Abstract
NMR paramagnetic relaxation enhancement experiments (PREs) have been applied to the intrinsically disordered protein α-synuclein, the primary protein in Parkinson’s disease, to directly characterize transient intermolecular complexes at neutral and low pH. At neutral pH, we observe weak N- to C-terminal inter-chain contacts that are driven by electrostatic interactions while at low pH, C- to C-terminal inter-chain interactions are significantly stronger and driven by hydrophobic contacts. Characterization of these first inter-chain interactions will provide fundamental insight into the mechanism of amyloid formation.
Protein aggregation and amyloid fibril formation are associated with a wide range of neurodegenerative diseases including Alzheimer’s, Parkinson’s and Prion disease1. Despite numerous studies on protein aggregation, the mechanism by which proteins convert from their normally soluble form to insoluble amyloid fibrils is still not well understood. Amyloid fibril formation for α-synuclein (αSyn), the primary protein component in Parkinson’s disease2 begins from an ensemble of heterogeneous intrinsically disordered conformations. To date, solution NMR has played an important role in characterizing the monomeric intrinsically disordered αSyn thereby beginning to define the starting point for aggregation3. However, the next steps in the mechanism of αSyn aggregation have not yet been characterized experimentally as these early self-associated species are transient and exist at very low populations. Here we report the use of NMR paramagnetic relaxation enhancement (PRE) experiments to provide a direct visualization of transient inter-chain contacts and describe the earliest events in the self-assembly of these intrinsically disordered proteins (IDP).
αSyn that like other IDPs is characterized by a low sequence complexity, low overall hydrophobicity and high net charge4. The charged residues are unevenly distributed within the sequence (Fig. 1a) and result in a net charge of −9 at neutral pH. Aggregation rates are very sensitive to pH and have been shown to be slower at neutral pH than at low pH5. We compare the transient inter-chain interactions observed at neutral and low pH to understand the role of the distribution of hydrophobicity and charge in driving the aggregation process.
Figure 1.
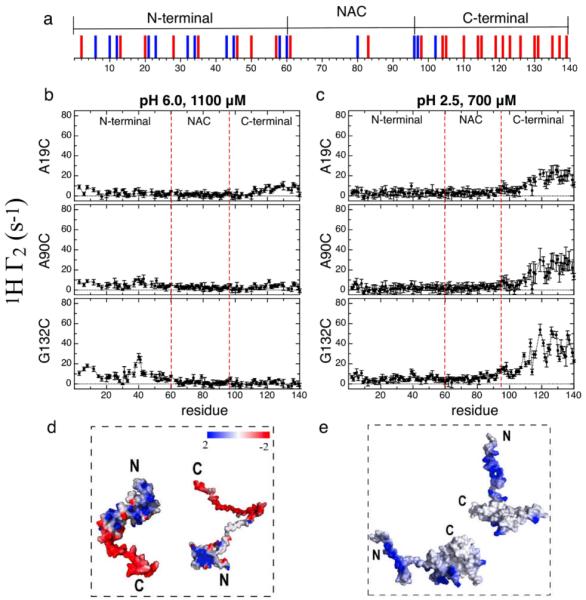
Transient encounter complexes in the intrinsically disordered protein αSyn. The distribution of positively and negatively charged residues is shown with blue and red bars respectively (a). Inter-chain NMR PRE profiles for intrinsically disordered αSyn at pH 6.0 (b) and pH 2.5 (c). 1H Γ2 values with MTSL labels at A19C, A90C and G132C are shown for both sets of conditions. Models that illustrate the inter-chain interactions observed from the PRE experiments at neutral (d) and low pH (e). Monomer conformations were selected from the REMD ensemble generated at neutral and low pH in ref. 5b and are shown as electrostatic surface potentials. The surface potential was calculated by Delphi7 and the color gradient from red to blue indicates surface potential from −2 to 2 kT/e.
It has recently been shown that NMR PREs can be used to detect transient lowly populated encounter complexes for native state protein-protein and protein-DNA interactions6. Here we apply these experiments for the first time to IDP to determine transient long-range inter-chain contacts in αSyn at neutral and low pH5b. By mixing samples that contain a 1:1 mixture of 15N labeled αSyn and 14N labeled αSyn with the MTSL spin label, the broadening of the signal in the 15N labeled chain will be limited only to the residues that are interacting with the MTSL on the 14N labeled chain thereby detecting only inter-chain interactions (Supp. Fig. S1, S2).
