Abstract
Object
To date, glioma immunotherapy has been focused mostly on stimulating antitumor peripheral lymphocyte responses; however, some data suggest that microglia and/or macrophages (not lymphocytes) are the predominant inflammatory cells infiltrating gliomas. To study this hypothesis further, the authors analyzed inflammatory cell infiltrates in fresh human malignant glioma specimens and primary cultures.
Methods
Single-cell suspensions from fresh operative malignant glioma specimens, obtained by stereotactic localization, were analyzed for CD11b and CD45 by using flow cytometry. A comparison was made with peripheral blood mononuclear cells. In a subset of patients, a more detailed flow cytometry analysis of Class I and II major histocompatibility complex, B7-1, B7-2, CD11c, and CD14 expression was performed. Macrophage-like cells in primary glioma cultures were similarly assessed.
Results
Operative samples were obtained from 9 newly diagnosed malignant gliomas. The mean percent of CD45+/CD11b− cells (lymphocytes) was 2.48% (range 0.65–5.50%); CD45dim/CD11b+ cells (microglia), 1.65% (range 0.37–3.92%); and CD45bright/CD11b+ (monocytes/macrophages), 6.25% (range 1.56–15.3%). More detailed fluorescence-activated cell sorting suggested that macrophage-like cells expressed Class I and II major histocompatibility complex, B7-2, and CD11c but not CD14 or B7-1. Primary human glioma cultures contained significant numbers of macrophage-like (CD45bright/CD11b+) cells, but these cells were lost with successive passages. These cells maintained the immunomarker profiles of macrophage-like cells from fresh specimens only if they were cultured in serum-free media.
Conclusions
The CD45+/CD11b+ cells are the predominant inflammatory cell infiltrating human gliomas. Of this type, the CD45bright/CD11b+ cells, a phenotype compatible with circulating macrophages in rodent models, and not microglia, are the most common. Their immunomarker profile is compatible with an immature antigen-presenting cell. They are present in primary glioma cultures but are lost in successive passages. Their role is enigmatic, and they may prove an important target for future glioma immunotherapy studies.
Keywords: glioma, immunotherapy, lymphocyte, macrophage, microglia
Malignant gliomas are the most common primary tumors of the CNS. Despite significant advances in surgery, radiation therapy, and chemotherapy over the past 3 decades, the median patient survival remains < 1 year from lesion diagnosis.26,27,31 This finding has prompted the investigation of novel therapeutic strategies, including immunotherapy. So far, glioma immunotherapy efforts largely have been focused on stimulating antitumor lymphocyte responses. For example, several types of glioma vaccines using various strategies have recently been developed and tested.28,30,36,46,48,50 These vaccines have occasionally led to measurable antiglioma lymphocyte responses, but clinical responses have been less impressive.42,47 This result in part may reflect the nature of the small pilot and Phase I studies that have been designed to evaluate the feasibility and safety of the therapy but not its efficacy. Nonetheless, it is known that the microenvironment within gliomas is immunosuppressive because of the expression of several factors (transforming growth factor–β2, prostaglandin E2, interleukin-6, and Fas ligand) that downregulate immune responses.13,24,34 Local glioma immunosuppression has not been adequately addressed in most vaccine trials.
The local immune microenvironment within gliomas is dependent on both glioma cells and other cell types such as infiltrating inflammatory cells. It is noteworthy that the lymphocyte’s role in the glioma microenvironment may be limited. Some data suggest that microglia (the brain’s resident antigen-presenting cells) and/or macrophages—not lymphocytes—are the predominant inflammatory cells infiltrating gliomas.20,21,23,38 There is evidence from rodent models that glioma-associated microglia do not participate in effective antiglioma immune responses and may actually secrete immunosuppressive factors such as interleukin-10 and Fas ligand.6,23,43,52 Targeting these cells may be an important immunotherapeutic strategy.
It has been reported that inflammatory cell subpopulations can be characterized in normal and inflamed rat brain on the basis of CD45 (leukocyte common antigen) and CD11b (complement receptor 3) 2-color flow cytometry.41 Four populations can be distinguished: CD45−/CD11b− (noninflammatory cells), CD45+/CD11b− (lymphocytes), CD45dim/CD11b+ (microglia), and CD45bright/CD11b+ (monocytes/macrophages). Distinguishing resident microglia from infiltrating systemic macrophages by relative CD45 expression is based on bone marrow transplant experiments. Animals were irradiated to ablate bone marrow and then received allogeneic bone marrow transplants. Brain inflammation was subsequently induced with increases in both CD45dim/CD11b+ and CD45bright/CD11b+ infiltrates. Haplotyping showed that the CD45dim population retained the parental haplotype (suggesting they were resident microglia), whereas the CD45bright population had the transplant haplotype (suggesting they were infiltrating systemic macrophages).41
The CD45/CD11b flow cytometry has been used to differentiate lymphocytes, peripheral monocytes/macrophages, and microglia in rodent models of experimental allergic encephalitis.40,41 More recently, Badie and colleagues3,7 have used this technique to analyze inflammatory cell infiltrates in several rat glioma models. They found marked microglial (CD45dim/CD11b+) infiltration into both tumor and adjacent cortex. In many cases, this progression was also associated with significant lymphocytic infiltration. As similar studies have not been conducted in human gliomas, we used CD45/CD11b flow cytometry to quantitate the number of lymphocytes, microglia, and monocytes/macrophages in human glioma specimens. We also performed more detailed flow cytometry characterization of surface immunomarker expression in a subset of glioma specimens. Finally, we sought to establish a method of culturing human glioma–derived microglia to facilitate future studies of glioma-microglia interactions.
