution F200Y is present at elevated levels and that its frequency is positively correlated with the intensity of farming and with geographic location (sub-Himalayan>sub-tropical>temperate). It would appear that this substitution is typically found in BZ resistant H. contortus in Europe but that the substitutions F167Y and E198A, although present, are much less common in this species (Silvestre and Cabaret, 2002; Ghisi et al. 2007; Hoglund et al. 2009; von Samson-Himmelstjerna et al. 2009). Interestingly, in vitro-selection for BZ resistance in H. contortus revealed changes only at position 198 of the beta-tubulin protein, but not at either position 167 or 200 (Rufener et al. 2009a).
There are situations in which exposure to BZ does not lead to the appearance of the substitutions usually associated with resistance. This is the case for Trichostrongylus tenuis in birds (Webster et al. 2008), Necator americanus in school children from the Zanzibar Archipelago, Ancylostoma duodenale and Ancylostoma caninum (Schwenkenbecher et al. 2007) and Ascaris lumbricoides from Kenya, East Africa and South America (Diawara et al. 2009). We know that the substitutions F167Y, E198A and F200Y can cause significant BZ resistance; so, the fact that they are not readily detected implies that they were not present in the original parasite population and do not appear by mutation during the course of BZ treatment.
The fact that substitutions that lead to BZ resistance exist at different locations in the beta-tubulin sequence clearly indicates that several independent mutations must have occurred. The prevalence of substitution F200Y in BZ resistant H. contortus worldwide may argue that a single genetic origin for this change might predominate throughout a majority of H. contortus populations worldwide (Silvestre and Cabaret, 2002). However, there is evidence consistent with multiple independent origins of the substitution F200Y in H. contortus and the related parasite, Trichostrongylus colubriformis (see Silvestre and Humbert, 2002). This evidence is based on the presence of unique alleles in isolated parasite populations and could be explained by inbreeding. When comparing different species, it seems most likely that novel mutations within each species are responsible for resistance.
Substitutions at both positions 167 and 200 have not been observed in the same allelic sequence of H. contortus of small ruminants or cyathostomins of equids, and appear to be mutually exclusive (de Lourdes Mottier and Prichard, 2008; Hodgkinson et al. 2008; Silvestre et al. 2009). One explanation might be that occurrence of both substitutions simultaneously is lethal in some way. Alternatively, current observations suggest that the creation of either substitution is a rare event and both together on the same sequence might simply never have occurred. For a single sequence to carry substitutions F167Y and F200Y, both mutations would have to occur consecutively in the same molecule. Alternatively, such a sequence could be created by recombination between these positions in a double heterozygote. On a genome-wide scale, markers are randomly mixed by recombination in H. contortus (see Silvestre et al. 2009) but within a single gene, recombination appears to have little effect in H. placei (see Mes, 2004). The one occasion when recombination has been observed between positions 167 and 200 of the beta-tubulin gene of H. contortus, both parental sequences carried the susceptible form at both positions (Silvestre et al. 2009).
Although sequence changes in beta-tubulin are thought to be the major cause of BZ resistance, changes in the drug transporter P-glycoprotein have also been linked with it (Blackhall et al. 2008). As is the case with ML resistance, one might imagine that Pgp plays a role in the transport of anthelmintic away from the principal site of drug action. Although the specific allele associated with BZ resistance is different from that associated with ML resistance, the possibility of a mechanistic link between resistance to BZ and MLs is disturbing. This would mean that selection for resistance with one drug could alter the development of resistance to the second drug. More evidence for this link between BZ and ML resistance comes from the finding that the beta-tubulin substitutions known to lead to BZ resistance are also selected for by exposure to MLs (de Lourdes Mottier and Prichard, 2008). It is not yet clear whether changes in the beta-tubulin protein can lead to ML resistance or if they are simply associated with it by genetic ‘hitch-hiking’. In a very real sense it does not matter, since, whatever the basis for these genetic changes, the conclusion that selection with MLs could predispose toward BZ resistance is unavoidable.
CHOLINERGIC RECEPTOR ANTHELMINTICS
Several different anthelmintics target members of the nicotinic acetylcholine gated cation-channels that mediate fast synaptic signalling in the neuromusculature of nematodes. These include the imidazothiazoles of which levamisole (LEV) is widely used, the tetrahydropyrimidines, such as pyrantel, the quaternary amines, such as bephenium and the newly described amino acetonitrile derivatives (AADs), including monepantel (MPTL). All of these act as agonists, increasing the flow of cations that leads to a rigid paralysis. The macfortines, including paraherquamide and the newly developed derquantel, act as antagonists preventing the activation of cation channels.
The ligand-gated ion-channels share a pentameric quaternary structure (Fig. 1) in which a single subunit type can produce a homomeric channel, but, more commonly, different subunit types combine to make a heteromeric channel. The natural ligand typically binds at the interface between an alpha-type and its adjacent subunit, causing a change in physical structure of the channel, opening a gate that allows ion flow into, or out of, the cell (Absalom et al. 2004) (see Fig. 2). In Ascaris suum, channels that respond to acetylcholine fall into 3 functional classes, defined by their conductance and sensitivity to LEV (L-subtype), nicotine (N-subtype) or bephenium (B-subtype) (Qian et al. 2006). The N-subtype has a relatively small conductance (G=22pS), is not antagonized by paraherquamide and nicotine, oxantel and methyridine are selective agonists. The L-subtype has an intermediate conductance (G= 33pS), is antagonized by paraherquamide and derquantel (2-deoxoparaherquamide), and levamisole and pyrantel are selective agonists. The B-subtype has the largest conductance (G=43pS) and is most sensitive to derquantel, and bephenium is a selective agonist. The activity of thenium appears to be intermediate between that of bephenium and pyrantel (Martin et al. 2004).
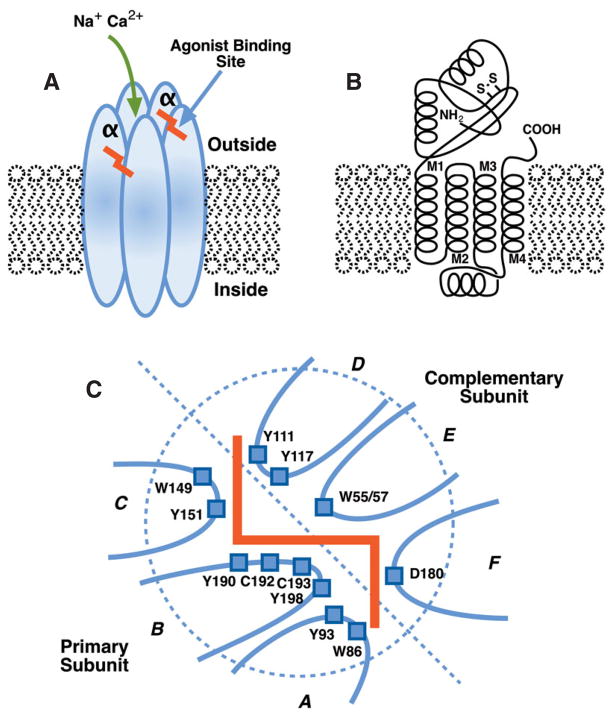
Fig. 1.
(A) Diagram of 5 transmembrane subunits, 2 α and 3 non-α, forming a nicotinic acetylcholine channel with at least 2 agonist-binding sites as indicated. (B) Diagram of an α subunit, characterized by a disulfide bridge between 2 vicinal cysteines, with 4 transmembrane regions. (C) Diagram of the agonist binding site between an α subunit forming the primary binding site with loops A, B and C and the complimentary site formed by the adjacent subunit with loops D, E and F. Amino acids that play a critical role in ligand binding are indicated.
