Abstract
We asked whether different forms of inhibition are altered differently by aging using a Motor and Perceptual Inhibition Test (MAPIT) based on Nassauer and Halperin (Nassauer & Halperin, 2003). Ninety-eight individuals participating in studies of balance and attention were separated into younger (mean age 25 years) and older participants (mean age 73). Older participants showed less Perceptual and Motor Inhibition than younger participant with moderation of this effect by gender. The two scores were uncorrelated in the young but significantly correlated in the older group. Overall, the MAPIT appeared to yield reliable measures of two aspects of inhibition that demonstrate a differential impact of age.
Keywords: Reaction time, executive function, reliability, human performance, processing speed, response choice
Lessened inhibition has been theoretically linked to aging, at least since the influential chapter of Hasher and Zacks (L. Hasher & Zacks, 1988). The theory is controversial, however, and has recently been updated (Lustig, Hasher, & Zacks, 2007). Most importantly from the current perspective, Lustig et al. (Lustig et al., 2007) review three types of inhibition influenced by aging: a) controlling access to attention's focus, b) deleting irrelevant information from attention and working memory, and c) suppressing or restraining strong but inappropriate responses. Their review highlights aging influences on all three of their forms of inhibition and discusses the yet unresolved issue of the relationship among these forms of inhibition. Their review of both behavioral and brain imaging results suggests that these forms of inhibition overlap, sharing a core process or set of processes. Each, however, has distinctive features that may vary for specific tasks and due to individual differences. An analogy might be drawn to intelligence that is commonly thought to have verbal and performance aspects that are correlated, but also have distinctive features and predictivity to other variables.
The inhibitory deficit view is not without its critics (Burke & Osborne, 2007; McDowd, 1997). These critics point out difficulties showing inhibitory deficits in particular tasks, e.g., negative priming tasks, and also note the importance of disentangling basic sensory and response speed deficits from presumed inhibitory deficits. The latter concern has been addressed to some extent. In a well examined sample, Christ and colleagues (Christ et al., 2001; McAuley et al., 2006) addressed this concern and established that the inhibitory deficit in the older participants could not be explained by response slowing. They went on to show with ex-Gaussian analysis that inhibitory limitations in older adults differed in quality from those in children.
Considerably more attention has been paid to whether inhibition is a unitary construct and whether aging is characterized by a decline in all forms of inhibition or only a decline in particular forms of inhibition. The critics noted above clearly suggest that the aging deficit isn't unitary, i.e. a single process that can be shown to be deficit across task domains. Empirically, the critics review evidence of lack of a deficit for what we would term perceptual inhibition with verbal processing tasks (as well as irrelevant information in such tasks). Although the suppression of prepotent responses (motor inhibition) was not as thoroughly critiqued, early studies, e.g., (Kramer, Humphrey, Larish, & Logan, 1994) demonstrated aging related deficits in this inhibitory function, but also suggested that this inhibitory process might not be a single, unitary process based on low correlations among different inhibitory tasks. More recently, Friedman and Miyake (Friedman & Miyake, 2004) argued that low correlations were not sufficient proof of independent processes and proposed analysis of the latent structure of correlations between multiple inhibitory tasks using structural equation modeling. Their analyses suggested two factors. One factor was labeled Prepotent Response Inhibition—combining tasks related to resistance to interference as well. The other, largely independent factor was Resistance to Proactive Inhibition. Interestingly, negative priming—a task frequently said to require inhibition, failed to relate to either of these factors. In short, their results suggested that different types of inhibition could be empirically defined, would relate differently to other tasks, and could clarify relationships obscured by the diffuseness of concept of inhibition. Their paper as well as that of Germaine and Collette (Germain & Collette, 2008) provide a more thorough review of current competing taxonomies of inhibition and evidence for these.
Different forms of inhibition might be expected to `age' differently if we accepting the concept of inhibition as function that may be implemented differently in different task contexts. Stated differently, variability in the degree of aging deficit might be expected between different tasks. For example, the inhibition of prepotent responses may be differently influenced by age than the controlling of access to attention or resisting proactive interference. Furthermore, even in the presence of maintained inhibitory capability, aging can impact physiological processes, e.g., sensory processing, that may result in weaker representations that would then be more readily inhibited than representions in a younger person with intact sensory function. On the other hand, a communality of aging deficits across inhibition tasks might be expected due to general effects of aging such as response slowing, e.g., (Salthouse & Salthouse, 2000) or dedifferentiation of abilities, e.g. (Li et al., 2004). There is no reason to suspect that inhibitory functions would be immune to general slowing or show a communality of effect as tasks are processed more similarly in older participants. Based on these arguments, one would expect aging effects that might have both a common effect as well as specific effects across tasks.
