Abstract
Lipid peroxidation (LPO) end-product 4-hydroxynonenal (4-HNE) has been implicated in the mechanism of retinopathy. Lately it has been shown that besides being cytotoxic, 4-HNE plays an important role in oxidative stress-induced signaling. In this study, we have investigated the effect of 4-HNE on epidermal growth factor receptor (EGFR)-mediated signaling, its potential functional consequences, and the regulatory role of the 4-HNE metabolizing isozymes, glutathione S-transferase A4-4 (GSTA4-4) on this signaling in retinal pigment epithelial (RPE) cells. Our results showed that consistent with its known toxicity at relatively higher concentrations, 4-HNE induced cell death in RPE. However, at lower concentrations (as low as 0.1 μM) 4-HNE triggered phosphorylation of EGFR and activation of its downstream signaling components ERK1/2 and Akt that are known to be involved in cell proliferation. These effects of 4-HNE on EGFR could be attenuated by the over expression of GSTA4-4 that reduces intra cellular levels of 4-HNE. Our results also indicated that 4-HNE-induced activation of EGFR is a protective mechanism against oxidative stress because EGFR, MEK, and PI3K inhibitors potentiated the toxicity of 4-HNE and also inhibited wound healing in a RPE cell model. These studies suggest that as an initial response to oxidative stress, 4-HNE induces protective mechanism(s) in RPE cells through EGFR-mediated signaling.
Keywords: 4-Hydroxy-2-nonenal, Glutathione S-transferases, Epidermal growth factor, Epidermal growth factor receptor, Retinal pigment epithelium cells, Oxidative stress
Introduction
Oxidative stress-induced lipid peroxidation (LPO) has been implicated in the pathogenesis of many degenerative ocular diseases including cataractogenesis, age related macular degeneration (ARMD), and retinopathy (Bressler et al., 1988; Kowluru and Chan, 2007). Retina is particularly susceptible to oxidative stress because it is constantly exposed to reactive oxygen species (ROS) produced by UV and high-energy visible light (Sickel, 1972). Retinal pigment epithelial (RPE) cells that maintain and support the photoreceptors by phagocytosis and degradation of the photoreceptor outer segment membranes, are known to be rich in polyunsaturated fatty acids (PUFA) (Young, 1967; Tate et al., 1993; Liang and Godley, 2003). Consequently, ROS-initiated LPO of PUFA in RPE cells leads to the formation of relatively higher amounts of LPO products including relatively stable and toxic electrophiles such as 4-HNE that may contribute to retinopathy (Kapphahn, et al., 2006). Previous studies have suggested that in retina, the alpha class glutathione S-transferases (GSTs) may serve as an important defense mechanism against the toxicity of LPO products (Saneto, et al., 1982; Singh, et al., 1984). The alpha class GST isozyme, GSTA4-4, which has substrate preference for 4-HNE, has been shown to provide protection against 4-HNE-induced cataractogenesis in lens cultures (Awasthi, S. et al., 1996; Srivastata et al., 1996). An important role of GSTs as antioxidant enzymes in negating the toxicity of LPO products in ocular tissues is suggested by these and other studies (Singhal, et al., 1999; Sharma, A., et al., 2008).
In recent years, 4-HNE has been recognized as an important signaling molecule that can modulate various signaling pathways in a concentration-dependent manner (Awasthi, Y.C., et al., 2008; Chaudhary, et al., 2010; Li et al., 2006; Sharma, R., et al., 2008; Jacobs and Marnett, 2007). 4-HNE promotes cell proliferation (Sharma, R. et al., 2004; Ruef et al., 2000) at lower concentration but at higher concentration it causes necrosis and apoptosis (Li et al., 2006; Sharma, R., et al., 2008). A role of 4-HNE in signaling mechanisms in ocular tissues is indicated by studies showing that it induces apoptosis in human lens epithelial (HLE B-3) and RPE cells through the activation of the death receptor, Fas mediated pathway as well as through the pathway involving p53 (Li et al., 2006; Sharma, A. et al., 2008). It has been shown that apoptosis caused by H2O2, UV or naphthalene exposure in HLE B-3 cells is in fact mediated by 4-HNE and that it can be attenuated by GSTs (Yang, et al., 2002). Lowering of the intracellular levels of 4-HNE in HLE B-3 cells upon transfection with hGSTA4 lead to profound changes in the expression of the key cell cycle genes suggesting a role of 4-HNE in cell cycle signaling and the regulatory role of GSTA4-4 in maintaining 4-HNE homeostasis in these cells (Patrick, et al., 2005). 4-HNE has also been shown to affect the epidermal growth factor receptor (EGFR)-mediated signaling in human skin cells (Liu, et al., 1999). EGFR belongs to the ErbB membrane receptors of tyrosine kinase receptor family and plays a key role in cell cycle signaling in response to exogenous stressors (Huang, et al., 1996). In general, EGFR acts like a mitogenic stimulator that can be activated by its ligands including EGF and IGF (Bogdan and Klambt, 2001; Harris, et al., 2003; Liu, et al. 1999). Activation of EGFR by its ligand EGF can up regulate several signaling cascades in RPE cells including the activation of phosphatidylinositol 3-kinase (PI3K), and extracellular signal-regulated kinase (ERK) that are involved in cell proliferation (Defoe and Grindstaff, 2004). Available evidence suggests that 4-HNE can cause concentration dependent, ligand independent induction and activation of EGFR in some cell types (Suc, et al., 1998; Liu, et al., 1999; Negre-Salvayre, et al., 2003). Present studies were designed to systematically examine the effects of 4-HNE on EGFR-mediated signaling, its physiological significance, and the role of GSTA4-4 in regulation of this signaling in RPE cells.
Materials and Methods
Cell line
The simian virus SV40-transformed human fetal male RPE 28 cells (Coriell Institute, Camden, NJ) that exhibit epithelioid morphology and retain physiological functions characteristic of the primary human RPE cells were cultured in standard medium containing 10% fetal bovine serum and antibiotics in a humidified incubator at 37°C in 5% CO2 atmosphere as described before (Sharma, A., et al., 2008). The cells were trypsinized and passaged every 3–4 days.