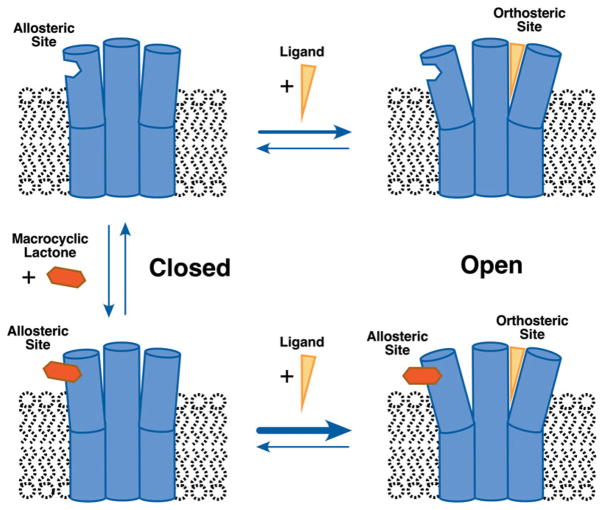
Fig. 2.
Schematic of allosteric modulation by macrocyclic lactones. Cut-away illustration of a ligand-gated channel showing 3 of the 5 subunits. The channel is in the open state when ligand is bound to the orthosteric site. Binding at an allosteric site, which can be anywhere on the channel, stabilizes the open state and potentiates agonist action.
Numerous genes encode cation-channels in C. elegans; they can be grouped into 5 clusters named after specific subunits: acr-16, acr-8, unc-38, unc-29 and deg-3, and, in addition, many subunits whose function is not clearly defined (Jones et al. 2007). The pharmacology of individual channels depends on their subunit composition. To a large extent, the subunits that contribute to different channel subtypes is not yet known, although exciting progress is being made in this area. Subunits in the C. elegans acr-16 cluster can form homomeric acetylcholine receptors that respond to nicotine (Ballivet et al. 1996). Elegant work has defined 8 C. elegans genes that are essential to reconstitute functional LEV sensitive channels in Xenopus oocytes. Three of these genes encode ancillary proteins involved in folding and transport of channel subunits from the endoplasmic reticulum to the cell surface. The remaining five, LEV-1, ACR-13 (previously known as LEV-8; Jones et al. 2007), UNC-29, UNC-38 and UNC-63 combine to produce an acetylcholine channel sensitive to LEV (Boulin et al. 2008; Qian et al. 2008).
As a complement to C. elegans, the nematode A. suum provides a tractable parasite model for which the response of excised muscle strips to various molecules can be measured (Robertson et al. 2002). Genes encoding UNC-29 and UNC-38 have been cloned from Ascaris and used to reconstitute a functional acetylcholine receptor in Xenopus oocytes. Unlike C. elegans, LEV-1, ACR-13 and UNC-63 are not required. Functional receptors are not produced with either protein alone. The heteromeric receptor, with a 5:1 ratio of UNC-38 to UNC-29, is sensitive to LEV (L-subtype) and confirms the role of these two genes in the sensitivity of Ascaris to the anthelmintic (see Fig. 3). A surprising finding is that when the ratio of UNC-29 to UNC-38 is reversed (1:5) the pharmacology of the receptor mimics the N-subtype channel (Williamson et al. 2009). In addition to the presence of specific subunits, it is the specific composition of a channel that determines its pharmacology; there is a very fine level of control over the nematode neuromusculature.
Fig. 3.
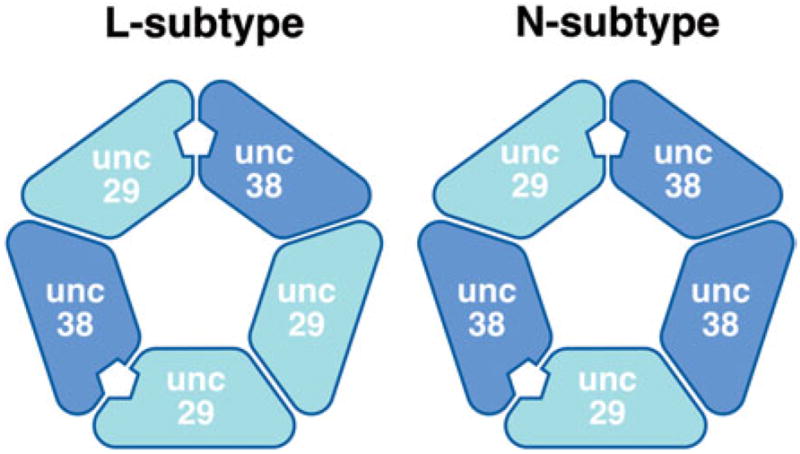
Different stoichiometric arrangements of receptor subunits alter receptor subtypes and relative sensitivity to different nicotinic anthelmintics. Putative arrangements for Clade III (Ascaris) subunits are illustrated. Binding sites are indicated between subunits.
Recent work has revealed a quadruplication of the unc-29 gene in the trichostrongylid nematodes H. contortus, Teladorsagia circumcincta and Trichrostrongylus colubriformis (see Neveu et al. 2010). This new evidence, together with the differences in L-AchR composition between C. elegans and A. suum, suggests that the L-AchR subunit composition may change significantly between closely related species and thus provide a moving target for anthelmintic treatment among species. More research is required to understand the details of these changes.
Examination of the unc-29, unc-38 and unc-63 genes in A. caninum found no evidence for specific amino acid substitutions associated with resistance to pyrantel. This evidence is in sharp contrast to the well-documented substitutions responsible for BZ resistance. Unlike tubulin, resistance to pyrantel is associated with a significant decrease in expression for only these 3 genes linked with the mechanism of action for pyrantel (Kopp et al. 2009). This information is consistent with the observation that, in LEV resistant Oesophagostomum dentatum, the L-subtype channel is absent (Robertson et al. 1999). The cDNA corresponding to an abbreviated form of the acr-8 gene is found only in LEV-resistant isolates of H. contortus. This form is present in addition to the full length acr-8 transcript but contains stop codons and could not encode a functional protein (Fauvin et al. 2010). A similar situation has been observed for the unc-63 gene in some LEV-resistant H. contortus (Neveu et al. 2010). It is not yet clear whether these unusual transcripts can cause resistance or whether they are only associated with it, but such transcripts have been shown to interfere with normal channel expression (Saragoza et al. 2003). In C. elegans, deletion or inactivation of many ion-channel genes results in no overt phenotypic difference, suggesting that, while individual ion-channel subunit genes can confer specific pharmacology on the nematode neuromusculature, there is sufficient plasticity that other channels may compensate for their loss. This inference does raise hopes that understanding anthelmintic resistance at the molecular level can indeed provide concrete means to improve parasite control. If loss of specific ion-channel subunits can lead to resistance to one subtype-selective anthelmintic, these resistant nematodes may be less able to survive other subtype-specific nicotinic anthelmintics. Thus, it may be desirable to consider combinations of different subtype-selective anthelmintics, such as oxantel and pyrantel, or pyrantel and monepantel, for instance. An example of this can be seen in C. elegans, where the loss of acr-16 leads to disappearance of the nicotine-sensitive acetylcholine channel, loss of the unc-29 gene causes a loss of the LEV-sensitive channel subtype. Each individual mutant displays acetylcholine-dependent control of the neuromusculature, whereas the double mutant is essentially immobile.
It should also be considered that ion-channels that bind anthelmintics are part of a ‘larger physiology’. For example, the response of Ascaris muscle to LEV is modulated by both ryanodine and the neuro-peptide AF2, via changes in intracellular calcium triggered by, but independent from, ion flow through the L-AchR (Puttachary et al. 2010; Robertson et al. 2010). It is likely that changes in ryanodine or AF2 receptors themselves could affect anthelmintic resistance.