Currently we examine aging, as well as gender, as another approach to assessing the communality and specificity of inhibitory function. If factors such as age and gender have indistinguishable effects on tasks presumed to require inhibitory function than a common process or set of processes can be inferred. In contrast, differential effects of factors such as age or gender on different inhibitory tasks suggest specificity. Nassauer and Halperin (Nassauer & Halperin, 2003) recently provided a tool for investigating inhibition. This new tool may be used to better understand the relationship of inhibition and other factors such as aging because differences in the tasks other than the inhibition required are controlled, i.e., the different forms of inhibition are assessed within the context of very similar stimulus and response demands. Differences in stimuli/responses and over task requirements have confounded prior attempts to isolate different forms of inhibition. Via a structured set of reaction time (RT) tasks, Nassauer and Halperin (Nassauer & Halperin, 2003) were able to show that perceptual inhibition could be disassociated from motor inhibition. Perceptual inhibition was operationally defined in a RT task as the ability to resist the tendency to push a response key that is spatially compatible with the location of the stimulus, i.e. a task similar to that introduced by Simon (xxx). Motor inhibition was operationally defined as the ability to resist the tendency to push the response key on the side toward which the arrow pointed, when responding on the opposing side was required. Using additive factor logic (Sternberg, 1969), Nassauer and Halperin demonstrated in a young sample that perceptual and motor inhibition were separable processes as their effects were additive—not interactive. In the taxonomy introduced by Lustig et al. (Lustig et al., 2007), perceptual inhibition would relate to controlling access to attention while motor inhibition would relate to suppressing or restraining strong, but inappropriate, responses although both would seem related to the Resistance to Prepotent Responses of Friedman and Miyake (Friedman & Miyake, 2004).
Accepting the separability of these forms of inhibition raises the question of whether a decline in inhibition with aging will be in one or both of these processes. Very recently, Germain and Collette (Germain & Collette, 2008) asked this question using exactly the task sequence proposed by Nassauer and Halperin (Nassauer & Halperin, 2003). Their results suggest a communality of inhibition across the perceptual and motor tasks that were more evident in older relative to younger participants. Concurrently as part of a program of research on aging, attention, and balance, we adapted the Nassauer and Halperin techniques to yield measures of perceptual and motor inhibition (Mendelson, Redfern, Nebes, & Jennings, submitted; Redfern, Jennings, Mendelson, & Nebes, In Revision). We termed our adaptation of the Nassauer and Halperin approach the Motor and Perceptual Inhibition Test—MAPIT. We then asked whether relatively younger participants differed from older participants for these scores and whether perceptual and motor inhibition continued to be separable processes among an elderly group. Our hypothesis was that inhibitory ability would decline with age, and that perceptual and motor inhibition would show differential decline. We further tested the internal consistency reliability of our difference scores as a partial test of their suitability as individual difference measures. Relative to the Germain and Collette report (Germain & Collette, 2008) our sample is somewhat larger, covers a larger age range, and is carefully characterized for their sensory capabilities.
Methods
Participants
Scores were available from older and college-aged adults taking part in different experiments composing a research program relating psychological factors to balance and vestibular function. Participants were recruited from the community using mailings and word of mouth. For older participants, most had been entered into a registry of individuals interested in participating in research. Younger participants were college students or immediate acquaintances of these students. Table 1 shows the participant characteristics. All subjects were screened for cognitive, sensory, and musculoskeletal health. Cognition was evaluated with a mini-mental status examination (MMSE) paradigm cutoff of less than 24. Vestibular function was evaluated through caloric and rotational vestibular testing (Furman & Cass, 1996). Clinical Dynamic Posturography (Equitest, Inc) was also performed, with subjects required to perform within age-adjusted clinical norms. In addition, a neurologist performed a neurological examination. Subjects were also excluded if they had: 1) a medical history including neurological conditions, vestibular dysfunction, psychiatric, or cardiac conditions, 2) musculoskeletal dysfunction affecting prolonged standing (arthritis, contractures, orthopedic implants, muscle weakness), 3) binocular visual acuity (with corrective lenses) worse than 20/40, 5) hearing loss, or 6) somatosensory neuropathy. Cutaneous pressure threshold was determined using Semmes-Weinstein monofilaments, with an exclusionary cut-off of 5.07. All participants provided informed consent for their original investigation and de-identified data were used for the current project. Original and current studies were both approved by the University of Pittsburgh Institutional Review Board.
Table 1.