Methods
Obtaining Human Glioma Specimens
Fresh surgical specimens from patients with untreated, newly diagnosed primary gliomas were collected at the time of craniotomy and processed immediately. Using an intraoperative image guidance system based on preoperative Gd-enhanced MR imaging (Stealth, Medtronic), samples were taken from enhancing tumor (9 samples) and overlying “normal” cortex (5 samples, when cortical resection was indicated). All specimens from enhancing areas were histopathologically confirmed as GBM (World Health Organization Grade IV astrocytoma). Specimens were obtained in accordance with a University of California, San Francisco, Committee on Human Research–approved tissue banking protocol at the Brain Tumor Research Center.
Preparation of Single-Cell Suspensions and Primary Cultures
Single-cell suspensions and primary cultures were obtained from fresh operative specimens as previously described.33 Briefly, specimens were minced and washed repeatedly with PBS, and obvious hematoma was removed. The resulting slurry was subjected to partial enzyme digestion (DNAse, pronase, and collagenase, Roche Bioscience and GibcoBRL), passed through 100-μm nylon mesh, and spun on a density gradient (Ficoll-Paque, Amersham Biosciences). Cells at the interface were collected and washed twice in PBS. The resulting single-cell suspensions were either used directly for flow cytometry or plated in DMEM/F12 medium supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin G (Cell Culture Facility, University of California, San Francisco) or in serum-free adaptive immunotherapy medium (AIM V, GibcoBRL) and cultured at 37°C, 5% CO2.
Isolation of PBMCs
Peripheral blood mononuclear cells were obtained from healthy donors via leukophoresis tubing. Samples were spun on a density gradient (Ficoll-Paque Plus, Amersham Biosciences). Cells at the interface were harvested, washed once in PBS, and used immediately for flow cytometry analysis.
Flow Cytometry
Single-cell suspensions from operative specimens were washed once in PBS, resuspended in PBS, counted on a hemocytometer with trypan blue staining, and divided into 106-cell aliquots for flow cytometry. Cells in these aliquots were washed again in PBS, resuspended in 100 μl of PBS with 1% human AB serum, and incubated at room temperature for 10 minutes for Fc-receptor blocking. Thereafter, 10 μl of antihuman CD45-FITC (eBioscience) and 10 μl of antihuman CD11b-PE (eBioscience) were added to each sample, and the samples were incubated at room temperature in the dark for 15 minutes. Similar staining was performed with isotype-matched control antibodies (eBioscience). Cells were washed in PBS, resuspended in 100 μl of PBS with 5 μl of 7-AAD (BD Pharmingen), and incubated at room temperature in the dark for 10 minutes. An additional 300 μl of PBS was added to each sample, and the samples were immediately read on a flow cytometer on the FL-1, -2, and -3 parameters with the aid of CellQuestPro software (both Becton Dickinson). An analysis was performed using the Flow-Jo software (TreeStar, Inc.). Dot plots for CD45 versus CD11b expression were generated after excluding debris by forward- and side-scatter gating and 7-AAD+ cells by leaving out dead cells.
For a subset of samples, more detailed flow cytometry analysis was performed. In addition to the CD45/CD11b staining described above, samples were stained with 10 μl of antihuman HLA-ABC–FITC (BD Pharmingen) and 10 μl of antihuman CD11c-PE (eBioscience), 10 μl of antihuman HLA-DR–FITC (Caltag Laboratories), 10 μl of antihuman B7-1–FITC (eBioscience, 10 μl of antihuman B7-2–PE (eBioscience), and 10 μl of antihuman CD14-FITC (Miltenyi Biotec). Staining (including 7-AAD staining) and analysis proceeded as above.
Analysis of Primary Human Glioma Cultures
Primary (Passage 1) and early-passage (Passages 2–4) human GBM cultures at 80% confluence were harvested by scraping after an incubation for 15 minutes in 5 mM EDTA at 37°C. They were washed, stained for CD11b, CD14, and CD45 as above, and analyzed immediately by flow cytometry. The CD11b+ cells were purified from primary human glioma cultures by harvesting in EDTA as above and then separating the CD11b+ cells by using antihuman CD11b-coated microbeads according to the manufacturer’s instructions (Miltenyi Biotec).