Chemicals
4-HNE and the inhibitors of EGFR (AG1478), MEK (U0126) and PI3K (LY294002) were purchased from Cayman Chemical (Ann Arbor, MI). Bradford reagent, bis-acrylamide, and SDS for SDS–PAGE were obtained from BioRad (Hercules, CA). Western blot stripping buffer was from Pierce Co. (Rockford, IL). The apoptosis detection system (CaspACE FITC-VAD-FMK in situ marker) was purchased from Promega Inc. (Madison, WI). The cell culture medium DMEM, Lipofectamine 2000 transfection reagent, and fetal bovine serum were from GIBCO (Invitrogen, Carlsbad, CA). All other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Antibodies
The antibodies against EGFR (1005) sc-03, ERK1 (C-16) sc-93, p-ERK (E-4) sc-7383, GAPDH (6C5) sc-32233 were procured from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies for p-EGFR (Y1068) (3777), Akt (9272), p-Akt (Ser 473) (9271) were obtained from Cell Signaling Technology, Inc. (Boston, MA). Polyclonal antibodies developed against hGSTA4-4 in chicken have been characterized and used by us previously (Zimniak, L. et al., 1997). Horseradish peroxidase (HRP)-conjugated secondary antibodies and those against GAPDH were purchased from Southern Biotech (Birmingham, AL).
Cell viability assay
The sensitivity of the RPE against 4-HNE was measured by the 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium Bromide (MTT) assay as described by Mosmann (1983) with minor modifications. Briefly, 2 ×104 cells in 190 µl of medium were seeded in 96-well microtiter plates and allowed to attach for 24 h. The next day, 10 µl of PBS containing the desired concentration of 4-HNE was added. After 12 h incubation, 10 µl of a stock solution of MTT (5 mg/ml in PBS) was added to each well; the plates were incubated for additional 4 h at 37°C, centrifuged, and the medium was decanted. Cells were subsequently dissolved in 100 µl DMSO with gentle shaking for 2 h at room temperature, followed by measuring absorbance at 562 nm in a microplate reader (El×808 BioTek Instruments, Inc). A dose-response curve was plotted and the concentration of 4-HNE causing a 50% reduction in formazan crystal formation (IC50) was determined.
Transient transfection with hGSTA4
RPE cells at a density of 5×105 cells per 100 mm Petri dish were plated and the dishes having >70% confluent cells were used for the transfection with 24 μg of either empty pTarget-T vector (VT) or the pTarget vector containing the open reading frame (ORF) of the restored Kozak hGSTA4 sequence (hGSTA4-Tr). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) was used for transfection as per the manufacturer’s instructions.
Western Blot Analysis
The cells with or without specified treatment(s), were pelleted, washed thrice with PBS, re-suspended in RIPA lysis buffer (50 mM Tris-HCl, pH 7.5; 1% NP-40; 150 mM NaCl; 1 mg/ml aprotinin; 1 mg/ml leupeptin; 0.5 mM phenylmethylsulfonyl fluoride; 1 mM Na3VO4; 1 mM NaF) at 4°C for 30 min, and lysed by sonication. Cell debris was removed by centrifugation at 14,000 g for 30 min at 4°C to obtain clear extracts. Western blot analyses were performed with the extracts containing 25–75 µg protein as described previously (Sharma, A. et al. 2008). Protein was determined by the method of Bradford (Bradford, 1976) throughout these studies. Band intensities were compared by densitometry.
In situ caspase-3 assay for Apoptosis
RPE cells (8×104) were plated on glass cover slip in 12 well plates. Cells were treated with the desired concentrations of 4-HNE for 12 h at 37°C. Apoptotic cells were detected by staining with 5 µM Caspase FITC-VAD-FMK (Promega) in situ marker for 30 min in the dark. The slides were fixed with 4% paraformaldehyde for 30 min, rinsed thrice with PBS containing calcium and magnesium (Gibco, Invitrogen, Carlsbad, CA) for 10 min, and mounted in a medium containing 1.5 µg/ml DAPI (Vector Laboratories, Inc., Burlingame, CA). Images were taken on Olympus AX70 fluorescence microscope. Percentage of activated caspase-3 was determined as described earlier (Duan et al., 2003).
Wound healing assay
Wound healing assay was performed as described earlier (Xu and Yu, 2007). Briefly RPE cells (8×104) were plated on glass cover slip in 12 well plates. After 48 h when 100% confluence (monolayer) was reached, the cells were wounded by a scratch with a 10µl pipette tip. Wounded cells were allowed to heal for 24 h in the presence or absence of 4-HNE (5 µM), AG1478 (5 µM), and combination of both as specified. Images were taken in Nicon microscope after staining with crystal violet dye. Wound closure was measured using the NIH Image J program (http://rsbweb.nih.gov/ij/).
Statistical Analysis
Statistical analyses were performed using Student’s t-test. P-value <0.05 is considered significant. Representatives of p value in figures include “*”< 0.05 and “**” < 0.01. Analysis of variance (ANOVA) was used for analysis of cell migration in wound healing assay and for analysis of densitometries of Western Blots (n=3 in each of the experiment).