Description of the sample of younger and older participants.
| Young | Old | |
|---|---|---|
| N | 47 | 51 |
| Age (sd) | 24.8 (3.3) | 73.3 (4.8) |
| Number female (percent) | 32 (63%) | 28 (50%) |
| Years of Education | 16.0(1.1) | 14.9 (3.9) |
| Simple RT (ms) | 345(32) | 437(69) |
| Motor Congruous RT (ms) | 340(38) | 436(62) |
| Perceptual Congruous RT (ms) | 432(71) | 549(93) |
| Perceptual Incongruous RT(ms) | 443(68) | 594(108) |
| Motor Incongruous RT(ms) | 385(56) | 561(138) |
Procedure
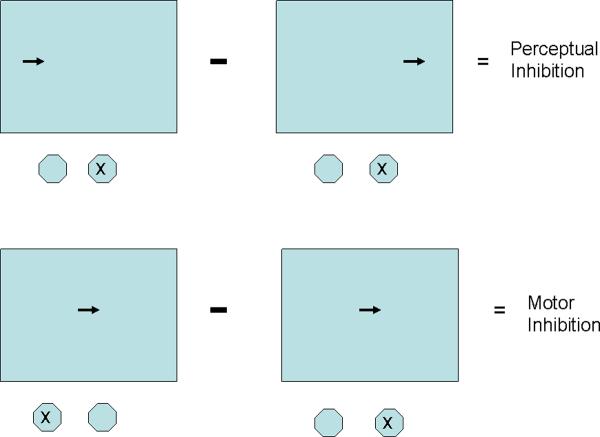
Participants were scheduled for a separate session during which the trials for the MAPIT as well as other inventories were administered. For example, 72 of the participants also were administered the RBANS (Repeatable Battery for the Assessment of Neuropsychological Status, PAR, Inc.). The MAPIT is based upon RTs to different tasks with computer screen presentation and reaction key responses. The stimuli consisted of an arrow 3.7 cm long pointing either to the right or left. Participants held the index fingers of their right and left hands on two response keys. The stimuli were presented until the participant responded. A visual fixation point, indicated by a central cross, disappeared with onset of the stimulus. Figure 1 illustrates the MAPIT tasks showing stimuli and correct responses illustrating the different conditions.
Figure 1.
Diagram illustrating the appearance of the computer screen and the correct response on the RT keys. Only tasks used in the inhibition score correlations are shown. In the Perceptual Inhibition comparison, all trials use the same stimulus-response mapping—respond on the key consistent with the direction of the arrow, but the arrow may appear on the side of the screen either congruent or incongruent with the direction of the arrow. In the Motor Inhibition comparison, arrows are always in mid screen, but for incongruent blocks the instruction is to respond in the opposite direction from the direction the arrow points while in congruent blocks the response is in the same direction. See text for details on the exact procedures for collecting the RTs and the task order.
For the tasks used to assess Perceptual Inhibition (PI), a right- or left-pointing arrow appeared 8.5 cm to the right or left of the central fixation. The participant was instructed to press the button on the side toward which the arrow pointed. There were two conditions: Congruous and Incongruous. In the Congruous condition, the spatial location of the arrow (i.e., the side of the screen on which it appeared) was the same as the direction the arrow pointed (e.g., a left-pointing arrow appeared to the left of fixation). In the Incongruous condition, the location of the arrow conflicted with the direction it pointed (e.g., a right-pointing arrow appeared to the left of fixation). In this condition, the participant had to inhibit processing the arrow's location, focusing only on the direction it pointed. There were 40 congruous and 40 incongruous trials randomly intermixed. Prior to this experimental block, a block of unscored trials reinforced the prepotent spatial response: subjects respond to a rectangle on the left or right of the screen on the spatially congruent key. Note that for both perceptually congruent and incongruent trials the participant always responded on the key congruent with the direction in which the arrow pointed. The participant's median reaction time (RT) and accuracy were determined for each condition.
The tasks used to assess Motor Inhibition were presented in separate blocks of trials in which the arrow always appeared in the center of the screen. In one block (Incongruous) subjects were instructed to respond on the key incompatible with the direction which the arrow pointed; right and left points arrows were randomly interspersed with the constraint that 40 of each direction appeared (20 trials with each arrow direction were presented within the 40 trial set). Thus, in this condition the participant must inhibit an over-learned spatially compatible response in order to make a response that is spatially incompatible with the presented stimulus. A comparison condition (Congruous) used in assessing both perceptual and motor inhibition consisted of two 40 trial blocks. For these blocks, arrows were centered and the response required was always compatible with the direction which the arrow pointed. The procedures followed as closely as possible those of Nassauer and Halperin (Nassauer & Halperin, 2003) with the single exception that we omitted a block in which trials of perceptual and motor interference items were intermixed.
As a control for sensorimotor speed, a simple reaction time task was also administered in which the participant pressed the button held in the dominant hand as soon as a 1 cm dot appeared in the center of the computer screen. The interstimulus interval was preset and varied randomly between 1.5 and 3 seconds so that the participant could not anticipate stimulus onset.
The tasks were presented in a fixed order: simple RT, 40 trial set for motor congruous/comparison, 80 trials set for perceptual congruous/incongruous, second 40 trial set for motor congruous/comparison, and 40 trial set for motor incongruous. Fixed presentation permits a valid assessment of individual differences; varying order would confound order and individual inhibitory measures. With the exception of the simple RT task, all tasks used an interval between the completion of the RT and the appearance of the next stimulus of 1.5 s.