Results
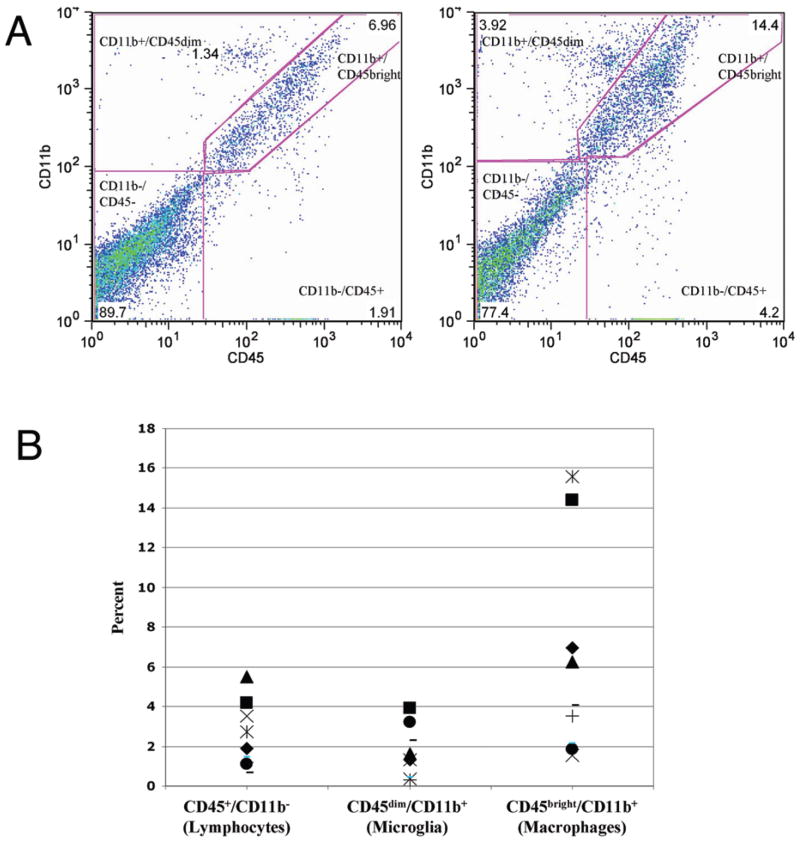
Fresh operative specimens were obtained from 9 patients harboring GBMs. Based on intraoperative image guidance (Stealth), samples were harvested from enhancing tumor or cortex overlying tumor (Fig. 1). As expected, flow cytometric analysis of CD45/CD11b expression patterns in fresh enhancing human GBM specimens revealed 4 distinct populations (CD45−/CD11b−, CD45+/CD11b−, CD45dim/CD11b+, and CD45bright/CD11b+) corresponding to the noninflammatory cells, lymphocytes, microglia, and macrophages identified in rodent models (Fig. 2A). Note, however, that the macrophage-like population (CD45bright/CD11b+) was the most common inflammatory cell identified (mean [± SD] 6.25 ± 5.30% of viable cells, range 1.56–15.3%). Lymphocytes (CD45+/CD11b−: mean 2.48 ± 1.64% of viable cells, range 0.65–5.50%) and microglia-like cells (CD45dim/CD11b+: mean 1.65 ± 1.28% of viable cells, range 0.37–3.92%) were less common (Fig. 2B). Macrophage-like cells were significantly more common than lymphocytes (p < 0.05, Tukey 2-tailed analysis of variance), whereas microglia-like cells were not. This finding was relatively surprising, as microglia and lymphocytes have been reported to be the most common inflammatory cells infiltrating rat glioma models.7
Fig. 1.

Representative T1-weighted Gd-enhanced MR image of a GBM demonstrating areas of resected overlying cortex (plain arrow) and enhancing tumor (arrow with diamond).
Fig. 2.
A: Two representative dot plots showing CD45 and CD11b staining for enhancing GBM specimens by flow cytometry. Each dot represents a single stained cell. Four distinct populations can be seen: CD45−/CD11b−, CD45+/CD11b−, CD45dim/CD11b+, and CD45bright/CD11b+. Double negative gates are based on isotype-matched controls (not shown). B: Summary scatter plot showing the percentage of total viable cells that were CD45+/CD11b−, CD45dim/CD11b+, and CD45bright/CD11b+. Each shape represents a tumor specimen taken from a specific patient.
Similarly, 4 distinct populations could be identified in “normal” cortex samples based on CD45/CD11b staining and flow cytometry (Fig. 3A). Macrophage-like cells (CD45bright/CD11b+) were the most common infiltrating inflammatory cells (mean 0.49 ± 0.50% of viable cells, range 0.11–1.30%), followed by microglia-like cells (CD-45dim/CD11b+: mean 0.32 ± 0.30% of viable cells, range 0.03–0.82%) and lymphocytes (CD45+/CD11b−: mean 0.22 ± 0.16% of viable cells, range 0.06–0.43%; Fig. 3B). Interestingly, the amount of infiltrating inflammatory cells in the cortex was an order of magnitude lower than in enhancing tumor (Fig. 2). This finding was also relatively surprising, as previous reports on rat glioma models have suggested that “normal” cortex in glioma-bearing animals has increased inflammatory cell infiltrates compared with truly normal cortex in non–glioma-bearing animals.7
Fig. 3.
A: Two representative dot plots showing CD45 and CD11b staining for cortex overlying tumor by flow cytometry. Similar types of CD45 and CD11b positive and negative cells can be seen as for enhancing tumor, but the absolute number of inflammatory cells is much lower. B: Summary scatter plot demonstrating the percentage of viable cells that are CD45+/CD11b−, CD45dim/CD11b+, and CD45bright/CD11b+. Each shape represents a tumor specimen taken from a specific patient.
