Abstract
We present 1HN, 15N, 13Cα, 13Cβ and 13C′ assignments and 15N transverse relaxation rates (R2) of a Parkinson’s disease-related intrinsically disordered protein, α-synuclein, in the presence of 2 M (360 g/liter) glucose solution.
Keywords: Synuclein, intrinsically disordered protein, glucose, NMR resonance assignments
Biological context
Human α-synuclein (αSyn) is the primary protein component of the Lewy body deposits found in Parkinson’s disease (PD). Aggregation of αSyn into fibrils is thought to play an important role in PD and the mechanism of conversion from soluble αSyn to amyloid fibril is not well understood. αSyn (UnitProtKB/SwissProt entry ID: P37840) is an intrinsically disordered protein (IDP) composed of 140 amino acids with 3 defined regions: an N-terminal region (residues 1–60), a central hydrophobic NAC (non-amyloid β component) region (residues 61–95) and a highly negatively charged C-terminal region (residues 96–140). Establishing the conformation and dynamics of the intrinsically disordered protein under different solution conditions will help elucidate the mechanism of fibril formation. To date, extensive NMR studies of αSyn in water have shown transient helical conformation in the N-terminal region and weak long-range transient tertiary N- to C-terminal intramolecular interactions (Bisaglia et al. 2009) at physiological pH. Recently, we have used NMR paramagnetic relaxation enhancement to determine the existence of weak N- to C-terminal inter-chain contacts of αSyn that are driven primarily by electrostatic interactions at neutral pH (Wu and Baum 2010).
In the cell, macromolecules can reach very high concentrations (Serber et al. 2005) and viscous or macromolecular solutes have been used to model the effects of crowding. It has been shown that the intrinsically disordered protein FlgM gains helical structure in the presence of high concentrations of glucose (2.5 M) (Dedmon et al. 2002) and recent reports by White et. al indicate that the growth kinetics of fibrillar proteins in crowded environments (e.g. poly (ethlyne glycol), glucose) are significantly accelerated (White et al. 2010). This implies that viscous or crowded environments may enhance or stabilize the secondary structures of IDP and may also induce self-association (Shtilerman et al. 2002; Uversky et al. 2002).
Here we describe the backbone assignments, secondary structure propensities and relaxation dynamics of αSyn in 2 M (360g/L) glucose solution in order to determine the effect of increased viscosity on these parameters and to begin to provide information that may be related to the molecular behavior of αSyn inside the cell.
Methods and experiments
Expression and purification of uniformly 15N-labeled αSyn and [15N/13C]-labeled αSyn were carried out as described previously (Wu et al. 2009). 2 M glucose was dissolved in PBS buffer at pH 7.4 (Sigma Inc., MO) for NMR experiments (Wu and Baum 2010). Backbone assignments of ~300 μM doubly labeled αSyn were carried out using 15N-15N connections using the 3D HNN spectrum (complex points: 1024:H (F3)/100:N (F2) /60:N (F1)) (Panchal et al. 2001) and then were verified by 13Cα-13Cα, 13Cβ-13Cβ and 13C′-13C′ connections using paired triple resonance 3D experiments [HNCACB (1024:H/104:C/60:N), CBCA(CO)NH (1024:H/84:C/48:N)] and [HNCO (1024:H/104:C /72:N), HN(CA)CO (1024:H /88:C/44:N)] (Ferentz and Wagner 2000). DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) was used to directly calibrate the 1H chemical shifts; calibrations of 13C and 15N chemical shifts were calculated according to the gyromagnetic ratios (Wishart et al. 2002). Interleaved transverse relaxation rate (R2) experiments of αSyn in 2 M glucose solution were acquired using the CPMG (Carr-Purcell-Meiboom-Gill) pulse train (Farrow et al. 1994) and the relaxation delay times and recycle delays were 10, 30, 50, 70, 90, 130, 170, 210 and 250 milliseconds and 2 seconds, respectively. Dried αSyn was dissolved in 2 M glucose in the presence of 20–30 % D2O in order to avoid crystallization of glucose when acquiring NMR data at 15°C. All NMR spectra were acquired on a Varian 800 MHz spectrometer at 15°C, processed using NMRPipe (Delaglio et al. 1995) and analyzed using Sparky (Goddard and Kneller). Secondary structural propensities (SSP) were calculated using SSP developed by the Forman-Kay group (Marsh et al. 2006).