Median RTs for each participant for each task were calculated. Medians were used instead of means to minimize the influence of large outlier RTs in some participants. Individuals that had less than 2/3rds of their responses correct on any of the tasks were excluded from analyses based on a concern that they may not have understood the tasks (3 participants were not included for this reason). The motor inhibition (MI) and perceptual inhibition (PI) scores were derived as follows:
| (1) |
| (2) |
The PI score was further broken down into components that would reflect potentially separable performance on the perceptually congruous and incongruous trials. PI scores are derived from the block of trials requiring both congruous and incongruous RTs. Performance overall on this block would be expected to be slowed by the mixing of stimuli with and without spatial incongruity. The PI score controls for this overall slowing, but not for any speeding of RT on the congruous trials due to the spatial compatibility of stimulus and response. Possible speeding due to congruity can be assessed by comparing congruous RTs from this block to control/comparison conditions in which the arrow stimulus was presented at the center of the screen, i.e., a neutral condition from the perspective of perceptual congruity. Thus, these scores adjust for the impact of spatial placement speeding congruous trials, but do so using neutral trials from a different block. Added slowing due to perceptually incongruous RTs from the mixed blocked can be similarly evaluated by comparing incongruous RTs to the same neutral trials from a different block (see similar score in Nassauer and Halperin (Nassauer & Halperin, 2003). Thus, two further perceptual inhibition related scores were created, note that the first of these captures not inhibition but is a facilitation score reflecting congruous presentation:
| (3) |
| (4) |
Statistical computations were uniformly performed with Statistica (Statsoft, Tulsa, OK).
Results
Perceptual and Motor Inhibition Performance
Analyses focused on the difference scores assessing inhibition, i.e. degree of interference. Table 1 shows the characteristics of the participants and the mean of the median RTs for each experimental condition. An initial analysis of variance assessed age and gender differences in the PI and MI scores. These scores were entered as a repeated measures factor. Age group and gender were between subject factors. Significant effects were found for Age group (F(1,94)=26.6, p<.001), PI vs MI (F(1, 94)=52.2, p<.001), and Age group by PI vs MI (F(1,94)=8.8, p=.003). Comparisons computed on these means showed that the MI score was significantly greater than the PI score for both young and old groups, and that age groups differed significantly on both scores (See means in Table 2 relevant to this and the next analyses).
Table 2.
Perceptual and Motor Inhibition scores for the young and older subjects. Mean and (standard deviation).
| Age Group | MI(ms) | MI P(C) | PI(ms) | PI P(C) | PFc(ms) | PFcP(C) | PII(ms) | PIIP(C) |
|---|---|---|---|---|---|---|---|---|
| Young | 44(43) | 99.6(1.9) | 10(27) | 98.5(3.6) | 91(53) | 98.0(4.3) | 101(45) | 99.5(3.0) |
|
| ||||||||
| Old | 125(98) | 98.8(4.2) | 45(55) | 97.5(5.4) | 113(72) | 96.3(7.2) | 158(76) | 98.8(4.7) |
Note. P(C) is percent correct. MI is motor inhibition. PI is perceptual inhibition is PFC is reaction time to the congruous items in the perceptual inhibition block relative to control RTs. PII is reaction time to the incongruous items in the perceptual inhibition block relative to control RTs.
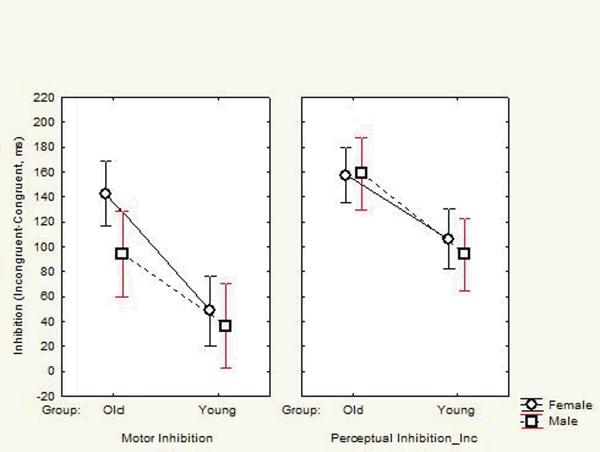
The source of the Perceptual Inhibition difference was investigated though analysis of variance with Age, Gender, and the PFC and PII as factors. Age showed a significant interaction with PFC vs PII (F(1,94)=12.6, p<.001). Comparisons between age groups then showed that the PI difference was primarily due to the slowing of older individuals on the perceptually incongruent RTs (PII F(1,94)=19.4, p<.001), rather than differences on the congruent RTs (PFC F(1,94)=3.8, p>.05). A final analysis of variance contrasted the MI with the PII score. In this analysis, the PII was significantly larger than the MI score (F(1,94)=58.8, p<.001) and Age, Gender, and MI vs PII showed a significant interaction (F(1,94)=3.9, p<.05). These effects as well as the significant overall Age effect are illustrated in Figure 2. Individual comparisons supported the impression from the figure; older females showed greater MI scores than older men (F(1,94)=4.9, p=.03) and they were the only group in which PII was not greater than MI (F(1,94)=1.9, ns).