It is possible that some of the inflammatory cells identified in our flow cytometry study represent contaminating PBMC blood retained on and within the tumor specimen. However, CD45/CD11b staining of and flow cytometry for human glioma specimens reveal a distinct pattern when compared with PBMCs (Fig. 4). As expected, PBMCs do not include significant numbers of CD45−/CD11b− (noninflammatory) or CD45dim/CD11b+ (microglia) cells. Furthermore, far more CD45+/CD11b− cells (lymphocytes) are present than CD45bright/CD11b+ cells (monocytes/macrophages). The ratio of CD45+/CD11b− cells (lymphocytes) to CD45+/CD11b+ cells (monocytes/macrophages/microglia) is significantly higher in healthy donor PBMCs (mean ± standard deviation 3.71 ± 1.01, 5 patients) than in glioma specimens (0.48 ± 0.54, 9 patients; p < 0.001, Student 2-tailed t-test). These data strongly suggest that the inflammatory cells identified in our flow cytometry study represent a distinct population of glioma-associated cells and not simply contaminating PBMCs.
Fig. 4.
A: Two representative dot plots showing CD45 and CD11b staining for normal PBMC samples by flow cytometry. Only CD45+/CD11b− and CD45bright/CD11b+ cells are visible. B: Ratio of CD45+/CD11b− to CD45+/CD11b+ cells for PBMCs and enhancing tumor specimens (GBMs). Marked differences are seen, suggesting that the CD45+ and CD11b+ cells in tumor specimens do not simply represent residual PBMCs. Each shape represents a tumor specimen taken from a specific patient.
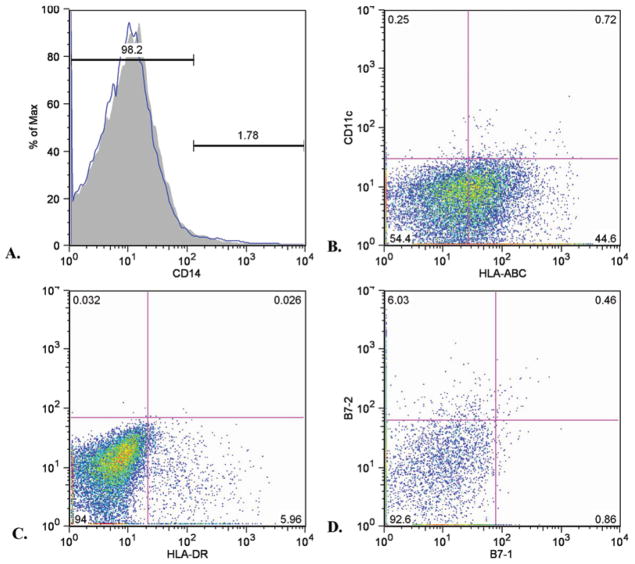
A more extensive flow cytometry study was performed on a subset of glioma samples (Fig. 5). Human leukocyte antigen–ABC (Class I MHC) consistently demonstrated a biphasic expression pattern in which most cells (presumably tumor cells) showed moderate expression and a sub-set (presumably inflammatory cells) showed high expression. Most cells were HLA-DR (Class II MHC) negative, but a subset was HLA-DR positive. This subset roughly corresponded numerically to the monocyte/macrophage and microglial cells identified by CD45/CD11b flow cytometry. Furthermore, HLA-DR+ cells were also positive for CD11b (data not shown). The CD11c and B7-2 cells were moderately positive in a subset of cells roughly corresponding numerically to the monocyte/macrophage and microglial cells identified by CD45/CD11b flow cytometry. The B7-1 and CD14 staining was negative.
Fig. 5.
Representative flow cytometry histogram (A) and dot plots (B–D) of more detailed immunomarker profiles of single-cell suspensions of fresh glioma specimens performed in a subset of patients. Expression of CD14 (A, essentially absent on all cells). Class I MHC (HLA-ABC) and CD11c expression (B). Note a biphasic Class I MHC expression (mostly moderately positive with a strongly positive subset) and limited CD11c expression. Class II MHC (HLA-DR) expression (C); a subset of cells is positive. Expression of B7-1 and B7-2 (D). Expression of the former is very limited, but B7-2 expression is seen as a subset of cells. Note that the percentage of Class I MHChigh, Class II MHC+, and B7-2+ cells roughly corresponds numerically to the number of CD45bright/CD11b+ cells for this sample (6.24%, data not shown)
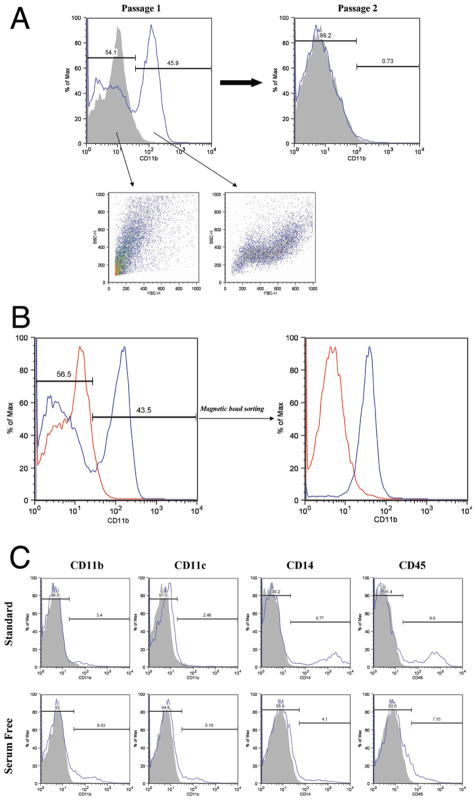
To facilitate further testing of monocyte/macrophage/microglia interactions with human gliomas, we sought techniques to establish cultures of glioma-derived monocyte/macrophage/microglia cells. A number of techniques for establishing microglial cultures from brain specimens have been reported.12,17,19,49 Several of these procedures (including separation on a density gradient, rotary shaking, and serum starvation) have been attempted with human glioma single-cell suspensions and primary cultures but did not yield significant numbers of CD11b+ cells (data not shown). However, we noted that primary cultures (Passage 1) from human glioma specimens often contained significant numbers of CD11b+ cells (Fig. 6A). This macrophage-like population was lost with subsequent passages but could be separated into a pure population of CD11b+ cells by sorting with magnetic beads (Fig. 6B). Attempts to sort pure populations of CD11b+ cells from fresh (uncultured) human glioma single-cell suspensions were unsuccessful, possibly because of the presence of contaminating debris (data not shown). It was apparent that different culture conditions could considerably modify immunomarker expression in macrophage-like cells in primary human glioma cultures. Primary cultures plated in standard medium (DMEM/F12 supplemented with 10% fetal bovine serum) showed reduced numbers of cells expressing CD11b or CD11c but increased CD14+ and CD45+ cells compared with primary cultures plated in serum-free AIM V medium (Fig. 6C). The immunomarker expression pattern in cells cultured in serum-free medium more closely resembled the pattern seen in infiltrating inflammatory cells in fresh gliomas specimens (Fig. 5).