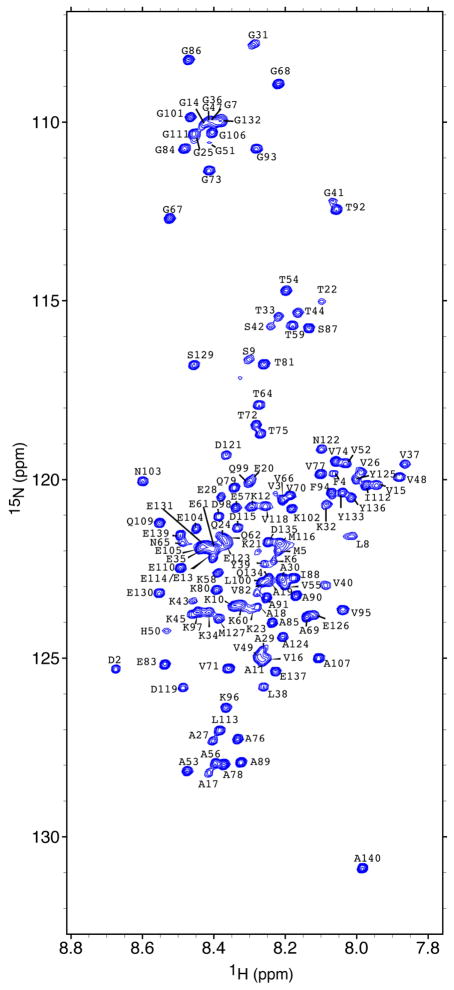
Assignments, data deposition and dynamics
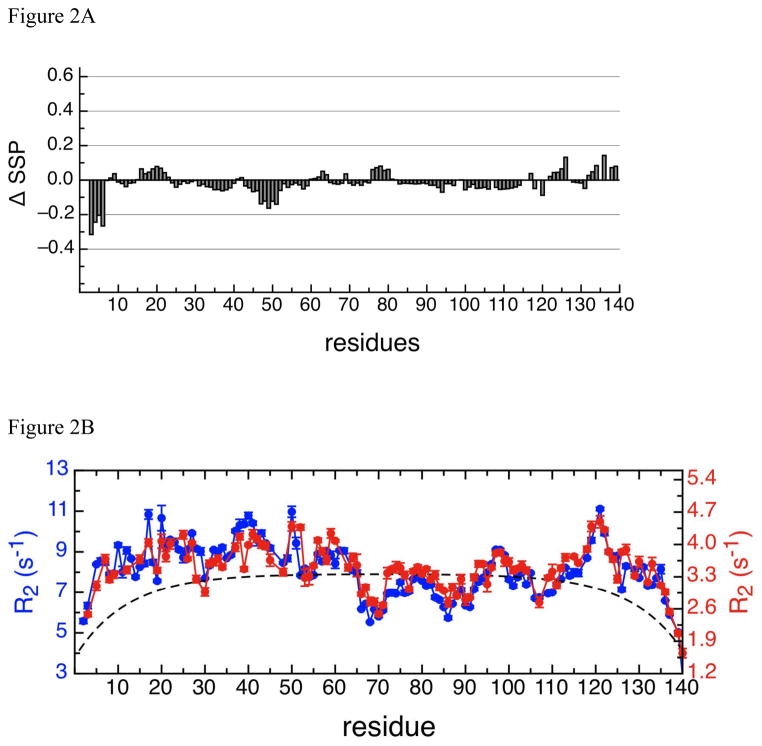
We have measured the translational diffusion coefficients of glucose from 1 mM to 2.5 M in our previous study to determine the changes of solvent viscosity (Wu and Baum 2010). The viscosity of 2 M glucose solution is approximately 3.5 times that of 1 mM glucose solution. The 1H-15N HSQC spectrum of αSyn in 2 M glucose solution at 15°C (figure 1) shows the narrow chemical shift dispersion in the 1H dimension that is typical of intrinsically disordered proteins. 1H and 15N assignments for all 135 non-proline amide protons except Met1 are assigned in the 1H-15N HSQC spectrum and indicated in figure 1. The 3D HNN spectrum was used primarily for sequential assignments and the 13C-13C connections were used to confirm the assignments. A total of 97 % of the 13Cα, 98% of the 13Cβ, and 90% of the 13C′ chemical shifts were assigned. Comparison of the secondary structural propensities (SSP) of αSyn between the viscous solution in 2 M glucose and the dilute solution in PBS indicates that the C-terminal and NAC regions show very small differences in SSP values, as the difference is close to zero, and that the N-terminal region at residues 30 to 60 shows slightly greater differences on the order of -0.1 (Figure 2a). Overall the SSP difference spectrum shows that the secondary structure propensities are similar to one another and that the viscous glucose solution does not appear to alter the conformation relative to water.