A novel class of anthelmintic based on AADs targets a different class of nicotinic acetylcholine receptors than LEV (Kaminsky et al. 2008). Sensitivity to the AAD monepantel (MPTL) is associated with the DEG-3 family of subunits unique to the nematodes, including deg-3, des-2, acr-20 and acr-23 (Kaminsky et al. 2008; Rufener et al. 2009b). A screen for mutations in C. elegans that lead to MPTL resistance found a majority of mutations in the acr-23 gene, but not the closely related acr-20, des-2 or deg-3 genes (Kaminsky et al. 2008). With limited quantities of MPTL available, a screen for resistance in H. contortus was based on several rounds of exposure of the free-living stage of the parasite to the drug, followed by passage through sheep. In the resistant parasites, molecular analysis identified many mutations in the genes des-2 and mptl-1 that inactivate splice junction sequences; mptl-1 is orthologous to the acr-20/acr-23 gene pair in C. elegans. These altered transcripts encode truncated proteins with premature stop codons that presumably lead to loss of function (Rufener et al. 2009b). In addition, transcription levels from the genes mptl-1 and des-2 of H. contortus were much reduced in MPTL-resistant parasites. Expression of the deg-3 orthologue was either increased or decreased in the resistant isolates. It would appear that loss of the MPTL-sensitive channel is the basis for resistance, a characteristic shared with resistance to LEV. In addition, the loss of 2 subunits in the DEG-3 group appears to lead to altered regulation of closely related subunits, possibly as a compensatory mechanism.
MACROCYCLIC LACTONES
The macrocyclic lactones (MLs), including ivermectin (IVM) and moxidectin (MOX), are allosteric modulators of nematode channels including glutamate and GABA-gated chloride channels, causing an inhibition of pharyngeal pumping, motility and egg-laying (Martin and Pennington, 1989; Cully and Paress, 1991; Martin, 1996; Dent et al. 2000; Feng et al. 2002; Forrester et al. 2002). One gene, avr-14, also known as GluClalpha3B (Beech et al. 2010), in particular, has been investigated in several related parasitic nematode species. In Cooperia oncophora, a single amino acid substitution L256F was found to be responsible for an altered response to both the natural ligand glutamate, and the anthelmintic IVM (Njue and Prichard, 2004). In an effort to dissect the molecular basis for ML resistance, the avr-14 homologue from H. contortus was expressed in Xenopus oocytes to produce IVM-sensitive chloride currents (McCavera et al. 2009). Several different amino acid substitutions that had previously been observed in ML-resistant parasites were evaluated and, as with C. onchophora, only the substitution L256F was found to have an effect, making the channel less sensitive to IVM and reducing affinity of the channel for the drug. In the lgc-37 gene, previously known as HG1 (Beech et al. 2010) that encodes a subunit of a GABA-gated chloride channel, a substitution of K169R was found to have a similar effect, decreasing sensitivity to GABA and MOX (Feng et al. 2002). A substitution (of A169 V) in the H. contortus glc-5 gene that encodes an alpha-subunit of a glutamate-gated chloride channel has also been observed, but it is not known whether this affects the channel response to MLs. Clearly, individual amino acid substitutions can alter the response of individual channels to ML.
One might expect that mutations known to reduce ML sensitivity would be found in parasites that have developed resistance to the drug. A survey of ML resistant field isolates for the K169R substitution had previously found there was no significant appearance of this substitution (Galazzo, 2004). There are two possible explanations for this finding. One possibility is that substitutions in the genes encoding the ML sensitive ion-channels can indeed ameliorate the effects of the drug, but that, at the elevated drug concentrations used in field application, other genes have a more significant effect. Secondly, as with BZ resistance, the substitutions capable of expressing resistance may simply not be present in the parasite population under selection.
Although the principal effects of the MLs are mediated by the glutamate and GABA-gated chloride channels, they are known to be allosteric modulators of many different anion- and even cation-channels (Krause et al. 1998). Recent cloning and characterization of the H. contortus ggr-3 gene has shown that it forms a dopamine-gated anion-channel, is expressed predominantly in the cervical papillae and is likely to be involved in tactile sensation. A single SNP in the 3′-untranslated region of the gene was found to be polymorphic with a significant difference in frequency between a susceptible isolate PF23 and 2 congenic ML-resistant isolates, IVF23 and MOF23, derived from the same parental stock as PF23 (Molento et al. 1999; Rao et al. 2009). Comparison of transcription levels by quantitative PCR found a significant reduction in ggr-3 expression in the ML-resistant isolates, although whether this results in differences in the level of expressed protein is not known. It is not clear what, if any, role the ggr-3 gene may play in ML resistance.
C. elegans appears to be a suitable model for rhabditid nematodes (clade IV) but may be less suitable for phylogenetically distant nematodes, such as Trichuris (clade I) and Ascaris and Brugia (clade III) (for clades, see Blaxter et al. 1998). The rate of pharyngeal pumping in C. elegans is very sensitive to IVM, being affected by as little as 0·15 nM of drug and producing complete paralysis using as little as 4·9 nM IVM. MOX has significantly less effect on pharyngeal pumping, with complete paralysis requiring 39 nM MOX (Ardleli et al. 2009). Although both drugs act on glutamate and GABA-gated chloride channels and binding assays for the genes examined so far have found the same binding sites, there appears to be a difference in response to the two drugs in terms of larval development rates, pharyngeal pumping and motility. Different channels may have different affinities for the two drugs. Complete loss of ML sensitivity in C. elegans can be achieved by deletion of 3 chloride channel subunit genes: avr-14, avr-15 and glc-1 when pharyngeal pumping is unaffected by either IVM or MOX. The difference in sensitivity between IVM and MOX disappears in C. elegans, from which the glc-2 gene is deleted. This gene has not previously been implicated in ML resistance, but its presence appears to be responsible for some degree of protection for the pharyngeal neuromusculature from the effects of MOX but not IVM. A similar, but less pronounced effect is seen with deletions of the glc-3 and glc-4 genes.
The effect of ML on C. elegans body movement velocity is complex. At 2·5 nM IVM produces an increase in movement relative to untreated controls, peaking at 1·5 h following exposure, which then diminishes and is gone by 2·5 h following treatment. This effect is not seen with MOX and is less pronounced with larger concentrations of IVM. Again, the difference in effect between IVM and MOX disappears in the glc-2 deletion mutants, so the temporary increase in movement is produced by both IVM and MOX (Ardleli et al. 2009). Exposure to IVM and MOX also affects transcription of ion-channel subunit genes. In some cases transcription was increased by drug exposure; in others transcription was significantly reduced and this effect could reverse with increased drug concentrations. Some genes were affected by one drug, but not the other and, again, this effect could reverse at higher drug concentrations. Overall, it seems clear that IVM and MOX can affect C. elegans in different ways. The exact molecular details remain to be identified but the glc-2 gene may play a critical role in these differences.
The search for genes involved in ML sensitivity in C. elegans has only identified those involved in glutamate-gated nerve signal transmission (Dent et al. 2000). In parasitic nematodes such as H. contortus; however, the P-glycoprotein (Pgp) multi-drug transporters have been implicated. To date, no single amino acid substitutions have been linked with ML resistance; only an increase in Pgp expression is thought to lead to increased drug transport and removal of the drug from the site of action. Recently, a variant of the bovine half-transporter, ABCG2, has been found with a single amino acid replacement, designated S581Y (Merino et al. 2009). ABCG2 is capable of transporting the fluorescent mitoxantrone that can thus be used to measure the transport activity in vivo. The 581Y variant was inhibited by both IVM and selamectin, more than the wild-type 581S, although only the effect of selamectin was significantly different. While no amino acid substitution has been found linked with anthelmintic resistance, it is clear that such a mechanism is at least in principle a possibility.