Fig. 6.
A: Histogram (Passage 1) showing CD11b expression in a primary human glioma culture. Distinct CD11b− and CD11b+ populations can be seen that have distinct forward scatter (FSC) and side scatter (SSC) properties. Note that the CD11b+ population is lost by Passage 2. B: Histograms showing purification of CD11b+ cells from glioma primary culture by sorting with anti–CD11b-coated microbeads. C: Histograms showing percentage of inflammatory cell marker-positive cells seen in primary glioma cultures established from a single specimen in the presence or absence of serum.
Discussion
As expected, based on previous reports in rodent glioma models,4,7,23 monocyte-lineage cells formed the most prominent group of inflammatory cells infiltrating human gliomas. Unexpectedly, we found that these cells were predominantly CD45bright/CD11b+. This phenotype corresponds to infiltrating systemic macrophages, not resident microglia (CD45dim/CD11b+), in rodent models.7,41 However, the validity of distinguishing microglia and macrophages based on the CD45dim/CD11b+ or CD45bright/CD11b+ phenotype has not been established in humans. Therefore, we believe that these data suggest that systemic macrophages (not resident microglia) are the most common inflammatory cell infiltrating human gliomas, although we recognize that we cannot be dogmatic in this assertion. In any event, monocyte-lineage cells are clearly more common than lymphocytes in human gliomas. This finding contrasts with data on rat 9L gliosarcoma and C6 glioma (the most common glioma models used for testing immunotherapies) in which lymphocytes can account for up to one-third of viable cells.7
Similarly, we were somewhat surprised that inflammatory cell infiltrates were not more numerous in “normal” cortex overlying malignant gliomas given that previous studies have suggested that “normal” cortex in rats bearing glioma or gliosarcoma xenografts demonstrate increased inflammatory cell infiltrates compared with cortex in non–tumor-bearing animals.7 The reason for this difference is not entirely clear. It is possible that commonly used rat glioma or gliosarcoma models are inherently immunogenic.35 This explanation would be in keeping with the increase in lymphocytic infiltration into tumors in these models7 compared with our results in fresh, human surgical specimens. That being the case, the increased inflammatory cell infiltrate in neighboring cortex in these models, compared with human specimens, may simply reflect an increased immune response against the tumor resulting in more inflammatory cells homing to the CNS.
In rodent models in which CD45/CD11b flow cytometry is used to analyze inflammatory cells infiltrating the CNS, animals are killed and perfused with saline prior to harvesting brains for analysis.7,41 This step is performed to reduce contaminating the PBMCs retained within CNS vasculature, which might confound analysis of parenchymal inflammatory cells. For obvious reasons, this step cannot be performed prior to harvesting glioma specimens from human patients. It is possible that some of the inflammatory cells we identified using CD45/CD11b flow cytometry represent contaminating PBMCs were retained with the tumor specimens. However, CD45+/CD11b− cells are more common than CD45+/CD11b+ cells in PBMCs but much less common in fresh glioma specimens (Fig. 4). This finding strongly suggests that we identified a distinct population of glioma-associated inflammatory cells, and not simply contaminating PBMCs.
The more detailed flow cytometry study used to examine surface immunomolecules was performed in a small subset of patients and thus must be interpreted with caution (Fig. 5). Nevertheless, some interesting observations can be made. As expected, most cells (presumably tumor cells) had modest Class I MHC expression and no Class II MHC. This finding is in keeping with previous reports on MHC expression in human gliomas.2,25,32,39 A small subset of cells was positive for Class II MHC and highly positive for Class I MHC. This population corresponds numerically to the monocyte-lineage (CD45+/CD11b+) cells and appears to be double positive for Class II MHC and CD11b+ cells (data not shown). Similarly, a subset of cells (corresponding numerically to CD45+/CD11b+ cells) was moderately positive for CD11c and the T cell costimulatory molecule B7-2. Note that B7-1 (another T cell costimulatory molecule) and CD14 (bacterial lipopolysaccharide receptor) were absent on all cells.