Figure 1.
1H-15N HSQC spectrum of αSyn in 2 M (360 g/L) glucose and PBS buffer (10 mM Na2HPO4/NaH2PO4, 137 mM NaCl, and 2.7 mM KCl) at 15 °C obtained at a 1H frequency of 800 MHz. Assigned residues are labeled with the 1-letter amino acid code.
Figure 2.
Figure 2A:
The SSP (Marsh et al. 2006) difference scores of αSyn in dilute solution and in viscous solution. 13Cα and 13Cβ chemical shifts were used to calculate the SSP score for αSyn and reference offsets were also checked by the SSP program. ΔSSP was calculated according to the formula: ΔSSP =SSP (glucose) – SSP (PBS), where SSP in phosphate buffer saline was calculated previously (Wu et al. 2009).
Figure 2B:
Relaxation dynamics of αSyn in dilute solution (10 mM MES, 100 mM NaCl, pH 6) at 15°C (Wu et al. 2009) ( ) and in viscous solution at 15°C (
) and in viscous solution at 15°C ( ) show very similar trends although the magnitudes are very different. Left (colored in blue) and right (colored in red) Y-axis indicates the scales of the R2 rates in viscous and in dilute solutions, respectively. The R2 rates of αSyn in 2 M glucose solution are approximately 2.4-fold greater than the R2 rates of αSyn in dilute solution. The dashed line is the theoretical R2 values of an 140-aa random coil protein calculated as previously described (Wu et al. 2008).
) show very similar trends although the magnitudes are very different. Left (colored in blue) and right (colored in red) Y-axis indicates the scales of the R2 rates in viscous and in dilute solutions, respectively. The R2 rates of αSyn in 2 M glucose solution are approximately 2.4-fold greater than the R2 rates of αSyn in dilute solution. The dashed line is the theoretical R2 values of an 140-aa random coil protein calculated as previously described (Wu et al. 2008).
1H-15N NMR relaxation experiments have been performed on wild type αSyn and mutants in order to define the dynamics of the intrinsically disordered state (Wu et al. 2008). Here we investigate the effects of increased viscosity on the dynamics by performing 1H-15N R2 relaxation experiments of αSyn in 2 M glucose solution (Figure 2b). Comparison to published 15N R2 values of αSyn in dilute solution at 15°C (Wu et al. 2009) indicates that the R2 profile is identical under viscous and non-viscous conditions but that the overall magnitude of the R2 values for each residue is increased. The average increase in R2 is approximate 2.6 times while the viscosity elevates approximately 3.5 times in the presence of 2 M glucose. These data suggest that the tumbling time of the protein is affected by the glucose environment but that the local dynamics do not change; the five clusters of restricted motion that were identified in water (Wu et al. 2009) remain intact in glucose. The relaxation data combined with the secondary structure propensity data indicate that the conformation and dynamics of αSyn in the highly viscous 2 M glucose solvent are very similar to those in water suggesting similar structural ensembles in both conditions. Our analysis of the monomer state in 2 M glucose can be used in combination with published literature which shows accelerated aggregation kinetics of α-synuclein in viscous or crowding solution to investigate the misfolding mechanism to amyloid fibrils.