Results
Cytotoxicity of 4-HNE to RPE cells
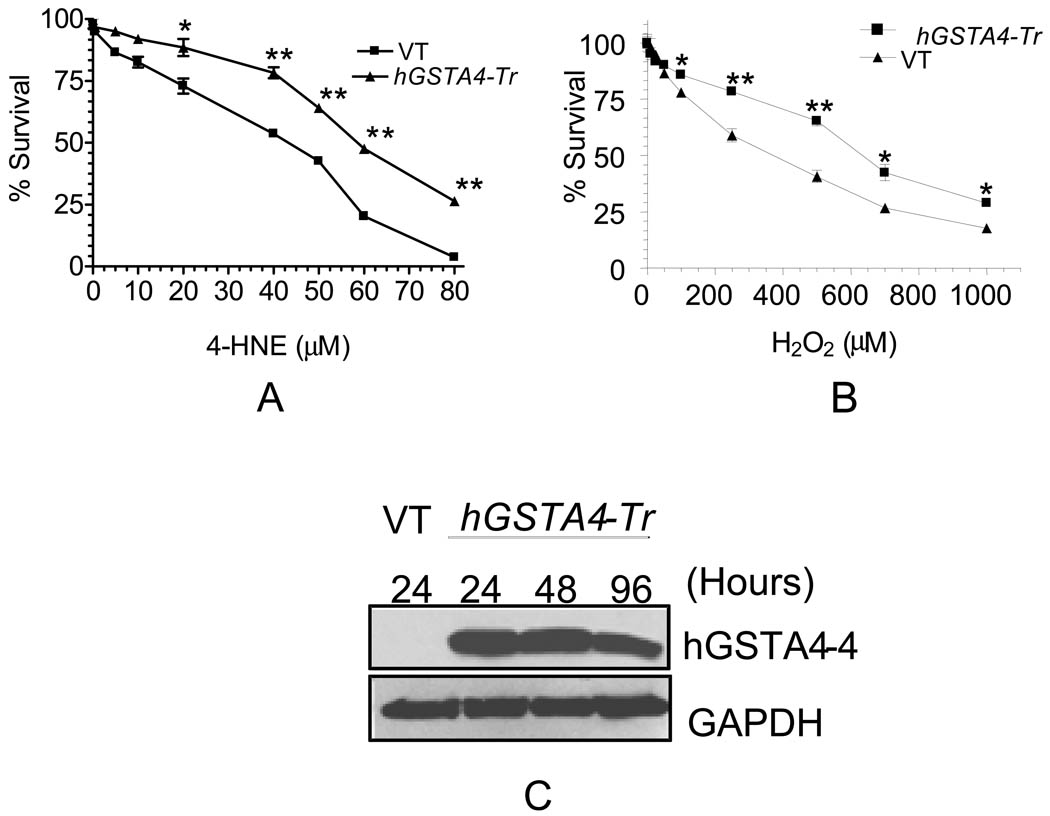
In view of the possible high accumulation of 4-HNE in RPE cells during oxidative stress, we examined the cytotoxic effects of 4-HNE on RPE cells in culture by determining the percentage of cells surviving upon exposure of varying concentrations of 4-HNE for 12 h using MTT assay. Results presented in Fig. 1A indicated that the treatment of 4-HNE significantly decreased cell viability in a dose dependent manner. At relatively lower (less than 5 µM) physiologically relevant concentrations, the toxicity of 4-HNE was only minimal but increased remarkably at higher concentrations. The IC50 value for 4-HNE was found to be 40 µM ± 3.7 that was in the similar range to that reported previously (Sharma, A., et al., 2008). 4-HNE toxicity was also examined in cells transiently transfected with hGSTA4. Cells over expressing hGSTA4-4 were significantly protected against 4-HNE toxicity (Fig. 1A) as indicated by a shift in 4-HNE IC50 value (IC50 values for the vector, and hGSTA4 transfected cells; 40 µM ± 3.7 µM, and 58 ± 4.2 µM, respectively). Cells over expressing hGSTA4-4 were also protected against H2O2 toxicity (IC50 value of H2O2, 360 ±11.4 µM for the control and 625 ± 14.8 µM for hGSTA4 transfected cells, respectively) (Fig. 1B). Since hGSTA4-4 specifically detoxifies 4-HNE and does not contribute to the detoxification of H2O2, these results suggest that H2O2 toxicity is, at least partially, mediated by 4-HNE generated by H2O2 exposure. The over expression of hGSTA4-4 in transiently transfected cells was confirmed by Western blot analysis where a robust expression of hGSTA4-4 was seen even 96 h after transfection (Fig. 1C).
Figure 1. Effect of hGSTA4 transfection on the toxicity of 4-HNE and H2O2 in RPE cells.
RPE cells were transiently transfected with empty p-Target vector (VT) and p-Target vector containing the ORF of hGSTA4 sequence (hGSTA4-Tr). VT and hGSTA4-Tr cells were treated with 0–80 µM of 4-HNE (A) or 0–1000 µM H2O2 (B) for 12 h and assayed for cytotoxicity by MTT assay. The plots show the percent cell survival (mean ± SD, n = 4) at different concentrations of 4-HNE. Over-expression of hGSTA4 in transfected cells was confirmed by western blot analysis (C).
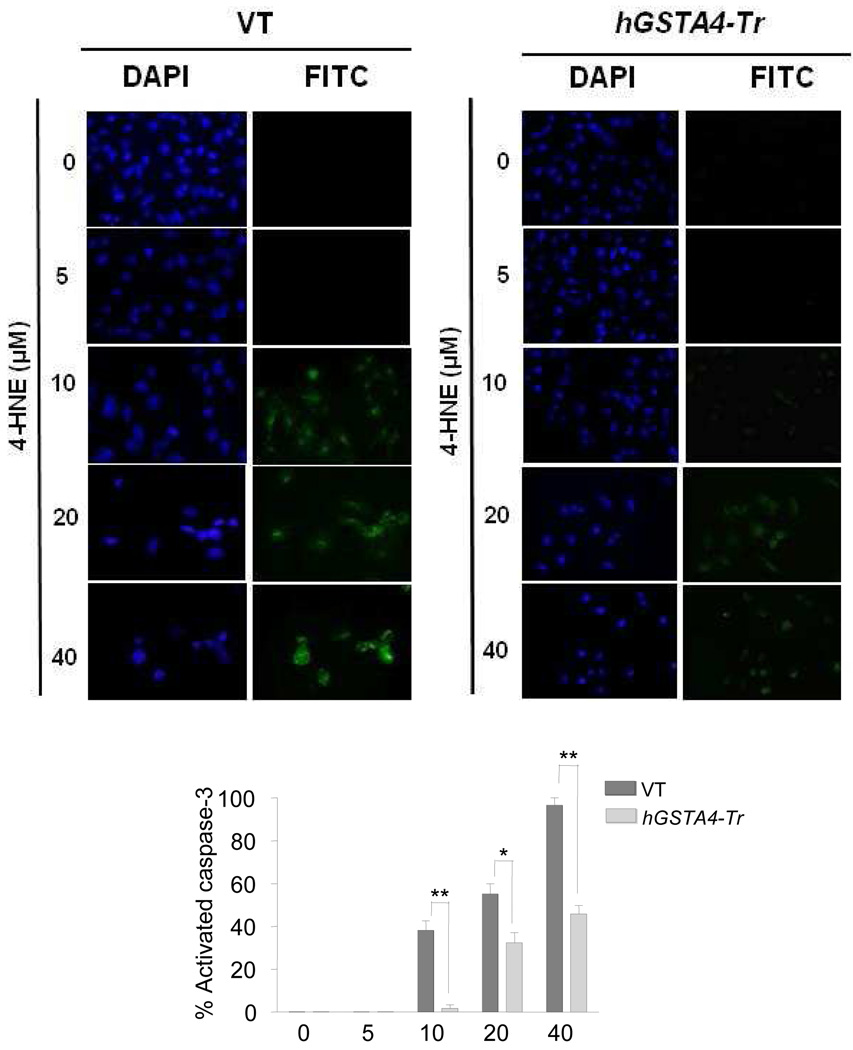
To examine whether 4-HNE toxicity to RPE cells involved the onset of apoptosis, cells treated with increasing concentrations of 4-HNE were analyzed for apoptosis by immunofluorescence using CaspACE™ FITC-VAD-FMK in situ marker. Result of these experiments showed no detectable apoptosis up to 5µM concentration of 4-HNE. There after, the percentage of apoptotic cells increased at the concentrations of 4-HNE up to 40 µM used in these experiments (Fig. 2). These results were consistent with the results in Fig. 1A showing that at concentrations up to 5 µM, 4-HNE exerts only a minimal toxicity. 4-HNE-induced apoptosis was significantly inhibited in hGSTA4 transfected cells (Fig. 2) affirming the protective role of hGSTA4-4.