Taken together, these findings suggest that the macrophage-like cells infiltrating human gliomas are Class I MHC+/Class II MHC+/CD11b+/CD11c+/CD14−/CD45+/B7-1−/B7-2+. This phenotype is compatible with immature macrophages as more mature, activated macrophages would be expected to have increased CD14 and B7-1 expression and decreased B7-2 expression; most lymphocytes would be Class II MHC− and CD11c−.15,16,37 The absence of CD14 expression is particularly striking given that its expression is nearly universally increased in macrophages and microglia in CNS inflammatory disorders.8–11,18,22,51 The combination of marked infiltration of monocyte-lineage cells and CD14 downregulation has not been reported in other CNS disorders. This combination may have important implications for proposed therapeutic strategies utilizing lipopolysaccharide or cytidine phosphate guanosine–containing oligonucleotides to stimulate antiglioma immune responses14,45 as CD14 is the receptor for both of these agents. However, we are reluctant to characterize these apparently immature glioma-associated macrophages as “inactive” based on the present study. We have not examined other aspects of their immune function such as phagocytic ability, antigen-presenting ability, and cytokine secretion. It is possible that glioma-conditioned macrophages and dendritic cells have an actively immunosuppressive phenotype characterized by the secretion of immunosuppressive cytokines, ability to induce apoptosis or anergy in activated T cells, and/or stimulation of immunosuppressive T regulatory cells.1,5,29,43,44 Further study of glioma-associated macrophage and microglia function is required to clarify these issues.
The data in this paper are descriptive. It would be desirable to develop model systems in which glioma/glioma-associated macrophage interactions can be tested more rigorously. Most monocyte-lineage cells infiltrating gliomas in this study had a macrophage-like (CD45bright/CD11b+) as opposed to a microglia-like (CD45dim/CD11b+) phenotype. This result suggests that examining interactions between peripheral blood monocytes (macrophage precursors) and glioma cells could be highly relevant. The coculture of human glioma cells with peripheral blood monocytes is a simple model that may be adequate to test these interactions. This coculture model has several advantages, including the existence of many immortalized human glioma cell lines and the relative ease with which monocytes can be isolated from PBMCs.
Note, however, that there are also distinct disadvantages to using monocyte/glioma interactions as a model for glioma interactions with glioma-associated macrophages in situ. First, the system described above is allogeneic. This feature may not be relevant when examining pure glioma/monocyte interactions but could significantly cloud results if allogeneic lymphocytes are added to the system to examine the functional effects of glioma/monocyte interactions such as antigen presentation. Using an autologous system of patient PBMCs and explanted tumor cultures may avoid this particular drawback. Still, infiltrating macrophage-like cells may be irreversibly altered by interactions with glioma cells in situ in ways that cannot be replicated by short-term coculture of glioma cells and peripheral blood monocytes. Furthermore, the distinction between peripheral macrophages and resident microglia on the basis of the CD45bright/CD11b+ and CD45dim/CD11b+ phenotype has not been established in humans.41 It is possible that the CD45bright/CD11b+ cells infiltrating gliomas are microglia after all. In that case, using peripheral blood monocytes to model microglial reactions may only be partially accurate.
For all of these reasons, it would be desirable to culture glioma-infiltrating macrophage-like cells so that autologous glioma explant/glioma-associated macrophage/lymphocyte coculture systems could be developed. We applied several standard techniques for establishing microglial cultures from normal human brain tissue (rotary shaking, serum starvation, or separation on a density gradient) to fresh glioma specimens and cultures, but we were unable to generate microglial cultures given the overwhelming presence of tumor cells (data not shown). Similarly, we were unable to isolate microglia cultures by sorting fresh glioma single-cell suspension with CD11b-magnetic beads (data not shown), perhaps because of the presence of considerable debris.
We did find that primary human glioma cultures (Passage 1) contained significant numbers of CD11b+ (macrophage-like) cells (Fig. 6). This population disappeared with successive passages. These cells could be easily sorted into a pure viable CD11b+ population by using magnetic beads, but this population did not survive as an independent culture. Although our present inability to establish pure glioma-associated macrophage cultures is somewhat limiting, the presence of these cells in primary explant cultures suggests that the cultures could be used as the basis for an autologous model of glioma/glioma-associated macrophage/lymphocyte interactions. Efforts are ongoing to establish pure glioma-associated macrophage cultures by changing the sorting techniques and/or adding cytokines or glioma-conditioned medium. Our finding that inflammatory cells in primary cultures established in serum-free medium have immunomarker profiles more similar to those in fresh surgical specimens suggests that the presence of glioma-associated macrophages may be an important aspect of establishing these in vitro model systems.
Documenting that primary glioma explant cultures contain significant numbers of inflammatory cells as well as tumor cells is important for several reasons. It has been reported that primary glioma cultures have immunological characteristics that are lost in successive passages.2 Although this change has been believed to reflect a loss of innate glioma characteristics with prolonged exposure to in vitro conditions, our data instead suggest that it may reflect a loss of associated inflammatory cells. Furthermore, several investigators have suggested tailoring glioma therapy based on screening primary glioma cultures for chemosensitivity and/or genetic profile.31 These efforts are likely to be unrewarding unless primary culture heterogeneity is taken into account.