Assigned chemical shifts and transverse relaxation rates have been deposited on Biological Magnetic Resonance Bank (http://www.bmrb.wisc.eud) with accession number 16904.
Acknowledgments
K-PW was supported by an NIH Interdisciplinary Research Workforce Fellowship (5R90DK071502). This work has been supported by NIH grant GM087012 and NSF grants DBI-0403062 and DBI-0320746 to JB.
References
- Bisaglia M, Mammi S, Bubacco L. Structural insights on physiological functions and pathological effects of alpha-synuclein. FASEB J. 2009;23:329–340. doi: 10.1096/fj.08-119784. [DOI] [PubMed] [Google Scholar]
- Dedmon MM, Patel CN, Young GB, Pielak GJ. FlgM gains structure in living cells. Proc Natl Acad Sci U S A. 2002;99:12681–12684. doi: 10.1073/pnas.202331299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Farrow NA, Muhandiram R, Singer AU, Pascal SM, Kay CM, Gish G, Shoelson SE, Pawson T, Forman-Kay JD, Kay LE. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry. 1994;33:5984–6003. doi: 10.1021/bi00185a040. [DOI] [PubMed] [Google Scholar]
- Ferentz AE, Wagner G. NMR spectroscopy: a multifaceted approach to macromolecular structure. Quarterly reviews of biophysics. 2000;33:29–65. doi: 10.1017/s0033583500003589. [DOI] [PubMed] [Google Scholar]
- Goddard TD, Kneller DG. Sparky 3. University of California, San Francisco; [Google Scholar]
- Marsh JA, Singh VK, Jia Z, Forman-Kay JD. Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci. 2006;15:2795–2804. doi: 10.1110/ps.062465306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal SC, Bhavesh NS, Hosur RV. Improved 3D triple resonance experiments, HNN and HN(C)N, for HN and 15N sequential correlations in (13C, 15N) labeled proteins: application to unfolded proteins. J Biomol NMR. 2001;20:135–147. doi: 10.1023/a:1011239023422. [DOI] [PubMed] [Google Scholar]
- Serber Z, Corsini L, Durst F, Dotsch V. In-cell NMR spectroscopy. Methods in enzymology. 2005;394:17–41. doi: 10.1016/S0076-6879(05)94002-0. [DOI] [PubMed] [Google Scholar]
- Shtilerman MD, Ding TT, Lansbury PT., Jr Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry. 2002;41:3855–3860. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- Uversky VN, EMC, Bower KS, Li J, Fink AL. Accelerated alpha-synuclein fibrillation in crowded milieu. FEBS Lett. 2002;515:99–103. doi: 10.1016/s0014-5793(02)02446-8. [DOI] [PubMed] [Google Scholar]
- White DA, Buell AK, Knowles TPJ, Welland ME, Dobson CM. Protein Aggregation in Crowded Environments. J Am Chem Soc. 2010;132:5170–5175. doi: 10.1021/ja909997e. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Case DA, Thomas L, James Dotsch V, Schmitz U. Methods in Enzymology. Academic Press; 2002. Use of chemical shifts in macromolecular structure determination; pp. 3–34. [DOI] [PubMed] [Google Scholar]
- Wu KP, Baum J. Detection of Transient Interchain Interactions in the Intrinsically Disordered Protein alpha-Synuclein by NMR Paramagnetic Relaxation Enhancement. J Am Chem Soc. 2010;132:5546–5547. doi: 10.1021/ja9105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KP, Kim S, Fela DA, Baum J. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation. Journal of molecular biology. 2008;378:1104–1115. doi: 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KP, Weinstock DS, Narayanan C, Levy RM, Baum J. Structural Reorganization of alpha-Synuclein at Low pH Observed by NMR and REMD Simulations. Journal of molecular biology. 2009;391:784–796. doi: 10.1016/j.jmb.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]