Figure 2.
Mean scores of the two age groups for PIi and MI as separated by gender. Error bars show the 95% confidence intervals.
Percent correct was scored and analyzed comparably to RT. Accuracy was generally high, e.g. young subjects showed an average percent correct of 97.8 and older subjects, 96.7, a nonsignificant difference between ages. Only the main effect of PI vs. MI was significant (F(1,94)=5.7, p<.05); the congruent – incongruent difference in percent correct was 0.7 for MI and 2.1 for PI. Separate assessment of percent correct for the perceptual congruent and incongruent items showed that errors were predominantly on the perceptual incongruent items (F(1,94)=19.7, p<.001). The PI score was unrelated to corresponding percent correct scores, but MI and percent correct indices were correlated r(98)=.31, p<.01) indicating that greater interference was related to more errors.
Correlations between Perceptual and Motor Inhibition Scores
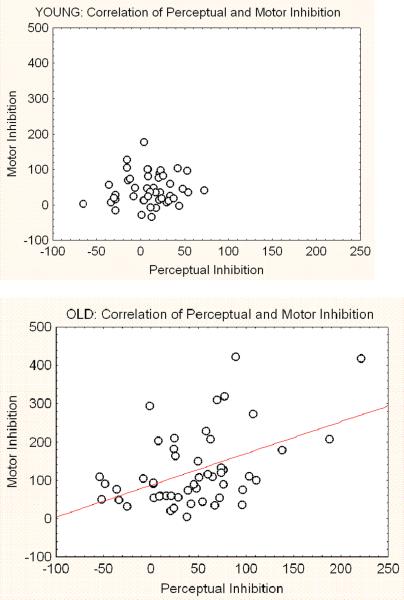
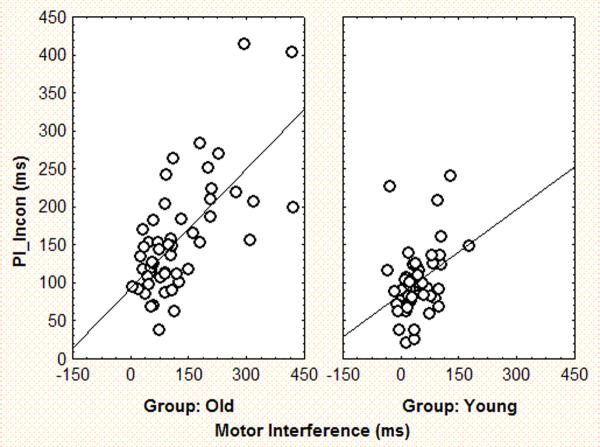
Pearson product-moment correlations were computed for the entire sample and separately by age group. PI and MI were correlated r=.51, p<.001 in the entire sample. Importantly, this correlation was due to the correlation among the older participants, r=.47, p=.001 rather than the younger participants, r=.09, ns. The correlation within the older group was significantly different (p<.05) from that in the younger group. Figure 3 shows the scatter diagrams corresponding to these results. Separate correlations of MI with the perceptual congruent (PFC) and incongruent (PII) scores were similar in the young and older adults for the congruent items (young r(47)=.26, ns; old r(51)=.36, p<.05, difference in r's ns). However, a larger correlation between MI and PII scores in the old (r(51)=.67, p<.001) than in the young (r(47)=.36, p<.01, difference in r's p<.05) was found. Figure 4 shows the scatter diagrams corresponding to this result.
Figure 3.
Scatter diagrams showing the joint distributions of Perceptual Inhibition and Motor Inhibition scores in the younger (r=.09) and older samples (r=.47). Note that the enhanced correlation in the older group appears largely due to the increased range of their inhibition scores.
Figure 4.
Scatter diagrams showing the joint distributions of Motor Inhibition and the Perceptual Incongruent score in the young (r=.36) and old (r=.67) groups.