Figure 2. In situ analysis of 4-HNE mediated activation of caspase-3 in VT and hGSTA4-Tr RPE cells.
RPE cells (1 × 105) were treated with 0–40 µM 4-HNE for 12 h. The activation of caspase-3 in these cells was examined by staining with 10 µM CaspACE™ FITC-VAD-FMK in situ marker according to the manufacturer’s instructions. The slides were mounted with Vectashield DAPI mounting medium and observed under a fluorescence microscope (Olympus) using the standard filter sets for DAPI and FITC. Percentage of caspase-3 activated cells presented in bar graph was determined as described previously (Duan et al., 2003). The data represent the mean ± SD (n =3).
4-HNE activates EGFR mediated signaling in RPE cells
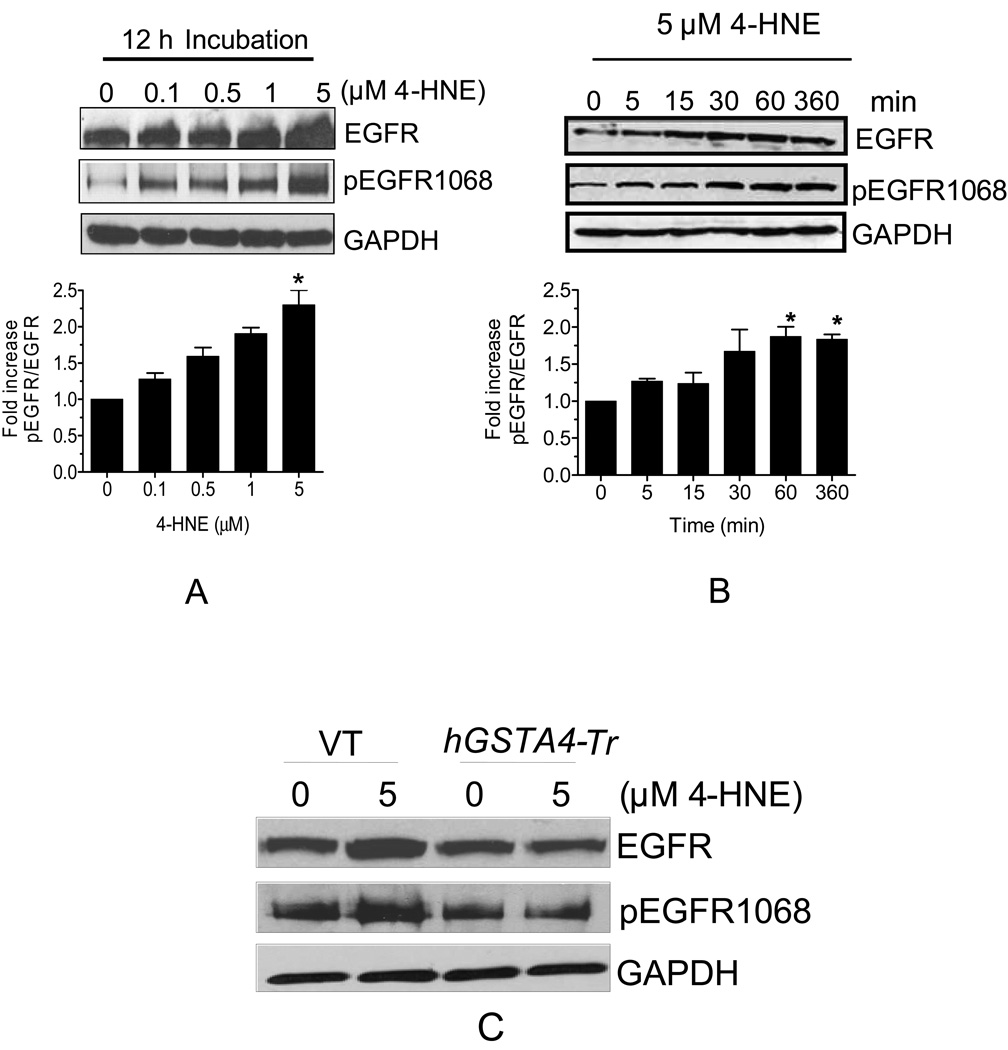
Four different isoforms of EGFR viz. EGFR1-4 (ErbB1-4) have been identified in various human cells (Ullrich, et al., 1984; Bargmann, et al., 1986; Plowman, et al., 1990, 1993; Kraus, et al., 1989). We examined the expression of these isoforms of EGFR in RPE cells. Result of Western blot analysis indicated that EGFR1 was the predominant isoform in RPE cells (Fig. 3A) and other isoforms were not detected under these conditions. These results were confirmed by immunofluorescence studies in which the isoforms ErbB2-4 were not detected (negative data for EGFR2-4 not presented). It was remarkable that the treatment of RPE cells even with 0.1 µM 4-HNE led to the induction of EGFR1. This induction of EGFR1 was dose and time dependent for 4-HNE concentrations of up to 5 µM and there after it leveled off (Fig. 3 A). Our results showing the induction of EGFR1 by 4-HNE well below the concentrations at which it is toxic suggest that 4-HNE causes the activation of EGFR1 in cells even before any toxic manifestations of oxidative stress. Phosphorylation of Ser and Thr residues present in the cytoplasmic domain of EGFR1 is one of the potential mechanisms for its activation. Therefore, we compared the phosphorylation status of Thr 1068 residue of EGFR1 in the control and 4-HNE treated cells. 4-HNE caused phosphorylation of EGFR1 at Thr 1068 in a time and concentration dependent manner (Fig. 3A and B) for concentrations up to 5 µM. We also compared the expression of EGFR1 in 4-HNE treated empty vector and hGSTA4 transfected cells. In hGSTA4 transfected cells, 4-HNE-induced activation and phosphorylation of EGFR1 was significantly inhibited as compared to the vector transfected cells (Fig. 3C). The intracellular levels of 4-HNE in hGSTA4 transfected cells are lower as compared to control cells (Sharma, A., et al., 2008). Our result showed that the basal level of phospho-EGFR1 was significantly lower in hGSTA4 transfected cells as compared to that of vector transfected cells suggesting that hGSTA4-4 inhibited activation of EGFR1 by lowering the intracellular concentration of 4-HNE.