Although no Pgp mutants of C. elegans have been found that are more ML tolerant, this is likely due to the fact that mutagenesis prior to selection for drug resistance typically produces loss of gene function. In the parasitic nematodes, drug resistance has been associated with an increase in Pgp expression, and presumably is the result of increased drug transport. In order to investigate the role of Pgp genes with ML resistance, selection with IVM of C. elegans without prior mutagenesis does produce some level of resistance. Unlike mutations in the avr-14, avr-15 and glc-1 genes, which together can produce a loss in sensitivity to a more than 1000-fold concentration of IVM, selection without prior mutagenesis produced up to 30-fold resistance (James and Davey, 2009). The most ML-resistant C. elegans were also cross-resistant to LEV and pyrantel, although not to the BZs. Several of the ABC-transporter genes are affected, including pgp-1, pgp-2 and genes involved in glutathione synthesis, a molecule intimately involved in the transport of drugs by members of the ABC-transporter family. The development of resistance was slow in this experiment, taking almost 1 year before the ability to survive a 10-fold increase in IVM was observed. It is possible that resistance through changes in gene expression may require multiple mutations that take time to accumulate, or an alternative explanation for this is that some sort of epigenetic modification could be responsible. Epigenetic modification of this kind can persist for many generations (Jablonka and Raz, 2009) and this phenomenon is likely to open a new area of research in the field of anthelmintic resistance.
Screens for allelic variation in ABC transporters in the human parasite, Onchocerca volvulus, have found evidence for frequency changes in areas where people have received treatment with IVM. As with the veterinary parasitic nematodes, specific alleles have been found that increase and others that decrease in abundance following drug treatment (Ardleli and Prichard, 2004; Bourguinat et al. 2008). Only diploid individuals were examined, so only genotype frequencies could be determined which, in some cases, rose in frequency by 15% or decreased by a similar amount. With human parasites, it is not possible to develop isogenic isolate pairs where one has been exposed to the drug and one has not. This makes it difficult to know with certainty that genetic changes are due to drug exposure rather than geographical differences. In order to minimize this, parasites have been collected from the same geographical region before and after treatment of the population (Bourguinat et al. 2008).
Expression of ABC transporter mrp genes in C. elegans responds to exposure of worms to both IVM and MOX (Ardleli et al. 2009). Overall, the pattern of expression is qualitatively similar between the two drugs, typically with the largest increase in expression within 30 min following exposure. Transcription generally decreases over the following 2 h. Resistance to the MLs is also associated with an increase in gene expression of 2 thioredoxin genes from H. contortus (Sotirchos et al. 2008).
GENOMICS AND GENETICS
There are a number of major research objectives that need to be accomplished to allow the application of genetic and genomic approaches to study anthelmintic resistance. These include the generation of assembled and annotated genome sequence from the important nematode species, the identification and characterization of genome-wide genetic markers and the characterization of genetic variation in both laboratory and field strains of parasites. Sophisticated genetic analysis of nematode parasite genetics is challenging due to the parasitic lifestyle requirement for a period inside a host. However, ongoing progress with parasitic nematode genome sequencing is beginning to provide tools that will make such analysis a possibility. For example, the genome project for H. contortus has produced some 280 Mb of assembled sequence (Berriman, 2009a), while that for T. circumcincta has 14 Mb (Berriman, 2009b). This sequence has been screened for microsatellite markers for both of these species, which are being used for routine strain typing and the investigation of genetic variation and population genetic structure. Micro-satellites consist of repeats of simple di-, tri- and tetra-nucleotides flanked by unique sequence (Bhargava and Fuentes, 2010). PCR primers that flank the repeat produce bands where the size reflects the number of repeats present. The high degree of variability of microsatellites means that it is possible to generate genetic fingerprints for parasites. A bioinformatic screen for candidate microsatellite loci identified 677 and 556 from H. contortus and T. circumcincta respectively. Of these 81 and 83, respectively, were tested experimentally with 52 and 58 failing to amplify reliably from a variety of different isolates (i.e. null alleles common in some populations) with 7 and 1 respectively being monomorphic and 13 in each case amplified more than 2 alleles from individual worms suggesting they were present at more than one locus in the genome (Grillo et al. 2007; Redman et al. 2008b). The high proportion of microsatellite markers that were discarded for routine use, mainly because of the high frequency of null alleles in some populations, suggests that finding large numbers of markers for genome-wide studies that work across many diverse populations will be a challenging task. However, panels have been developed that provide robust tools for strain typing, quality control and population genetic studies. For example, a core set of 15 microsatellites have been developed for H. contortus that can be combined into 5 multiplex PCR reactions. When amplified from DNA isolated from a population of worms these provide fingerprint profiles visualized on capillary sequencing chromatograms that characterize and discriminate many of the commonly used laboratory isolates of H. contortus. Another panel of 6 micro-satellite markers linked on a section of the X-chromosome has been developed and used to investigate mating patterns in H. contortus (Redman et al. 2008a). It has been found that female worms mate repeatedly with different male worms, such that a single female brood contains progeny with paternal genotypes originating from up to 4 different male worms. As well as providing a mechanism for increasing genetic variation in parasite populations, this has practical implications that have been exploited to improve the inbreeding of H. contortus (see below).
Sequencing projects for a number of parasitic helminths are progressing at the Genome Center at Washington University, St Louis, USA, the Sanger Centre in the UK (Beech et al. 2010). The filarial nematode, Brugia malayi was the first parasitic nematode genome published (Ghedin et al. 2007) and, recently, the trematodes Schistosoma mansoni and S. japonicum have been published (Berriman et al. 2009; Liu et al. 2009). The genome of the cestode Echinococcus multilocularis is almost complete and is currently in gap closure and finishing. Genome data for Loa loa, Wuchereria bancrofti and O. volvulus are publicly available. The nematodes, H. contortus, Nippostrongylus brasiliensis, Strongyloides ratti and Globodera pallida are in the shotgun sequencing, assembly phases and projects for Ascaris and Trichuris muris are planned.
The H. contortus genome project, which has particular relevance for anthelmintic resistance research, is ongoing at the Sanger Centre and was begun in 2004 with support from The Wellcome Trust as a pilot project intended to address expected problems with sequence variability in the parasitic nematodes (http://www.sanger.ac.uk/Projects/H_contortus/). In 2006, the project was included in the Sanger Centre’s 5-year core-project funding and so far has generated 1·3 million random sequence reads from capillary sequencing, totalling more than 900 Mb of sequence. This provides a valuable resource for the research community that is well used as evidenced by numerous publications. However, in addition, 21 BAC clones, ranging in size from 38 to 139 kb have been sequenced completely and fully annotated. Four of these clones overlap to form 350 kb of continuous sequence on the X-chromosome. Recently, a further 350 Mb of Roche 454 sequencing has been completed bringing the total sequence generated to more than 1·2 Gb. The original estimate of the H. contortus genome size of 53 Mb, based on flow cytometry, was smaller than that of C. elegans. It now seems certain that this is a significant underestimate, the current genome assembly contains 120000 contigs totalling almost 280 Mb. Examination of the pattern of overlapping reads suggests that the genome size could be 200–350 Mb in size, which would make the current data equivalent to almost 4x coverage. Annotation of the available BAC sequence reveals that genes in H. contortus are significantly larger than their orthologues in C. elegans, on average twice the length, with more and larger introns within the gene and larger intergenic distances (Laing R, Saunders G, Beech RN, Berriman M and Gilleard J, unpublished data). This again argues for a larger genome size for H. contortus than the 100 Mb size of C. elegans. In one case, the C. elegans mec-7 beta-tubulin gene is 1·8 kb translated into a protein of 440 amino acids. The orthologous gene in H. contortus spans over 10 kb but produces a protein of the same length, 441 amino acids.