As seen most clearly in Figure 3, older participants showed substantially more interference in both the perceptual and motor domains. This enhancement of range appears responsible for the greater correlation in the older group because excluding scores in the older group that exceeded the range of scores in the young yields a correlation of r=.12 (n=37, ns) among the remaining older participants. Excluded older participants for this analysis did not differ from the non-excluded older participants in age, gender or speed of their simple RT. They did differ in their percent correct on the MAPIT tasks; the groups showed equivalent accuracy, 98, for the congruent items, but for incongruent items percent correct was 93.1 for participants beyond the speed range relative to 96.6 for those older participants scoring in the younger group's speed range (F(1,47)=8.7, p<.001 for interaction of Congruent vs. Incongruent and In and Out of Range group). Due to the possibility that the excluded participants were showing initial evidence of dementia, we further examined neuropsychological results that were available on a portion of the participants. Among the 72 participants receiving the RBANS, 40 were in the older group and 11 had inhibition scores outside the range of the younger participants. None of the scores on the components of the RBANS differed between these 11 participants and the 29 older participants scoring within the young participants' range of scores. Thus, current cognitive status did not appear to differ between those scoring within and beyond the range of the younger sample's PI and MI scores.
Simple RT was unrelated to MAPIT scores in the young, but moderately related in older participants. In the older sample PI was correlated r=.24, p=.09 with simple RT and MI was correlated .36, p=.01. The corresponding correlations in the younger group were r=.06 and r=.02. The association of slower simple RT with poorer inhibition, however, did not moderate the correlation between PI and MI in the older group, r=.42, p=.002 when simple RT was partialled out of the correlation. The correlation in the younger group remained minimal (r=.06) when simple RT was partialled out.
Reliability of the Inhibition Scores
Internal consistency reliability statistics were computed using Cronbach's alpha index. Items to interrelate were formed by taking the median of sequential blocks of 10 trials within the corresponding congruent and incongruent trials for relevant RTs. Corresponding medians for these 10 trial blocks were then subtracted to form PI and MI scores as well as the PFC and PII scores. This scoring was used to minimize the influence between subjects of variations in missing data due to errors or outlier RT values. The resulting 4 `items' for each of the inhibition scores were then tested for internal consistency. The resulting standardized Cronbach's alpha reached acceptable levels for MI, .83 (.89 in young, .79 in old), PFC, .72 (.85 in young, .57 in old), and PII, .70 (.84 in young, .63 in old). A marginal Cronbach's alpha, .65 (.40 in young, in old, .65), was observed for the PI score.
Discussion
Age differences on the Perceptual and Motor Inhibition scores support both the concept that inhibition capability declines with age and that forms of inhibition may not be uniform—declining at different rates with age (Borella, Carretti, & De Beni, 2008; Colcombe, Kramer, Erickson, & Scalf, 2005; Kramer et al., 1994; Troyer, Leach, & Strauss, 2006). The concept that at least some forms of inhibition decline with age is well accepted (Borella et al., 2008; Grant & Dagenbach, 2000; Lynn Hasher, Lustig, & Zacks, 2007; Kane, Hasher, Stoltzfus, Zacks, & Connelly, 1994). Our results, however, support the separability of different aspects of inhibition as generally suggested by Lustig et al. (Lustig et al., 2007). We observed age differences common to both our perceptual and motor inhibition measures. In addition motor interference appeared greater than perceptual interference for the older participants when perceptual interference was defined relative to spatially congruent responses within a block with congruent and incongruent trials intermixed. When perceptual interference was defined relative to a comparison block in which responses were to a centered stimulus, then perceptual interference appeared greater than motor interference among the older men, but not the women. These interactions with type of interaction task were significantly less evident in the younger participants. In short aging and gender had different influences on perceptual and motor inhibition although the relative magnitude of perceptual and motor inhibition depended on the exact score employed. This conclusion as well as the correlations between forms of inhibition in the different age groups align closely those reported by Germain and Collette in their similar investigation (Germain & Collette, 2008). Our results suggest, but cannot yet prove, that inhibitory function is defined by a common executive control process supplemented by specific processes that are specific to the form of inhibition required, e.g., motor response processing. Aging may differentially impact the shared/common inhibitory process as evidenced by our results suggesting that the forms of inhibition are reasonably uncorrelated at a young age but significantly related in an older age group. Although our data are cross-sectional, they suggest that some individuals as they age lose some capability to cope with interference—a loss that is evident in both perceptual and motor inhibition. This interpretation is consistent with the hypothesis that mental skills in general become more highly correlated with increased age, e. g.,(Li et al., 2004).
Note that our results emphasize individual differences in the effects of aging on inhibition. Restricting the range of inhibition scores in the older group to the range in the young eliminated the correlation of Perceptual and Motor Inhibition. Older individuals with Perceptual and Motor Inhibition scores outside the range of scores in the young were responsible for the correlation with the overall older group. Given that perceptual and motor interference appeared increased in these individuals, a loss in some general process or factor common to both appears responsible. Such a loss, possibly in forebrain white matter or gray matter function (Colcombe et al., 2005), would explain our correlational results. Unfortunately, we were not able to find any other variable that distinguished individuals performing within and outside of the range of the younger group's inhibition scores: age, speed of processing, neuropsychological test performance, and gender were unrelated. Errors were greater among those outside the range, but this only established further the difficulty of inhibition for them as well as the absence of a speed-accuracy tradeoff in the data. Research identifying the common process subject to aging would be of great interest.