Figure 3. Effect of 4-HNE on p-EGFR and EGFR expression in RPE cells.
Cell extracts prepared as described in methods section containing 30 µg protein were subjected to Western blot analysis. (A). Concentration dependent effect of 4-HNE on the expression of EGFR and p-EGFR. (B). Time dependent effect of 5 µM 4-HNE on the expression of EGFR and p-EGFR. (C). Effect of 4-HNE on the expression of EGFR and p-EGFR in VT and hGSTA4-Tr RPE cells. Western blot analysis was performed with total cell lysates using indicated antibodies. GAPDH was used to control for loading differences. The densitometry data in bar graphs below the respective figures represents the mean ± SD (n =3).
Effect of 4-HNE on ERK and Akt signaling pathways
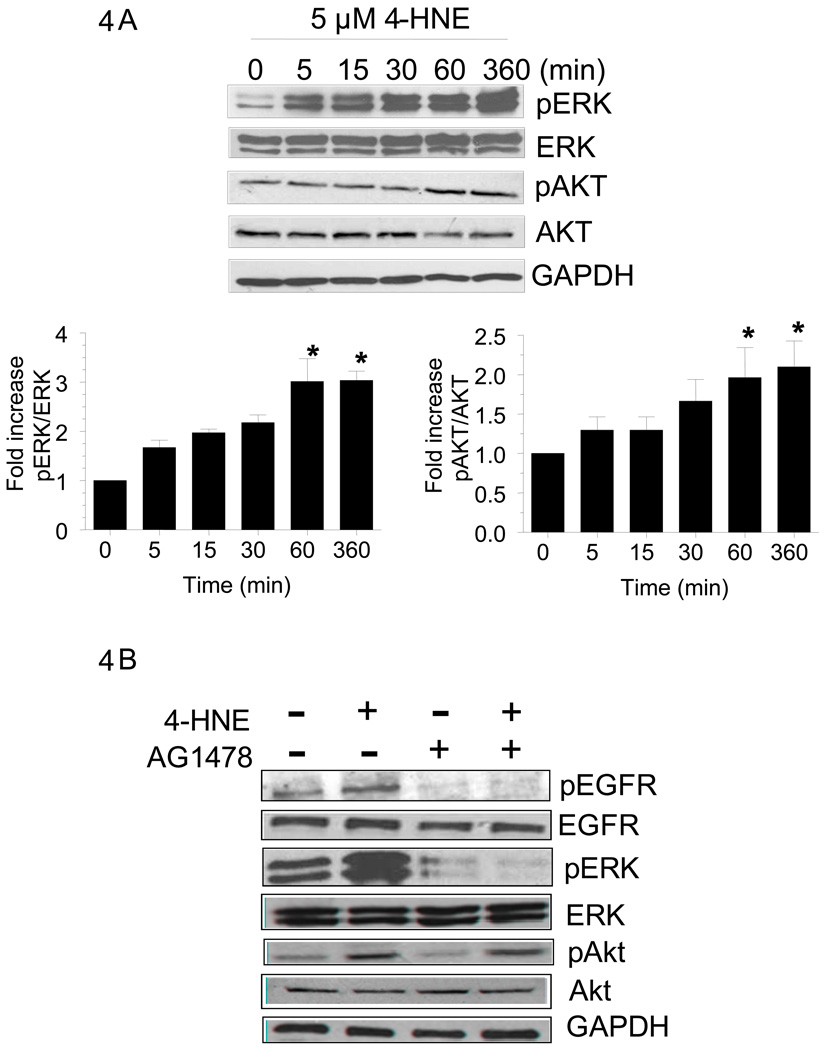
Previous studies have shown that EGFR1 activates ERK and/or Akt via phosphorylation and elicits a variety of cellular responses affecting cell growth and differentiation (Defoe and Grindstaff, 2004). We therefore examined the effect of 5 µM 4-HNE on these down stream signaling components of EGFR1 because optimal induction of EGFR1 was observed at this concentration of 4-HNE. When RPE cells were exposed to 5 µM 4-HNE, phospho-ERK levels were increased within 5 min (Fig. 4A) and this increased expression was sustained for 6 h of the total duration of this experiment. Total ERK levels were however not affected by 4-HNE treatment (Fig. 4A). An increase in the phosphorylation of Akt was also observed but this increase was seen only after 60 min of 4-HNE exposure (Fig. 4A). However, this increase also persisted for at least 6 h. No significant effect on total Akt levels was observed upon 4-HNE exposure (Fig. 4A). Results of these studies indicated that the activation of EGFR1 by 4-HNE was accompanied with the activation of its down stream pathways, perhaps in a time dependent manner because increase in p-ERK levels was observed as early as 5 min but the p-Akt levels were increased only after 60 min. Since ERK and Akt associated pathways are involved in cell survival and proliferation, these results suggest that as an initial response to oxidative stress, 4-HNE causes the activation of EGFR mediated protective mechanism.
Figure 4. Effect of 4-HNE on p-ERK and ERK and p-Akt and Akt expression in RPE cells.
Cell extracts prepared as described in methods section containing 30 µg protein were subjected to Western blot analysis. (A). Time dependent effect of 5 µM 4-HNE on the expression of p-ERK/ERK and p-Akt/Akt. (B) Cells were treated with or without 5 µM 4-HNE and or 5 µM AG1478 for 6 h. Western blot analysis was performed with total cell lysates using indicated antibodies. GAPDH was used to control for loading differences. The densitometry data in bar graphs below the figure represents the mean ± SD (n =3).
To study whether or not the activation of EGFR by 4-HNE leads to the activation of its downstream components, ERK and Akt, cells were treated with 5 µM 4-HNE in the presence of 5 µM EGFR inhibitor AG1478. Results of these experiments presented in Fig. 4B showed that 4-HNE-induced activation of EGFR as well as ERK was blunted by EGFR inhibitor. However, activation of Akt, that was suppressed in the presence of EGFR inhibitor alone, could still be activated by 4-HNE. This would suggest that in addition to EGFR mediated activation, Akt can also be activated by 4-HNE through alternate mechanism(s) that is/are independent of EGFR. Never the less, these results show that 4-HNE activates EGFR as well as ERK and Akt.