In addition to genome size there are other likely contributing factors to poor genome assembly. Repetitive DNA and sequence polymorphism, which we know to be high, can both interfere with genome assembly and also suggest the genome size is larger than it is. As an approach to deal with this latter problem, a more inbred version of the MHco3(ISE) strain has been developed to provide a template for the next round of genome sequencing. This was produced by transplantation of many adult females with a single male, which avoids the problem of polyandry demonstrated in H. contortus (Redman et al. 2008a). This inbred line has been genetically verified using the core set of microsatellites developed for H. contortus and it is hoped the reduced level of sequence polymorphism will enable shotgun sequence generated from this strain to be assembled more easily. Roche 454 sequencing of a paired end library is also planned and should provide information for scaffolding the available sequence together with deep sequencing using Illumina technology.
Finally, Illumina sequencing is being used to generate an extensive data set for the transcriptome. This will assist in the ultimate annotation of a completed genome for H. contortus. Illumina sequence reads of 54 bp from adult cDNA have already been mapped on to the genome assembly to allow the identification of transcribed sequence and L3 derived reads are currently in process (Laing R, Berriman M and Gilleard J, personal communication). These data, along with the H. contortus shotgun assembly will shortly be incorporated into WormBase to allow public access browsing.
PARASITE ISOLATES
A major concern for anyone working with a parasitic nematode is the reliable knowledge of the origin and characteristics of any particular isolate. Within each research establishment, isolates have been collected, characterized, catalogued and stored over the years, but there has been minimal genetic characterization and monitoring of the isolates used and exchanged among laboratories. This situation has resulted largely from a lack of appropriate tools. A case in point is the Chiswick avermectin resistant (CAVR) isolate of H. contortus, which was isolated in 1993 (Le Jambre, 1993) and has been used in a number studies in various laboratories around the world since its isolation (Kotze et al. 2002, 2006; Redman et al. 2008b; Varady et al. 2009). The development of microsatellite markers, based on genomic data, is one approach by which the status of any isolate can be established and subsequently monitored for integrity and quality assurance. A suitable fingerprint could be used to determine with certainty that an isolate is in fact the one it is otherwise only assumed to be.
In order to unequivocally identify different parasite isolates and provide a centralized repository for historical, genetic, biological and anthelmintic resistance data, a virtual database has been established and is hosted by the Victorian Bioinformatics Institute at Monash University in Melbourne (http://vbc.med.monash.edu.au/cars/cars.py/). The database holds information, relating to parasite species, the institute that holds the material, geographical origin, isolation date, country of origin, host species that the material was obtained from, drug resistance status, citations relating to characterization of the isolate, information on management history and what materials might be available. Information on use of the system can be obtained from the database manager, Peter Hunt (peter.hunt@csiro.au).
The individual collections are wonderful resources for researchers who are working in the area of anthelmintic resistance but this raises a number of questions when isolates/strains are shared amongst colleagues. Do isolates retain their genetic integrity when maintained in different laboratories from around the world? Can a standardized nomenclature be introduced throughout the different laboratories, which will easily provide useful information on the provenance of a particular isolate? Can the anthelmintic resistance status of isolates be easily determined in a meaningful way without resorting to the use of large animal hosts? Can isolates be more readily characterized using model systems such as jirds (Meriones unguiculatus)?
The establishment of a universal nomenclature for isolates would assist in the cataloguing of isolates and help to verify that material was derived from a known source. A system that details laboratory designation, species name, isolation date and passage number and geographical origin is important. A system to capture this information has also been proposed and details can be found on the new parasite isolate database (http://vbc.med.monash.edu.au/cars/cars.py/).
Currently, the tests that are available for phenotypic characterization of isolates rely on monitoring the effects of compounds on the biological actions of parasite populations in vitro and determining dose responses accordingly, for example, ED50 and ED99 estimates with the egg hatch test, in which parasite eggs are incubated in the presence of the drug and resistance is determined from the proportion of eggs that survive to the point of hatching. Experimental parasite material that is passaged through host animals before being characterized is costly in both time and animals. Studies conducted in jirds have been shown to be useful in preliminary evaluations of anthelmintic activity (Conder et al. 1990, 1991a,b) and characterization studies (Molento et al. 1999).
CONCLUSION
Much progress has been made in understanding the mechanisms by which parasite nematodes become resistant to anthelmintics in the 16 years since the molecular basis of BZ resistance in H. contortus was identified (Kwa et al. 1994). During this time, the problems posed by resistance worldwide have increased to the point where the presence of resistance is now commonplace. Management practices place increasing importance on refugia, where parasites that remain unexposed to drug treatment can preserve anthelmintic susceptibility. The use of physiological indicators of heavy parasitism, such as FAMACHA and weight loss, allow treatment to be targeted to the most severe cases (Kenyon et al. 2009), but this has been established only in small ruminants to date. It is interesting to speculate that treatment of only those animals showing physiological signs of infection may have the advantageous side-effect of selecting for parasites that minimize their impact on the host.
The spread and increased awareness of the problems caused by anthelmintic resistance draws our attention to a shift in our view of resistance. When resistance is a rare phenomenon, our focus is drawn to every new case. Now, it is often of less concern whether resistance is present, rather a question of how susceptible the existing population of parasites will be to the drugs we have available. The frequency of substitutions F167Y, E198A and F200Y in beta-tubulin can identify the presence of resistance, but, conversely, can also predict how effective BZ treatment can be. Where ligand-gated ion-channels are the principal drug target, it is becoming clear that the down-regulation of transcription and loss of the target receptor is a common mechanism. Testing for the presence of a LEV, ML or MPTL target receptor may be a useful way to predict whether investment in these drugs will be money well spent. Perhaps the clearest message from the present CARS meeting is that, as we understand resistance in more detail, the most effective practical tools we may be able to develop will be molecular markers not for anthelmintic resistance but for susceptibility.