The comparison of the neutral (pH 6.0) and low pH (pH 2.5) PRE experiments (Fig.1) indicates that the location of the transient inter-chain contacts are very different under conditions in which the total number of charged residues changes from 39 to 15. In addition, the PRE effects are significantly weaker at neutral pH (Fig. 1b) despite the fact that the concentration is 1.6 times greater than at low pH (Fig. 1c). αSyn can be divided into three regions4d-f consisting of an N-terminal region (residues 1-60) with a net charge of +4 at pH 7, a central hydrophobic region (NAC) (residues 61-95) which is proposed to be primarily responsible for aggregation and which forms the core of the amyloid fibrils and a C-terminal region (residues 96-140) which is highly acidic (net charge of −12) (Fig. 1a). At neutral pH, when the paramagnetic spin label is placed at the negatively charged C-terminus (G132C) there are inter-chain interactions between G132C and the N-terminal residues 3-15 and 35-50; when the spin label is placed at the N-terminus there are interactions between A19C and a broad region of the C-terminus including residues 110-140, as well as with a narrower range of residues, 10-15, at the N-terminus. This profile suggests that the charged C- to N-terminal inter-chain interactions dominate. Interestingly, when the spin label is placed in the more hydrophobic NAC region (residue A90C) the interactions between the NAC and the rest of the protein are minimal despite the fact that the NAC is thought to play a key role in initiating aggregation.
The inter-chain PRE profile at low pH shows stronger PRE effects than at neutral pH consistent with stronger and possibly shorter-range inter-chain interactions (Fig. 1c). At low pH the strongest interactions are between the C-terminal G132C and the essentially neutralized C-terminal end of another chain. When the spin label is placed at A90C in the NAC region, the interactions to the C-terminal end are weaker and when the spin label is placed at A19C, the interactions to the C-terminal are still weaker (Fig. 1c). The lack of symmetry between the stronger N-(A19C) to C-terminal interactions and the extremely weak C-(G132C) to N-terminal interactions may arise from the differences in the relative orientations of the spin labels at these sites due to differences in conformation. Similarly to the neutral pH state, NAC regions do not interact with one another.
The NMR PRE profiles represent an ensemble of encounter complexes that arise from interactions between disordered monomer ensembles in solution. Changes in solution conditions including concentration, viscosity of the solvent and ionic strength alter the magnitude of self-association of αSyn rather than the location of the interactions, as monitored by the strength of the PRE interaction (Fig. S3-S5). Solvent PRE effects6b-c have been ruled out for low and neutral pH from concentration dependent experiments (Fig. S4). At neutral pH, when the ionic strength of the solution is decreased, the strength of the interactions increases significantly supporting the fact that electrostatics are the basis of the interaction between the inter-chain N- and C- terminal ends (Fig. S4). These data under different solution conditions suggest that despite the heterogeneity of the monomer conformational ensembles in the intrinsically disordered protein, the encounter complex ensembles appear to have a non-random distribution of interactions.
We present a cartoon representation of a possible set of transient encounter complexes within the ensembles at neutral and low pH that highlight the strongest interactions in the PRE experiment (Fig. 1d, 1e). The weaker interactions seen in fig. 1b and 1c exist in the encounter complex ensembles but are not depicted here as their populations may be low. To portray the encounter complexes we have selected representative structures from the heterogeneous conformational ensembles of the monomer that were calculated previously5b. The conformations of the monomer have been shown to be more compact than expected for a random coil at both neutral and low pH.3c-f,5b-d At neutral pH, the monomer conformation highlights the largely extended, highly negatively charged C-terminal tail, and the more self-interacting N-terminal and NAC regions.3c-f The picture of two monomers interacting in a head to tail arrangement with the NAC region non-interacting is most consistent with the weak inter-chain interactions between the complementary charged N- and C-terminal regions (Fig. 1d). The weakness of these interactions may be due to the fact that the highly charged sequence would prefer to be solvated rather than interacting with another highly charged monomer. These preferences would suggest that αSyn has been evolved to be a relatively poor aggregator at pH 6.0.