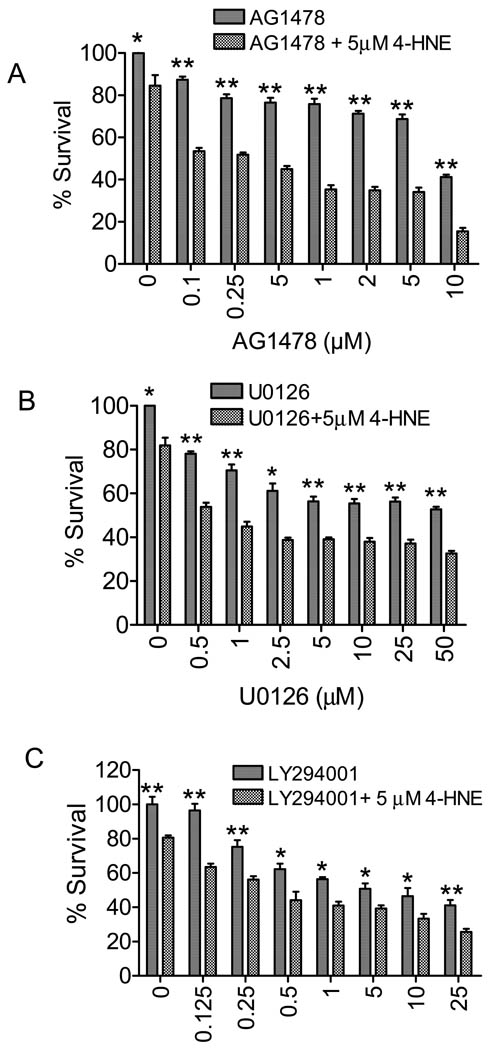
Inhibitors of EGFR pathway potentiate 4-HNE toxicity
In the next series of experiments, we studied the physiologic significance of 4-HNE-induced activation of EGFR1, ERK, and Akt by examining the toxicity of 4-HNE to RPE cells in the presence of EGFR inhibitor, and the inhibitors of MEK and PI3K that are upstream of ERK and Akt, respectively. Since 5 µM 4-HNE caused optimal induction of EGFR1 and only a minimal but measurable cell death (Fig. 1A) we examined the effect of 5 µM 4-HNE (12 h exposure) on cell survival in the presence of increasing concentrations (0.1–10 µM) of an EGFR inhibitor, AG1478. Results presented in Fig. 5A indicated that 0.1 µM inhibitor caused significant cell death that was consistent with the protective role of EGFR1 associated pathway(s). Exposure to 5 µM 4-HNE alone caused only about 14% cell death but this cytotoxic effect of 4-HNE was remarkably potentiated to about 53% cell death in the presence of 0.1 µM AG1478. In the presence of 10 µM AG1478, 5 µM 4-HNE caused 85% cell death (Fig. 5A). These results clearly indicated that EGFR associated pathway(s) protected RPE cells during oxidative stress and support our contention that as an initial response to oxidative stress, 4-HNE induces EGFR associated signaling pathways that provide protection against oxidant toxicity. Additional results (Fig. 5B and C) showing that the inhibitors of MEK and PI3K also potentiated 4-HNE cytotoxicity are also consistent with this idea. Together these results reaffirm a protective role of 4-HNE mediated signaling as an initial, rapid cellular response to oxidative stress.
Figure 5. Effect of HNE on cell growth in the presence and absence of EGFR, MEK and PI3K inhibitors.
RPE cells were plated in 96-well microplates (2 × 104 cells per well in 190 µl of DMEM) for 24 hours and then incubated with 5 µM 4-HNE in presence or absence of indicated concentrations of the inhibitors of EGFR (A), MEK (B) and PI3K (C) for 12 h. MTT assay was performed as described in material and methods. The data represent the mean ± SD (n =3).
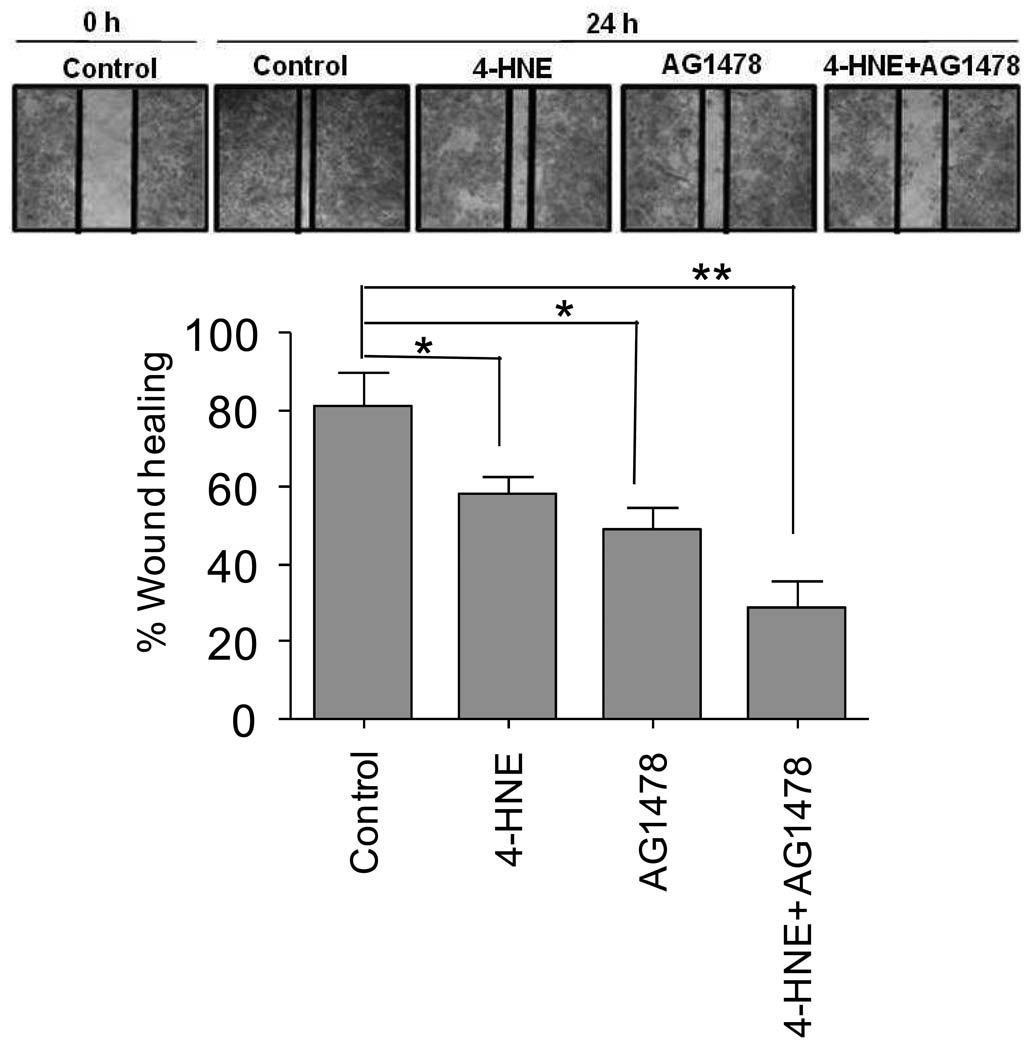
Effect of 4-HNE-mediated activation of EGFR on wound healing
The protective role of 4-HNE through EGFR induction was also examined in a model of wound healing. For these studies, a model similar to that used for wound healing in RPE cells was used (Xu and Yu, 2007) and wound closure in the presence and absence of 5 µM 4-HNE and 5 µM EGFR inhibitor was examined. As shown in Fig. 6 RPE cells were able to heal a scratch wound within 24 h suggesting that injured RPE cells can generate autocrine factors for wound healing. As expected, in presence of 5 µM 4-HNE the healing of the wound was significantly inhibited. This inhibition of wound healing was more robust in the presence of AG1478 (Fig. 6). These results are consistent with cell viability data showing the potentiation of 4-HNE induced cell death in the presence of EGFR, PI-3 kinase, and MEK inhibitors (Fig. 5) and suggest that activation of EGFR by 4-HNE and its down stream signaling targets is a protective mechanism against oxidative stress.