Our results support the reliability and discriminative validity of the Perceptual and Motor Inhibition scores based on the procedures introduced by Nassauer and Halperin (Nassauer & Halperin, 2003). The difference scores forming these separate indices showed acceptable internal consistency reliability with the exception of minimal reliability for the direct comparison of RTs from the block mixing perceptual congruent and incongruent (interfering and non-interfering) items (termed the Perceptual Inhibition score). Consideration should be given to increasing the number of trials used in this mixed block in future work. Our results suggested that older participants slowed their response to perceptually incongruent responses relative to younger individuals with the magnitude of this effect relative to motor inhibition dependent on choice of comparison items (whether congruent items from the same block or neutral items from a different block were compared). Obtaining reliable scores of both types would permit further exploration of age effects on the general (inhibitory?) slowing due to the mixing of congruent and incongruent trials in a block, see (Koch, Prinz, & Allport, 2005; Los, 1996). Given the marginal reliability of the Perceptual Inhibition score, we did not pursue this question in the current data set. Overall, the various inhibition scores differed in both their mean values and inter-correlations between age groups, providing some content validity to the distinction between perceptual and motor inhibition. Furthermore, we have found differential relationships between perceptual and motor inhibition and dual task performance in our studies of balance and aging (Mendelson et al., submitted) as well as a relationship between perceptual inhibition and balance maintenance during mild challenges (Redfern et al., In Revision).
Further investigation may be required to fully support the validity of the MAPIT test titles: perceptual inhibition and motor inhibition. Difficulty of the tasks rather than their specific perceptual or motor characteristics might distinguish the tasks. The best gauge of difficulty may be the comparison of the MI score with the PII because incongruent items are subtracted from the same control RTs. These scores are essentially equal for the young subjects but a substantial difference is seen for the older subjects. This observation supports the validity of the scores if one assumes that the motor characteristics of the motor inhibition created the enhanced difficulty in the older group. Another potential threat to validity is the fixed task order. Motor interference is assessed after perceptual interference. This influence has not been investigated. Nassauer and Halperin (Nassauer & Halperin, 2003) created the tasks based on perceptual and motor requirements and showed a differential correlation of perceptual inhibition but not motor inhibition with Stroop task performance. Further work relating other face valid tests of perceptual and motor inhibition to the MAPIT task would be useful. It remains unclear whether the nominally perceptual inhibition scores would relate to tasks without the explicit requirement for a speeded perceptual-motor response. A substantial literature on inhibition in older groups examines the influence of distraction during reading or presentation of items to remember or the inhibition in negative priming tasks (i.e. when the response to an item that was a distractor on a prior trial is slowed) (Burke & Osborne, 2007; Lustig et al., 2007). These former tasks would seem to be instances of perceptual inhibition while the latter involve inhibition of an association rather than a response. Relationships between such tasks and the Nassauer and Halperin (2003) indices would further define that nature of their measures—most particularly the validity of the perceptual and motor labels. The current research is not helpful in this regard. A related issue is the independence of the two inhibition scores. An issue already raised by Bruyer (Bruyer, 2003) by pointing out that the demonstration of independence of Nassauer and Halperin (Nassauer & Halperin, 2003) rests on additive factor assumptions and the absence of a statistical interaction. Indeed, individual differences yielded a correlation between perceptual and motor inhibition both in our sample and in Nassauer and Halperin's (Nassauer & Halperin, 2003) when data were not stratified by age. Our results strengthen to some extent the case for independence of the measures given that perceptual and motor inhibition were uncorrelated in the young sample ranging from 21 to 34 years, but significantly correlated to the older sample ranging from 65 to 82 years.
The current study has clear limitations. The results are cross-sectional so we cannot distinguish aging and cohort effects. The current sample size can contribute limited reliability and validity information on the MAPIT as a test of individual differences, but the sample is far too small to be considered normative data. Finally, the sample was composed of community dwelling individuals willing to volunteer for studies of balance regulation; this imposes a selection requirement that might bias our results in an unknown way.
Overall, the MAPIT has suggested different trajectories over age for different forms of inhibition. It appears to show acceptable reliability and validity as a test of different forms of inhibition. Further work on construct validity would be useful, however, to specify precisely what is assessed by the nominally perceptual and motor inhibition tests.