Conclusions
Glioma immunotherapy to date largely has been focused on stimulating antiglioma responses by peripheral lymphocytes without any attempts to address local immunosuppressive conditions. Comprehensive efforts to analyze the role of glioma-associated macrophage/microglia cells have been even less common. In this paper, we presented data suggesting that monocyte-lineage cells (not lymphocytes) are the most common inflammatory cells infiltrating human gliomas. These cells have characteristics compatible with infiltrating systemic macrophages, not resident microglia. Preliminary data indicate that they have an immunologically immature phenotype, but more investigation is required. Coculturing human glioma cells and peripheral blood monocytes may be a simple and relevant model for performing these studies given that glioma-infiltrating cells resemble peripheral macrophages. Nonetheless, it would still be desirable to isolate glioma-associated macrophages for further investigation. We have not been able to establish pure glioma-associated macrophage cultures, but we have found that primary human glioma cultures contain significant numbers of macrophage-like cells that are lost with successive passages. Therefore, primary glioma cultures themselves may represent a useful system for studying glioma-macrophage interactions, and attempts are ongoing to purify macrophages from these cultures. The cellular heterogeneity of primary glioma cultures has important implications for other studies based on primary explant cultures.
Acknowledgments
We acknowledge Christine Sison for her contribution to this work.
Abbreviations used in this paper
- DMEM/F12
Dulbecco modified Eagle medium and Hams F12 medium
- FITC
fluorescein isothiocyanate
- GBM
glioblastoma multiforme
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- 7-AAD
7-aminoactinomycin D
Footnotes
Disclaimer
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Disclosure
Dr. Parney has been a Neuro-Oncology Fellow for the Accelerate Brain Cancer Cure and is currently a clinical investigator for the Alberta Heritage Foundation for Medical Research. This work was funded in part by National Institutes of Health Grants No. 2P50 CA097257-06 and KO8 NS 046671 (A.T.P.).
References
- 1.Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclo-oxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–4359. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RC, Elder JB, Brown MD, Mandigo CE, Parsa AT, Kim PD, et al. Changes in the immunologic phenotype of human malignant glioma cells after passaging in vitro. Clin Immunol. 2002;102:84–95. doi: 10.1006/clim.2001.5152. [DOI] [PubMed] [Google Scholar]
- 3.Badie B, Bartley B, Schartner J. Differential expression of MHC class II and B7 costimulatory molecules by microglia in rodent gliomas. J Neuroimmunol. 2002;133:39–45. doi: 10.1016/s0165-5728(02)00350-8. [DOI] [PubMed] [Google Scholar]
- 4.Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54:106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 5.Badie B, Schartner J, Prabakaran S, Paul J, Vorpahl J. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001;120:19–24. doi: 10.1016/s0165-5728(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 6.Badie B, Schartner J, Vorpahl J, Preston K. Interferon-gamma induces apoptosis and augments the expression of Fas and Fas ligand by microglia in vitro. Exp Neurol. 2000;162:290–296. doi: 10.1006/exnr.1999.7345. [DOI] [PubMed] [Google Scholar]
- 7.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–962. doi: 10.1097/00006123-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Becher B, Fedorowicz V, Antel JP. Regulation of CD14 expression on human adult central nervous system-derived microglia. J Neurosci Res. 1996;45:375–381. doi: 10.1002/(SICI)1097-4547(19960815)45:4<375::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Beschorner R. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2003;13:231–232. doi: 10.1111/j.1750-3639.2003.tb00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beschorner R, Nguyen TD, Gözalan F, Pedal I, Mattern R, Schluesener HJ, et al. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- 11.Beschorner R, Schluesener HJ, Gözalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002;126:107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 12.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 13.Bodmer S, Huber D, Heid I, Fontana A. Human glioblastoma cell derived transforming growth factor-beta 2: evidence for secretion of both high and low molecular weight biologically active forms. J Neuroimmunol. 1991;34:33–42. doi: 10.1016/0165-5728(91)90096-p. [DOI] [PubMed] [Google Scholar]
- 14.Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- 15.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, et al. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 17.Dobrenis K. Microglia in cell culture and in transplantation therapy for central nervous system disease. Methods. 1998;16:320–344. doi: 10.1006/meth.1998.0688. [DOI] [PubMed] [Google Scholar]
- 18.Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- 19.Fedoroff S, Hao C. Origin of microglia and their regulation by astroglia. Adv Exp Med Biol. 1991;296:135–142. doi: 10.1007/978-1-4684-8047-4_14. [DOI] [PubMed] [Google Scholar]
- 20.Hao C, Parney IF, Roa WH, Turner J, Petruk KC, Ramsay DA. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2 and Th3 dysregulation. Acta Neuropathol. 2002;103:171–178. doi: 10.1007/s004010100448. [DOI] [PubMed] [Google Scholar]