Figure 6. Suppression of 4-HNE induced EGFR activation inhibits wound healing.
RPE cells at confluence were injured by a scratch with a 10 µL pipette tip. Wounded cells were allowed to heal for 24 h in the presence or absence of 4-HNE (5 µM), AG1478 (5 µM) and combination of both 4-HNE (5 µM) and AG1478 (5 µM). Inset shows representative micrograph of wound closure extent for untreated cells, or in the presence of either 4-HNE, AG1478 or a combination of both. The data represents the mean ± SD (n=3).
Discussion
Results of present studies reaffirm the idea that 4-HNE plays an important role in stress-induced signaling. The activation of EGFR-mediated proliferative pathway involving ERK and Akt by 4-HNE at concentration well below the toxic levels clearly suggest that it acts as a sensor to potentially deleterious effects of oxidative stress and evokes the signaling mechanism(s) that can negate these effects. It has been shown that at relatively high concentrations 4-HNE is necrotic and apoptotic, and even at low concentrations it shows significant toxicity (Sharma A, et al., 2008; Chaudhary, et al., 2010). Our present findings are in agreement with these earlier observations. Our results demonstrate that at low levels of 4-HNE the activation of EGFR-mediated pathway does indeed provide protection to cells because inhibitors of EGFR, MEK, and PI3K dramatically potentiate the toxicity of 4-HNE. For example, at 5 µM concentrations 4-HNE causes merely 14 % cell death that is increased to more than 80% in the presence of these inhibitors. The capacity to repair the wound healing in the presence of 4-HNE, an index of oxidative stress is also impaired in presence of these inhibitors. This would suggest that induction of EGFR pathway at low levels of 4-HNE generated during initial stages of oxidative stress contributes to the process of wound healing. Thus in RPE cells 4-HNE seems to act as an initial sensor of oxidative stress that activates defense mechanism to negate the deleterious effects of oxidative stress. This idea is consistent with the reported activation of HSF-1 by 4-HNE for protection against oxidative stress (Jacobs and Marnet, 2007; Chaudhary, et al., 2010).
Transfection of cells with hGSTA4 results in lowering of the intracellular concentration of 4-HNE (Sharma, et al, 2004). Our results also show that the toxic effect of H2O2 is attenuated in hGSTA4 transfected cells suggesting that at least a part of the toxicity of oxidants is mediated specifically by 4-HNE because GSTA4-4 has no activity towards H2O2 and unlike GSTA1-1 and A2-2, GSTA4-4 has only minimal glutathione peroxidase activity towards lipid hydroperoxides that are precursors of 4-HNE (Yang et al., 2003). 4-HNE formation is an inevitable consequence of oxidative stress and particularly in retina due to high levels of ω-6 fatty acids high levels of 4-HNE are expected. The antioxidant role of GSTs may be particularly important for retina because increased 4-HNE-protein adducts formation has been associated with retinopathy. In retina GSTA1-1 and A2-2 that inhibit LPO (Yang, et al., 2003) are constitutively expressed while GSTA4-4 or the isozyme GST 5.8 that have substrate preference for 4-HNE are induced in response to even low oxidative stress (Singhal et al., 1999). Thus by limiting the formation of 4-HNE and by accelerating its detoxification these enzymes play a major role in maintaining the 4-HNE homeostasis in this tissue during oxidative stress.
Maintaining 4-HNE homeostasis in retina may be crucial because too low 4-HNE levels may lead to undesired proliferation (Sharma, R., et al., 2004) and high levels may cause toxicity (Li et al., 2006; Sharma, R., et al., 2008). Since our results suggest that 4-HNE-mediated signaling contributes to toxicity as well as protective mechanism in a concentration dependent manner, it is reasonable to predict that a window of basal level of 4-HNE may be crucial for the normal functioning of retina. Defining this window in retina may be clinically relevant to the prevalent use of antioxidants in commercial eye nutrients (Kuzniarz, et al. 2001). However, it must be realized that it is difficult to exactly define this window of the basal 4-HNE levels due to the variability of the parameters involved in its formation (endogenous ROS, UV, xenobiotics, and drug exposure) and rapid induction of the enzymes involved in its metabolism (Singhal, et al., 1999; Cheng, et al., 2001; Yang, et al., 2003) and future studies should be focused to overcome this problem.
-
➢
In RPE cells, 0.1 µM 4-HNE activates EGFR mediated pathway.
-
➢
This effect of 4-HNE on EGFR can be blunted by the over expression of GSTA4-4.
-
➢
4-HNE-induced activation of EGFR is a protective mechanism against oxidative stress.
-
➢
Inhibition of 4-HNE induced EGFR activation attenuates wound healing process.
-
➢
4-HNE acts as an initial sensor of oxidative stress to induce defense mechanisms.