Acknowledgments
Funds supporting the annual meeting of the Consortium for Anthelmintic Resistance and Sensitivity (CARS) were made available by Pfizer Animal Health, Fort Dodge Animal Health, Bayer Animal Health and Merial. The project on cholinergic receptors was supported in part by Grant number R 01 AI 047194 from the National Institute of Allergy and Infectious Diseases to R.J.M. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
References
- Absalom NL, Lewis TM, Schofield PR. Mechanisms of channel gating of the ligand-gated ion channel superfamily inferred from protein structure. Experimental Physiology. 2004;89:145–153. doi: 10.1113/expphysiol.2003.026815. [DOI] [PubMed] [Google Scholar]
- Ardleli BF, Prichard RK. Identification of variant ABC-transporter genes among Onchocerca volvulus collected from ivermectin-treated and untreated patients in Ghana, West Africa. Annals of Tropical Medicine and Parasitology. 2004;98:371–384. doi: 10.1179/000349804225003415. [DOI] [PubMed] [Google Scholar]
- Ardleli BF, Stitt LE, Tompkins JB, Prichard RK. A comparison of the effects of ivermectin and moxidectin on the nematode Caenorhabditis elegans. Veterinary Parasitology. 2009;165:96–108. doi: 10.1016/j.vetpar.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Ballivet M, Alliod C, Bertrand S, Bertrand D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. Journal of Molecular Biology. 1996;258:261–269. doi: 10.1006/jmbi.1996.0248. [DOI] [PubMed] [Google Scholar]
- Beech RN, Prichard RK, Scott ME. Genetic variability of the beta-tubulin genes in benzimidazole-susceptible and -resistant strains of Haemonchus contortus. Genetics. 1994;138:103–110. doi: 10.1093/genetics/138.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech RN, Silvestre A. Mutations associated with anthelmintic drug resistance. Anti-Infective Agents in Medicinal Chemistry. 2010;9:105–112. [Google Scholar]
- Beech RN, Wolstenholme AJ, Neveu C, Dent JA. Nematode parasite genes, what’s in a name? Trends in Parasitology. 2010;26:334–340. doi: 10.1016/j.pt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Berriman M. The Haemonchus contortus sequencing project. 2009a http://www.sanger.ac.uk/Projects/H_contortus/
- Berriman M. The Teladorsagia circumcincta sequencing project. 2009b http://www.sanger.ac.uk/Projects/H_contortus/
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature, London. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A, Fuentes FF. Mutational dynamics of microsatellites. Molecular Biotechnology. 2010;44:250–266. doi: 10.1007/s12033-009-9230-4. [DOI] [PubMed] [Google Scholar]
- Blackhall WJ, Prichard RK, Beech RN. P-glycoprotein selection in strains of Haemonchus contortus resistant to benzimidazoles. Veterinary Parasitology. 2008;152:101–107. doi: 10.1016/j.vetpar.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature, London. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proceedings of the National Academy of Sciences, USA. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguinat C, Ardelli BF, Pion SD, Kamgno J, Gardon J, Duke BO, Boussinesq M, Prichard RK. P-glycoprotein-like protein, a possible genetic marker for ivermectin resistance selection in Onchocerca volvulus. Molecular and Biochemical Parasitology. 2008;158:101–111. doi: 10.1016/j.molbiopara.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Cabaret J. Pro and cons of targeted selective treatment against digestive-tract strongyles of ruminants. Parasite. 2008;15:506–509. doi: 10.1051/parasite/2008153506. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhao W, Lu Y, Wang J, Chen Y, Li H, Zhou M. High-throughput detection of highly benzimidazole-resistant allele E198A with mismatch primers in allele-specific real-time polymerase chain reaction. Pest Management Science. 2009;65:413–419. doi: 10.1002/ps.1691. [DOI] [PubMed] [Google Scholar]
- Conder GA, Jen LW, Marbury KS, Johnson SS, Guimond PM, Thomas EM, Lee BL. A novel anthelmintic model utilizing jirds, Meriones unguiculatus, infected with Haemonchus contortus. Journal of Parasitology. 1990;76:168–170. [PubMed] [Google Scholar]
- Conder GA, Johnson SS, Guimond PM, Cox DL, Lee BL. Concurrent infections with the ruminant nematodes Haemonchus contortus and Trichostrongylus colubriformis in jirds, Meriones unguiculatus, and use of this model for anthelmintic studies. Journal of Parasitology. 1991a;77:621–623. [PubMed] [Google Scholar]
- Conder GA, Johnson SS, Guimond PM, Geary TG, Lee BL, Winterrowd CA, Lee BH, DiRoma PJ. Utility of a Haemonchus contortus/jird (Meriones unguiculatus) model for studying resistance to levamisole. Journal of Parasitology. 1991b;77:83–86. [PubMed] [Google Scholar]
- Crowe ML. SeqDoC: rapid SNP and mutation detection by direct comparison of DNA sequence chromatograms. BMC Bioinformatics. 2005;6:133. doi: 10.1186/1471-2105-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully DF, Paress PS. Solubilization and characterization of a high affinity ivermectin binding site from Caenorhabditis elegans. Molecular Pharmacology. 1991;40:326–332. [PubMed] [Google Scholar]
- Cvilink V, Lamka J, Skalova L. Xenobiotic metabolizing enzymes and metabolism of anthelminthics in helminths. Drug Metabolism Reviews. 2009;41:8–26. doi: 10.1080/03602530802602880. [DOI] [PubMed] [Google Scholar]
- de Lourdes Mottier M, Prichard RK. Genetic analysis of a relationship between macrocyclic lactone and benzimidazole anthelmintic selection on Haemonchus contortus. Pharmacogenetics and Genomics. 2008;18:129–140. doi: 10.1097/FPC.0b013e3282f4711d. [DOI] [PubMed] [Google Scholar]
- Dent JA, Smith MM, Vassilatis DK, Avery L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proceedings of the National Academy of Sciences, USA. 2000;97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DA, Scott ME, Halpenny C, Stothard JR, Prichard RK. Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Neglected Tropical Diseases. 2009;3:e397. doi: 10.1371/journal.pntd.0000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, Dean E, Reilly E, Bergholz E, Chalfie M. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. Journal of Cell Biology. 1989;109:2993–3003. doi: 10.1083/jcb.109.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drudge JH, Szanto J, Wyant ZN, Elam GW. Field studies on parasite control of sheep: comparison of thiabendazole, ruelene and phenothiazine. American Journal of Veterinary Research. 1964;25:1512–1518. [PubMed] [Google Scholar]
- Fauvin A, Charvet C, Issouf M, Cortet J, Cabaret J, Neveu C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Molecular and Biochemical Parasitology. 2010;170:105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Feng XP, Hayashi J, Beech RN, Prichard RK. Study of the nematode putative GABA type-A receptor subunits: evidence for modulation by ivermectin. Journal of Neurochemistry. 2002;83:870–878. doi: 10.1046/j.1471-4159.2002.01199.x. [DOI] [PubMed] [Google Scholar]
- Forrester SG, Prichard RK, Beech RN. A glutamate-gated chloride channel subunit from Haemonchus contortus: expression in a mammalian cell line, ligand binding, and modulation of anthelmintic binding by glutamate. Biochemical Pharmacology. 2002;63:1061–1068. doi: 10.1016/s0006-2952(02)00852-3. [DOI] [PubMed] [Google Scholar]
- Galazzo D. MSc thesis. McGill University; Montreal, Canada: 2004. A comparison of laboratory and field resistance to macrocyclic lactones in Haemonchus contortus. [Google Scholar]
- Garg R, Yadav CL. Genotyping of benzimidazole susceptible and resistant alleles in different populations of Haemonchus contortus from Himalayan and sub-Himalayan regions of North-West India. Tropical Animal Health and Production. 2008;41:1127–1131. doi: 10.1007/s11250-008-9292-5. [DOI] [PubMed] [Google Scholar]