- 21.Hao C, Richardson A, Federoff S. Macrophage-like cells originate from neuroepithelium in culture: characterization and properties of the macrophage-like cells. Int J Dev Neurosci. 1991;9:1–14. doi: 10.1016/0736-5748(91)90067-v. [DOI] [PubMed] [Google Scholar]
- 22.Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalier L, Cartron PF, Pedelaborde F, Olivier C, Loussouarn D, Martin SA, et al. Increase in PGE2 biosynthesis induces a Bax dependent apoptosis correlated to patients’ survival in glioblastoma multiforme. Oncogene. 2007;26:4999–5009. doi: 10.1038/sj.onc.1210303. [DOI] [PubMed] [Google Scholar]
- 25.Lampson LA. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microsc Res Tech. 1995;32:267–285. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- 26.Laperriere N, Zuraw L, Cairncross G Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site Group. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol. 2002;64:259–273. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 27.Laws ER, Jr, Parney IF, Huang W, Anderson F, Morris A, Asher T, et al. Survival following surgery and prognostic factors for newly diagnosed malignant glioma: data from the Glioma Outcome Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 28.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 29.Nakano Y, Kuroda E, Kito T, Uematsu S, Akira S, Yokota A, et al. Induction of prostaglandin E2 synthesis and microsomal prostaglandin E synthase-1 expression in murine microglia by glioma-derived soluble factors. Laboratory investigation. J Neurosurg. 2008;108:311–319. doi: 10.3171/JNS/2008/108/2/0311. [DOI] [PubMed] [Google Scholar]
- 30.Parney IF, Chang LJ, Farr-Jones MA, Hao C, Smylie M, Petruk KC. Technical hurdles in a pilot clinical trial of combined B7-2 and GM-CSF immunogene therapy for glioblastomas and melanomas. J Neurooncol. 2006;78:71–80. doi: 10.1007/s11060-005-9058-0. [DOI] [PubMed] [Google Scholar]
- 31.Parney IF, Chang S. Current chemotherapy for glioblastoma. Cancer J. 2003;9:149–156. doi: 10.1097/00130404-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Parney IF, Farr-Jones MA, Chang LJ, Petruk KC. Human glioma immunobiology in vitro: implications for immunogene therapy. Neurosurgery. 2000;46:1169–1178. doi: 10.1097/00006123-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Parney IF, Farr-Jones MA, Petruk KC. Improved technique for establishing short term human brain tumor cultures. J Neurooncol. 1999;43:1–10. doi: 10.1023/a:1006115608103. [DOI] [PubMed] [Google Scholar]
- 34.Parney IF, Hao C, Petruk KC. Glioma immunology and immunotherapy: a review. Neurosurgery. 2000;46:778–792. doi: 10.1097/00006123-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Parsa AT, Chakrabarti I, Hurley PT, Chi JH, Hall JS, Kaiser MG, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–1000. doi: 10.1097/00006123-200010000-00050. [DOI] [PubMed] [Google Scholar]
- 36.Parsa AT, Miller JI, Eggers AE, Ogden AT, III, Anderson RC, Bruce JN. Autologous adjuvant linked fibroblasts induce anti-glioma immunity: implications for development of a glioma vaccine. J Neurooncol. 2003;64:77–87. doi: 10.1007/BF02700023. [DOI] [PubMed] [Google Scholar]
- 37.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 38.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92:288–293. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- 39.Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51:279–285. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- 40.Sedgwick JD, Schwender S, Gregersen R, Dorries R, ter Meulen V. Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med. 1993;177:1145–1152. doi: 10.1084/jem.177.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang J, Flomenberg P, Harshyne L, Kenyon L, Andrews DW. Glioblastoma patients exhibit circulating tumor-specific CD8+ T cells. Clin Cancer Res. 2005;11:5292–5299. doi: 10.1158/1078-0432.CCR-05-0545. [DOI] [PubMed] [Google Scholar]
- 43.Wagner S, Czub S, Greif M, Vince GH, Süss N, Kerkau S, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82:12–16. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–930. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 45.Won EK, Zahner MC, Grant EA, Gore P, Chicoine MR. Analysis of the antitumoral mechanisms of lipopolysaccharide against glioblastoma multiforme. Anticancer Drugs. 2003;14:457–466. doi: 10.1097/00001813-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Yamanaka R. Cell- and peptide-based immunotherapeutic approaches for glioma. Trends Mol Med. 2008;14:228–235. doi: 10.1016/j.molmed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka R, Honma J, Tsuchiya N, Yajima N, Kobayashi T, Tanaka R. Tumor lysate and IL-18 loaded dendritic cells elicits Th1 response, tumor-specific CD8+ cytotoxic T cells in patients with malignant glioma. J Neurooncol. 2005;72:107–113. doi: 10.1007/s11060-004-3550-9. [DOI] [PubMed] [Google Scholar]
- 48.Yamanaka R, Itoh K. Peptide-based immunotherapeutic approaches to glioma: a review. Expert Opin Biol Ther. 2007;7:645–649. doi: 10.1517/14712598.7.5.645. [DOI] [PubMed] [Google Scholar]
- 49.Yong VW, Antel J. Culture of glial cells from human brain biopsies. In: Fedoroff S, Richardson A, editors. Protocol for Neural Cell Culture. 2. Totowa, NJ: Humana Press; 1996. pp. 81–87. [Google Scholar]
- 50.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 51.Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–319. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Handel MV, Schartner JM, Hagar A, Allen G, Curet M, et al. Regulation of IL-10 expression by upstream stimulating factor (USF-1) in glioma-associated microglia. J Neuroimmunol. 2007;184:188–197. doi: 10.1016/j.jneuroim.2006.12.006. [DOI] [PubMed] [Google Scholar]