Acknowledgement
We gratefully acknowledge the support of NIH AG14116 and AG024827 and assistance from The Pittsburgh Mind-Body Center (PMBC; NIH grants HL076852/076858)
References
- Borella E, Carretti B, De Beni R. Working memory and inhibition across the adult life-span. Acta Psychologica. 2008;128(1):33–44. doi: 10.1016/j.actpsy.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Bruyer R. Are perceptual and motor inhibition processes really dissociated? A comment on Nassauer and Halperin (2003) Journal of the International Neuropsychological Society. 2003;9(5):811–812. doi: 10.1017/S1355617703950144. [DOI] [PubMed] [Google Scholar]
- Burke DM, Osborne G. Aging and inhibition deficits: Where are the effects? In: Gorfein DS, MacLeod CM, editors. Inhibition in cognition. American Psychological Association; Washington, D.C.: 2007. pp. 163–183. [Google Scholar]
- Christ SE, White DA, Mandernach T, Keys BA, Christ SE, White DA, et al. Inhibitory control across the life span. Developmental Neuropsychology. 2001;20(3):653–669. doi: 10.1207/S15326942DN2003_7. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The Implications of Cortical Recruitment and Brain Morphology for Individual Differences in Inhibitory Function in Aging Humans. Psychology and Aging. 2005;20(3):363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The Relations Among Inhibition and Interference Control Functions: A Latent-Variable Analysis. Journal of Experimental Psychology: General. 2004;133(1):101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Furman JM, Cass SP. Disorders of the Vestibular System. In: Baloh RW, Halmagyi M, editors. Laboratory testing. Electronystagmography and Rotational Testing. Oxford Press; New York: 1996. [Google Scholar]
- Germain S, Collette F. Dissociation of perceptual and motor inhibitory processes in young and elderly participants using the Simon task. Journal of the International Neuropsychological Society. 2008;14(6):1014–1021. doi: 10.1017/S135561770808123X. [DOI] [PubMed] [Google Scholar]
- Grant JD, Dagenbach D. Further considerations regarding inhibitory processes, working memory, and cognitive aging. American Journal of Psychology. 2000;113(1):69–94. [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks R, editors. Inhibitory mechanisms and the control of attention. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension, and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 22. Academic Press; New York: 1988. pp. 193–225. [Google Scholar]
- Kane MJ, Hasher L, Stoltzfus ER, Zacks RT, Connelly S. Inhibitory attentional mechanisms and aging. Psychology and Aging. 1994;9(1):103–112. doi: 10.1037//0882-7974.9.1.103. [DOI] [PubMed] [Google Scholar]
- Koch I, Prinz W, Allport A. Involuntary retrieval in alphabet-arithmetic tasks: Task-mixing and task-switching costs. Psychological Research/Psychologische Forschung. 2005;69(4):252–261. doi: 10.1007/s00426-004-0180-y. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD. Aging and inhibition: Beyond a unitary view of inhibitory processing in attention. Psychology and Aging. 1994;9(4):491–512. [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB, et al. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15(3):155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Los SA. On the origin of mixing costs: Exploring information processing in pure and mixed blocks of trials. Acta Psychologica. 1996;94(2):145–188. [Google Scholar]
- Lustig C, Hasher L, Zacks R. Inhibitory deficit theory: Recent developments in a `New view'. In: Gorfein DS, McLeod CM, editors. Inhibition in cognition. American Psychological Association; Washington, DC: 2007. pp. 145–162. [Google Scholar]
- McAuley T, Yap M, Christ SE, White DA, McAuley T, Yap M, et al. Revisiting inhibitory control across the life span: insights from the ex-Gaussian distribution. Developmental Neuropsychology. 2006;29(3):447–458. doi: 10.1207/s15326942dn2903_4. [DOI] [PubMed] [Google Scholar]
- McDowd JM. Inhibition in attention and aging. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1997;52B(6):265–273. doi: 10.1093/geronb/52b.6.p265. [DOI] [PubMed] [Google Scholar]
- Mendelson DN, Redfern MS, Nebes RD, Jennings JR. Inhibitory processes relate differently to balance/reaction time dual tasks in young and older adults. Aging, Neuropsychology, and Cognition. doi: 10.1080/13825580902914040. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassauer KW, Halperin JM. Dissociation of perceptual and motor inhibition processes through the use of novel computerized conflict tasks. Journal of the International Neuropsychological Society. 2003;9(1):25–30. doi: 10.1017/s1355617703910034. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Mendelson D, Nebes RD. Perceptual inhibition relates to sensory integration for standing postural control among older adults. doi: 10.1093/geronb/gbp060. In Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Salthouse TA. Aging and measures of processing speed. Biological Psychology. 2000;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Discovery of processing stages: Extensions of Donders' method. In: Koster WG, editor. Attention and performance II. Elsevier; Amsterdam: North Holland: 1969. pp. 276–315. [Google Scholar]
- Troyer AK, Leach L, Strauss E. Aging and Response Inhibition: Normative Data for the Victoria Stroop Test. Aging, Neuropsychology, and Cognition. 2006;13(1):20–35. doi: 10.1080/138255890968187. [DOI] [PubMed] [Google Scholar]