- Ghedin E, Wang S, Spiro D, Caler E, Zhao Q, Crabtree J, Allen JE, Delcher AL, Guiliano DB, Miranda-Saavedra D, Angiuoli SV, Creasy T, Amedeo P, Haas B, El-Sayed NM, Wortman JR, Feldblyum T, Tallon L, Schatz M, Shumway M, Koo H, Salzberg SL, Schobel S, Pertea M, Pop M, White O, Barton GJ, Carlow CK, Crawford MJ, Daub J, Dimmic MW, Estes CF, Foster JM, Ganatra M, Gregory WF, Johnson NM, Jin J, Komuniecki R, Korf I, Kumar S, Laney S, Li BW, Li W, Lindblom TH, Lustigman S, Ma D, Maina CV, Martin DM, McCarter JP, McReynolds L, Mitreva M, Nutman TB, Parkinson J, Peregrin-Alvarez JM, Poole C, Ren Q, Saunders L, Sluder AE, Smith K, Stanke M, Unnasch TR, Ware J, Wei AD, Weil G, Williams DJ, Zhang Y, Williams SA, Fraser-Liggett C, Slatko B, Blaxter ML, Scott AL. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisi M, Kaminsky R, Maser P. Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Veterinary Parasitology. 2007;144:313–320. doi: 10.1016/j.vetpar.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Gilleard JS, Beech RN. Population genetics of anthelmintic resistance in parasitic nematodes. Parasitology. 2007;134:1133–1147. doi: 10.1017/S0031182007000066. [DOI] [PubMed] [Google Scholar]
- Grillo V, Jackson F, Cabaret J, Gilleard JS. Population genetic analysis of the ovine parasitic nematode Teladorsagia circumcincta and evidence for a cryptic species. International Journal for Parasitology. 2007;37:435–447. doi: 10.1016/j.ijpara.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Harhay MO, Horton J, Olliaro PL. Epidemiology and control of human gastrointestinal parasites in children. Expert Review of Anti Infective Therapy. 2010;8:219–234. doi: 10.1586/eri.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson JE, Clark HJ, Kaplan RM, Lake SL, Matthews JB. The role of polymorphisms at beta tubulin isotype 1 codons 167 and 200 in benzimidazole resistance in cyathostomins. International Journal for Parasitology. 2008;38:1149–1160. doi: 10.1016/j.ijpara.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Hoglund J, Gustafsson K, Ljungstrom BL, Engstrom A, Donnan A, Skuce P. Anthelmintic resistance in Swedish sheep flocks based on a comparison of the results from the faecal egg count reduction test and resistant allele frequencies of the beta-tubulin gene. Veterinary Parasitology. 2009;161:60–68. doi: 10.1016/j.vetpar.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Quarterly Reviews in Biology. 2009;84:131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- James CE, Davey MW. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. International Journal for Parasitology. 2009;39:213–220. doi: 10.1016/j.ijpara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- James CE, Hudson AL, Davey MW. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends in Parasitology. 2009;25:328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Jones AK, Davis P, Hodgkin J, Sattelle DB. The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Invertebrate Neuroscience. 2007;7:129–131. doi: 10.1007/s10158-007-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, Weber SS, Wenger A, Wieland-Berghausen S, Goebel T, Gauvry N, Pautrat F, Skripsky T, Froelich O, Komoin-Oka C, Westlund B, Sluder A, Maser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature, London. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- Kenyon F, Greer AW, Coles GC, Cringoli G, Papadopoulos E, Cabaret J, Berrag B, Varady M, Van Wyk JA, Thomas E, Vercruysse J, Jackson F. The role of targeted selective treatments in the development of refugia-based approaches to the control of gastrointestinal nematodes of small ruminants. Veterinary Parasitology. 2009;164:3–11. doi: 10.1016/j.vetpar.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Kopp SR, Coleman GT, Traub RJ, McCarthy JS, Kotze AC. Acetylcholine receptor subunit genes from Ancylostoma caninum: altered transcription patterns associated with pyrantel resistance. International Journal for Parasitology. 2009;39:435–441. doi: 10.1016/j.ijpara.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Kotze AC, Dobson RJ, Tyrrell KL, Stein PA. High-level ivermectin resistance in a field isolate of Haemonchus contortus associated with a low level of resistance in the larval stage: implications for resistance detection. Veterinary Parasitology. 2002;108:255–263. doi: 10.1016/s0304-4017(02)00200-5. [DOI] [PubMed] [Google Scholar]
- Kotze AC, Le Jambre LF, O’Grady J. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Veterinary Parasitology. 2006;137:294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Molecular Pharmacology. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Kwa MSG, Jetty VS, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in β-tubulin isotype 1. Molecular and Biochemical Parasitology. 1994;63:299–303. doi: 10.1016/0166-6851(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Le Jambre LF. Ivermectin-resistant Haemonchus contortus in Australia. Australian Veterinary Journal. 1993;70:357. doi: 10.1111/j.1751-0813.1993.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhou Y, Wang ZQ, Lu G, Zheng H, Brindley PJ, McManus DP, Blair D, Zhang QH, Zhong Y, Wang S, Han ZG, Chen Z. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature, London. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ. An electrophysiological preparation of Ascaris suum pharyngeal muscle reveals a glutamate-gated chloride channel sensitive to the avermectin analogue, milbemycin D. Parasitology. 1996;112:247–252. doi: 10.1017/s0031182000084833. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Clark CL, Trailovic SM, Robertson AP. Oxantel is an N-type (methyridine and nicotine) agonist not an L-type (levamisole and pyrantel) agonist: classification of cholinergic anthelmintics in Ascaris. International Journal for Parasitology. 2004;34:1083–1090. doi: 10.1016/j.ijpara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Pennington AJ. A patch-clamp study of effects of dihydroavermectin on Ascaris muscle. British Journal of Pharmacolology. 1989;98:747–756. doi: 10.1111/j.1476-5381.1989.tb14602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCavera S, Rogers AT, Yates DM, Woods DJ, Wolstenholme AJ. An ivermectin-sensitive glutamate-gated chloride channel from the parasitic nematode Haemonchus contortus. Molecular Pharmacology. 2009;75:1347–1355. doi: 10.1124/mol.108.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCavera S, Walsh TK, Wolstenholme AJ. Nematode ligand-gated chloride channels: an appraisal of their involvement in macrocyclic lactone resistance and prospects for developing molecular markers. Parasitology. 2007;134:1111–1121. doi: 10.1017/S0031182007000042. [DOI] [PubMed] [Google Scholar]
- Merino G, Real R, Baro MF, Gonzalez-Lobato L, Prieto JG, Alvarez AI, Marques MM. Natural allelic variants of bovine ATP-binding cassette transporter ABCG2: increased activity of the Ser581 variant and development of tools for the discovery of new ABCG2 inhibitors. Drug Metababolism and Disposition. 2009;37:5–9. doi: 10.1124/dmd.108.022715. [DOI] [PubMed] [Google Scholar]
- Mes TH. Purifying selection and demographic expansion affect sequence diversity of the ligand-binding domain of a glutamate-gated chloride channel gene of Haemonchus placei. Journal of Molecular Evolution. 2004;58:466–478. doi: 10.1007/s00239-003-2569-4. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Lezhava A, Kawai Y, Kikuchi T, Oguchi-Katayama A, Kogo Y, Itoh M, Miyagi T, Takakura H, Hoshi K, Kato C, Arakawa T, Shibata K, Fukui K, Masui R, Kuramitsu S, Kiyotani K, Chalk A, Tsunekawa K, Murakami M, Kamataki T, Oka T, Shimada H, Cizdziel PE, Hayashizaki Y. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nature Methods. 2007;4:257–262. doi: 10.1038/nmeth1007. [DOI] [PubMed] [Google Scholar]
- Molento MB. Parasite control in the age of drug resistance and changing agricultural practices. Veterinary Parasitology. 2009;163:229–234. doi: 10.1016/j.vetpar.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Molento MB, Wang GT, Prichard RK. Decreased ivermectin and moxidectin sensitivity in Haemonchus contortus selected with moxidectin over 14 generations. Veterinary Parasitology. 1999;86:77–81. doi: 10.1016/s0304-4017(99)00131-4. [DOI] [PubMed] [Google Scholar]