In contrast to the highly charged neutral pH sequence, at low pH the percentage of charged residues decreases from 40 to 6.7. Previously we5b and others5c-d have reported that at low pH the C-terminus is primarily collapsed onto itself and the NAC region, rather than extended and solvated as seen at neutral pH. Using a representative conformation from the low pH REMD conformational ensemble as a starting point5b, the strong inter-chain C- to C-terminal contacts along with the lack of N- to N-terminal interactions in the PRE experiment can be represented by two monomers arranged with the C-terminal ends in contact while the N-terminal ends remain, on average, further apart (Fig. 1e). The suggested configuration optimizes both hydrophobic interactions in the C-terminal and charge repulsion between the N-terminal ends. In addition, the lack of NAC to NAC contacts are consistent with the optimized configuration as charge repulsion between the N terminal ends may make it difficult for the NAC regions to interact. The weak head to tail contacts driven by electrostatics at neutral pH versus the stronger tail to tail contacts driven by hydrophobicity at low pH highlight the importance of the distribution of charge and hydrophobicity in directing inter-chain interactions. The difference in the nature of the inter-chian interactions at the two pHs may be related to the faster fibril formation at low pH. 5b-d
There is increasing evidence that the early intermediates in the misfolding process may be more toxic than the final aggregates1, therefore characterizing the interactions that define the initial steps of amyloid formation in αSyn are of particular importance. At neutral pH, where the charged residues dominate the αSyn sequence at both the N- and C-terminal ends, the anti-parallel head to tail inter-chain interactions, under physiological conditions in vitro, are very weak. In living cells, similar anti-parallel contacts to those seen in vitro have been observed using biomolecular fluorescence complementation8 The results presented here show that 1H NMR paramagnetic relaxation experiments are a powerful tool for visualizing transient lowly populated initial encounter complexes in intrinsically disordered proteins. These experiments can be extended to other IDPs such Aβ in Alzheimer’s disease or Tau in Parkinson’s disease to understand the basic principles of self-assembly in aggregation or amyloid formation.
Supplementary Material
Acknowledgement
We thank Ron Levy, David Talaga, Sheena Radford, Seho Kim and Daniel Weinstock for helpful discussions. This work has been supported by NIH grants GM45302 and GM087012 and NSF grants DBI-0403062 and DBI-0320746 to JB.
Footnotes
Supporting Information Available: experimental details and figures S1-S6. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- (1).Chiti F, Dobson CM. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- (2).Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Proc Natl Acad Sci U S A. 1998;95:6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Mittag T, Forman-Kay JD. Curr. Opinion Struct. Biol. 2007;17:3–14. doi: 10.1016/j.sbi.2007.01.009. [DOI] [PubMed] [Google Scholar]; (b) Wright PE, Dyson HJ. Curr. Opinion Struct. Biol. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Eliezer D. Curr. Opinion Struct. Biol. 2009;19:23–30. doi: 10.1016/j.sbi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wu K-P, Kim S, Fela DA, Baum J. J Mol Biol. 2008;378:1104–15. doi: 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. J. Am. Chem. Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]; (f) Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Proc Natl Acad Sci U S A. 2005;102:1430–5. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Uversky VN, Gillespie JR, Fink AL. Proteins. 2000;41:415–27. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- (5).(a) Uversky VN, Li J, Fink AL. J Biol Chem. 2001;276:10737–44. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]; (b) Wu K-P, Weinstock DS, Narayanan C, Levy RM, Baum J. J Mol Biol. 2009;391:784–796. doi: 10.1016/j.jmb.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cho MK, Nodet G, Kim HY, Jensen MR, Bernado P, Fernandez CO, Becker S, Blackledge M, Zweckstetter M. Protein Sci. 2009;18:1840–6. doi: 10.1002/pro.194. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McClendon S, Rospigliosi CC, Eliezer D. Protein Sci. 2009;18:1531–40. doi: 10.1002/pro.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Iwahara J, Clore GM. Nature. 2006;440:1227–30. doi: 10.1038/nature04673. [DOI] [PubMed] [Google Scholar]; (b) Tang C, Iwahara J, Clore GM. Nature. 2006;444:383–6. doi: 10.1038/nature05201. [DOI] [PubMed] [Google Scholar]; (c) Tang C, Louis JM, Aniana A, Suh JY, Clore GM. Nature. 2008;455:693–6. doi: 10.1038/nature07342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rocchia W, Alexov E, Honig B. J Phys Chemi B. 2001;105:6507–6514. [Google Scholar]
- (8).Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ. PLoS ONE. 2008;3:e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