Acknowledgments
This work was supported in part by NIH Grant EY 04396 (Y.C.A.).
The abbreviations used are
- 4-HNE
4-hydroxy-2-nonenal
- GST
glutathione S-transferase
- LPO
Lipid peroxidation
- EGFR
Epidermal growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- ERK
Extracellular-signal-related kinase
- MEK
Mitogen-activated protein kinase kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awasthi S, Srivatava SK, Piper JT, Singhal SS, Chaubey M, Awasthi YC. Curcumin protects against 4-hydroxy-2-trans-nonenal-induced cataract formation in rat lenses. Am. J. Clin. Nutr. 1996;64:761–766. doi: 10.1093/ajcn/64.5.761. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free. Radic. Biol. Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Bogdan S, Klambt C. Epidermal growth factor receptor signaling. Curr. Biol. 2001;11:R292–R295. doi: 10.1016/s0960-9822(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv. Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J. Biol. Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S, Awasthi YC. Mechanisms of 4-Hydroxy-2-nonenal Induced Pro- and Anti-Apoptotic Signaling. Biochem. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe DM, Grindstaff RD. Epidermal growth factor stimulation of RPE cell survival: contribution of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. Exp. Eye Res. 2004;79:51–59. doi: 10.1016/j.exer.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EAG. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J. Pathol. 2003;199:221–228. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp. Cell. Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Huang RP, Wu JX, Fan Y, Adamson ED. UV activates growth factor receptors via reactive oxygen intermediates. J. Cell Biol. 1996;133:211–220. doi: 10.1083/jcb.133.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AT, Marnett LJ. Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J. Biol. Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, Ferrington DA. Retinal proteins modified by 4-hydroxynonenal: Identification of molecular targets. Exp. Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chan PS. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007;2007:43603–43614. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniarz M, Mitchell P, Cumming RG, Flood VM. Use of vitamin supplements and cataract: the Blue Mountains Eye Study. Am. J. Ophthalmol. 2001;132:19–26. doi: 10.1016/s0002-9394(01)00922-9. [DOI] [PubMed] [Google Scholar]
- Li J, Sharma R, Patrick B, Sharma A, Jeyabal PV, Reddy PM, Saini MK, Dwivedi S, Dhanani S, Ansari NH, Zimniak P, Awasthi S, Awasthi YC. Regulation of CD95 (Fas) expression and Fas-mediated apoptotic signaling in HLE B-3 cells by 4-hydroxynonenal. Biochemistry. 2006;45:12253–12264. doi: 10.1021/bi060780+. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, Kurokawa K, Uchida K, Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J. Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Vieira O, Escargueil-Blanc I, Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol. Aspects. Med. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- Patrick B, Li J, Jeyabal PV, Reddy PM, Yang Y, Sharma R, Sinha M, Luxon B, Zimniak P, Awasthi S, Awasthi YC. Depletion of 4-hydroxynonenal in hGSTA4-transfected HLE B-3 cells results in profound changes in gene expression. Biochem. Biophys. Res. Commun. 2005;334:425–432. doi: 10.1016/j.bbrc.2005.06.099. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Whitney GS, Neubauer MG, Green JM, McDonald VL, Todaro GJ, Shoyab M. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4905–4909. doi: 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, Bhatnagar A. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler. Thromb. Vasc. Biol. 2000;20:1745–1752. doi: 10.1161/01.atv.20.7.1745. [DOI] [PubMed] [Google Scholar]
- Saneto RP, Awasthi YC, Srivastava SK. Glutathione S-transferases of the bovine retina. Evidence that glutathione peroxidase activity is the result of glutathione S-transferase. Biochem J. 1982;205:213–217. doi: 10.1042/bj2050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch. Biochem. Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, Awasthi YC. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur. J. Biochem. 2004;271:1690–1701. doi: 10.1111/j.1432-1033.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Dwivedi S, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas. Biochemistry. 2008;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickel W. In: Handbook of Sensory Physiology. Fuortes MGF, editor. Springer-Verlag; 1972. pp. 667–727. [Google Scholar]
- Singh SV, Dao DD, Srivastava SK, Awasthi YC. Purification and characterization of glutathione S-transferases in human retina. Curr. Eye Res. 1984;3:1273–1280. doi: 10.3109/02713688409007413. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Godley BF, Chandra A, Pandya U, Jin GF, Saini MK, Awasthi S, Awasthi YC. Induction of glutathione S-transferase hGST 5.8 is an early response to oxidative stress in RPE cells. Invest. Ophthalmol. Vis. Sci. 1999;40:2652–2659. [PubMed] [Google Scholar]
- Srivastata SK, Awasthi S, Wang L, Bhatnagar A, Awasthi YC, Ansari NH. Attenuation of 4-hydroxynonenal-induced cataractogenesis in rat lens by butylated hydroxytoluene. Curr. Eye Res. 1996;15:749–754. doi: 10.3109/02713689609003458. [DOI] [PubMed] [Google Scholar]
- Suc I, Meilhac O, Lajoie-Mazenc I, Vandaele J, Jürgens G, Salvayre R, Nègre-Salvayre A. Activation of EGF receptor by oxidized LDL. FASEB J. 1998;12:665–671. doi: 10.1096/fasebj.12.9.665. [DOI] [PubMed] [Google Scholar]
- Tate DJ, Newsome DA, Oliver PD. Metallothionein shows an age-related decrease in human macular retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1993;34:2348–2351. [PubMed] [Google Scholar]
- Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, Downward J, Mayes ELV, Whittle N, Waterfield D, Seeburg PH. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Xu KP, Yu FS. Cross Talk between c-Met and Epidermal Growth Factor Receptor during Retinal Pigment Epithelial Wound Healing. Invest. Ophthalmol. Vis. Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Cheng JZ, Saini MK, Ansari NH, Andley UP, Awasthi S, Awasthi YC. Protection of HLE B-3 cells against hydrogen peroxide- and naphthalene-induced lipid peroxidation and apoptosis by transfection with hGSTA1 and hGSTA2. Invest. Ophthalmol. Vis. Sci. 2002;43:434–445. [PubMed] [Google Scholar]
- Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, Awasthi S, Awasthi YC. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J. Biol. Chem. 2003;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak L, Awasthi S, Srivastava SK, Zimniak P. Increased resistance to oxidative stress in transfected cultured cells overexpressing glutathione S-transferase mGSTA4-4. Toxicol. Appl. Pharmacol. Mar. 1997;143:221–229. doi: 10.1006/taap.1996.8070. [DOI] [PubMed] [Google Scholar]