- Munoz C, Gomez Talquenca S, Volpe ML. Tetra primer ARMS-PCR for identification of SNP in beta-tubulin of Botrytis cinerea, responsible of resistance to benzimidazole. Journal of Microbial Methods. 2009;78:245–246. doi: 10.1016/j.mimet.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Neveu C, Charvet C, Fauvin A, Cortet J, Beech R, Cabaret J. Genetic diversity of levamisole receptor subunits in parasitic nematodes and abbreviated transcripts associated with resistance. Pharmacogenetics and Genomics. 2010;20:414–425. doi: 10.1097/FPC.0b013e328338ac8c. [DOI] [PubMed] [Google Scholar]
- Njue AI, Prichard RK. Genetic variability of glutamate-gated chloride channel genes in ivermectin-susceptible and -resistant strains of Cooperia oncophora. Parasitology. 2004;129:741–751. doi: 10.1017/s0031182004006183. [DOI] [PubMed] [Google Scholar]
- Palcy C, Silvestre A, Sauve C, Cortet J, Cabaret J. Benzimidazole resistance in Trichostrongylus axei in sheep: Long-term monitoring of affected sheep and genotypic evaluation of the parasite. Veterinary Journal. 2008;183:68–74. doi: 10.1016/j.tvjl.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Puttachary S, Robertson AP, Clark CL, Martin RJ. Levamisole and ryanodine receptors. II: An electrophysiological study in Ascaris suum. Molecular and Biochemical Parasitology. 2010;171:8–16. doi: 10.1016/j.molbiopara.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB Journal. 2006;20:2606–2608. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- Qian H, Robertson AP, Powell-Coffman JA, Martin RJ. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB Journal. 2008;22:3247–3254. doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VT, Siddiqui SZ, Prichard RK, Forrester SG. A dopamine-gated ion channel (HcGGR3*) from Haemonchus contortus is expressed in the cervical papillae and is associated with macrocyclic lactone resistance. Molecular and Biochemical Parasitology. 2009;166:54–61. doi: 10.1016/j.molbiopara.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Redman E, Grillo V, Saunders G, Packard E, Jackson F, Berriman M, Gilleard JS. Genetics of mating and sex determination in the parasitic nematode Haemonchus contortus. Genetics. 2008a;180:1877–1887. doi: 10.1534/genetics.108.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman E, Packard E, Grillo V, Smith J, Jackson F, Gilleard JS. Microsatellite analysis reveals marked genetic differentiation between Haemonchus contortus laboratory isolates and provides a rapid system of genetic fingerprinting. International Journal for Parasitology. 2008b;38:111–122. doi: 10.1016/j.ijpara.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Robertson AP, Bjorn HE, Martin RJ. Resistance to levamisole resolved at the single-channel level. FASEB Journal. 1999;13:749–760. doi: 10.1096/fasebj.13.6.749. [DOI] [PubMed] [Google Scholar]
- Robertson AP, Clark CL, Burns TA, Thompson DP, Geary TG, Trailovic SM, Martin RJ. Paraherquamide and 2-deoxyparaherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. Journal of Pharmacology and Experimental Therapeutics. 2002;302:853–860. doi: 10.1124/jpet.102.034272. [DOI] [PubMed] [Google Scholar]
- Robertson AP, Clark CL, Martin RJ. Levamisole and ryanodine receptors. I: A contraction study in Ascaris suum. Molecular and Biochemical Parasitology. 2010;171:1–7. doi: 10.1016/j.molbiopara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaghi M, Uhlen M, Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- Rufener L, Kaminsky R, Maser P. In vitro selection of Haemonchus contortus for benzimidazole resistance reveals a mutation at amino acid 198 of beta-tubulin. Molecular and Biochemical Parasitology. 2009a;168:120–122. doi: 10.1016/j.molbiopara.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Rufener L, Maser P, Roditi I, Kaminsky R. Haemonchus contortus acetylcholine receptors of the DEG-3 subfamily and their role in sensitivity to monepantel. PLoS Pathogens. 2009b;5:e1000380. doi: 10.1371/journal.ppat.1000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragoza PA, Modir JG, Goel N, French KL, Li L, Nowak MW, Stitzel JA. Identification of an alternatively processed nicotinic receptor alpha7 subunit RNA in mouse brain. Brain Research Molecular Brain Research. 2003;117:15–26. doi: 10.1016/s0169-328x(03)00261-4. [DOI] [PubMed] [Google Scholar]
- Schwenkenbecher JM, Albonico M, Bickle Q, Kaplan RM. Characterization of beta-tubulin genes in hookworms and investigation of resistance-associated mutations using real-time PCR. Molecular and Biochemical Parasitology. 2007;156:167–174. doi: 10.1016/j.molbiopara.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Schwenkenbecher JM, Kaplan RM. Real-time PCR assays for monitoring benzimidazole resistance associated mutations in Ancylostoma caninum. Experimental Parasitology. 2009;122:6–10. doi: 10.1016/j.exppara.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Silvestre A, Cabaret J. Mutation in position 167 of isotype 1 beta-tubulin gene of Trichostrongylid nematodes: role in benzimidazole resistance? Molecular and Biochemical Parasitology. 2002;120:297–300. doi: 10.1016/s0166-6851(01)00455-8. [DOI] [PubMed] [Google Scholar]
- Silvestre A, Humbert JF. Diversity of benzimidazole-resistance alleles in populations of small ruminant parasites. International Journal for Parasitology. 2002;32:921–928. doi: 10.1016/s0020-7519(02)00032-2. [DOI] [PubMed] [Google Scholar]
- Silvestre A, Sauve C, Cortet J, Cabaret J. Contrasting genetic structures of two parasitic nematodes, determined on the basis of neutral microsatellite markers and selected anthelmintic resistance markers. Molecular Ecology. 2009;18:5086–5100. doi: 10.1111/j.1365-294X.2009.04421.x. [DOI] [PubMed] [Google Scholar]
- Sotirchos IM, Hudson AL, Ellis J, Davey MW. Thioredoxins of a parasitic nematode: comparison of the 16- and 12-kDA thioredoxins from Haemonchus contortus. Free Radical Biology and Medicine. 2008;44:2026–2033. doi: 10.1016/j.freeradbiomed.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Varady M, Corba J, Letkova V, Kovac G. Comparison of two versions of larval development test to detect anthelmintic resistance in Haemonchus contortus. Veterinary Parasitology. 2009;160:267–271. doi: 10.1016/j.vetpar.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Von Samson-Himmelstjerna G, Blackhall WJ, McCarthy JS, Skuce PJ. Single nucleotide polymorphism (SNP) markers for benzimidazole resistance in veterinary nematodes. Parasitology. 2007;134:1077–1086. doi: 10.1017/S0031182007000054. [DOI] [PubMed] [Google Scholar]
- Von Samson-Himmelstjerna G, Walsh TK, Donnan AA, Carriere S, Jackson F, Skuce PJ, Rohn K, Wolstenholme AJ. Molecular detection of benzimidazole resistance in Haemonchus contortus using real-time PCR and pyrosequencing. Parasitology. 2009;136:349–358. doi: 10.1017/S003118200800543X. [DOI] [PubMed] [Google Scholar]
- Walsh TK, Donnan AA, Jackson F, Skuce P, Wolstenholme AJ. Detection and measurement of benzimidazole resistance alleles in Haemonchus contortus using real-time PCR with locked nucleic acid Taqman probes. Veterinary Parasitology. 2007;144:304–312. doi: 10.1016/j.vetpar.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Webster LM, Johnson PC, Adam A, Mable BK, Keller LF. Absence of three known benzimidazole resistance mutations in Trichostrongylus tenuis, a nematode parasite of avian hosts. Veterinary Parasitology. 2008;158:302–310. doi: 10.1016/j.vetpar.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Williamson SM, Robertson AP, Brown L, Williams T, Woods DJ, Martin RJ, Sattelle DB, Wolstenholme AJ. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathogens. 2009;5:e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Research. 2001;29:E88–8. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]