Abstract
In cancer cell lines and rodent models calcium and vitamin D favorably modulate cell proliferation, differentiation, and apoptosis in colonic epithelia. These effects may be modulated by local expression of the calcium receptor (CaR), the vitamin D receptor (VDR), and the P450 cytochromes CYP27B1 and CYP24A1, however, they have yet to be investigated in humans. To address this gap, we conducted a randomized, double-blinded, placebo-controlled 2×2 factorial clinical trial. Patients with at least one pathology-confirmed colorectal adenoma were treated with 2 g/day elemental calcium and/or 800 IU/day vitamin D3 versus placebo over 6 months (N=92; 23/group). CaR, VDR, CYP27B1, and CYP24A1 expression and distribution in biopsies of normal-appearing rectal mucosa were detected by standardized automated immunohistochemistry and quantified by image analysis. In the calcium-supplemented group CaR expression increased 27% (p=0.03) and CYP24A1 expression decreased 21% (p=0.79). In the vitamin D3-supplemented group CaR expression increased 39% (p=0.01) and CYP27B1 expression increased 159% (p=0.06). In patients supplemented with both calcium and vitamin D3 VDR expression increased 19% (p=0.13) and CaR expression increased 24% (p=0.05). These results provide mechanistic support for further investigation of calcium and vitamin D3 as chemopreventive agents against colorectal neoplasms, and CaR, VDR, CYP27B1, and CYP24A1 as modifiable, pre-neoplastic risk biomarkers for colorectal neoplasms.
Keywords: calcium, vitamin D, colonic neoplasms, biomarkers, clinical trial
Introduction
Colorectal cancer remains the second leading cause of cancer deaths in the United States, despite advances in cancer screening and treatment (1). The molecular basis for colon carcinogenesis has become clearer (2), aiding in the development of much needed treatable pre-neoplastic biomarkers of risk for colorectal neoplasms.
There is strong biological plausibility and animal and human observational evidence for protective effects of calcium and vitamin D, acting separately and synergistically through, at least in part, their respective receptors against colorectal neoplasms. Colonic luminal calcium binding to the calcium receptor (CaR, also referred to as the calcium sensing receptor) may directly modulate the cell cycle of colonocytes, partly by 1) inhibiting the β-catenin/TCF transcription complex (3, 4),2) promoting activation of E-cadherin (3), and 3) reducing the concentration of 25-hydroxyvitamin D 24-hydroxylase (CYP24A1)(5). Luminal calcium is also hypothesized to bind pro-inflammatory secondary bile acids and ionized fatty acids (3, 6). Binding of vitamin D to the vitamin D receptor (VDR) may modulate the cell cycle, partly by 1) competitively binding β-catenin(7),2) up-regulating p21 (8)and E-cadherin expression (9), and 3) regulating growth factors (10). The VDR also promotes detoxification of the secondary bile acid lithocholic acid (10). Also, prospective cohort studies have consistently found higher total calcium intake to be associated with reduced risk for colorectal neoplasms (10), calcium supplementation reduces colorectal adenoma recurrence(modified by vitamin D status)(11), and higher circulating concentrations of 25(OH)D are inversely associated with colorectal neoplasms(10, 12, 13).
The CaR is expressed in all parts of the gastrointestinal tract and sporadically expressed in well-to moderately-differentiated colon carcinomas; however, little to no expression of the CaR is found in undifferentiated carcinomas (14). Colorectal mucosa expresses the VDR; the 1,25(OH)2D synthesizing enzyme, 25(OH)D-1α-hydroxylase (CYP27B1);and the catabolizing enzyme CYP24A1, suggesting an autocrine/paracrine function for vitamin D in colorectal mucosa that is separate from its role in systemic calcium homeostasis (15). Expression of the VDR and CYP27B1 was reported to be increased in well-to moderately-differentiated colon carcinomas; however, little to no expression was observed in undifferentiated colon carcinomas (16–19). Expression of CYP24A1 was reported to be increased in undifferentiated colon carcinomas relative to moderately differentiated colon carcinomas (17, 19).
CaR, VDR, CYP27B1, and CYP24A1 are appealing potential modifiable biomarkers of risk for colorectal neoplasms given the reported differences in their expression between cancerous and normal mucosa and their functional importance in modulating the disease-reducing actions of calcium and vitamin D. We know of no other reported human studies in which the effects of calcium and vitamin D supplementation on the expression of these markers in normal human colorectal mucosa were evaluated. To address this, as reported herein, we conducted a pilot, randomized, double-blind, placebo-controlled 2 × 2 factorial chemoprevention clinical trial of supplemental calcium and vitamin D3, alone and in combination versus placebo over 6 months, to estimate the efficacy of these agents on markers of calcium and vitamin D metabolism in the normal colorectal mucosa.
Study Participants and Methods
Participant population
A detailed description of the study protocol for recruitment procedures and detailed specific exclusions was published previously (20). Briefly, eligible participants were 30 to 75 years of age, in general good health, and had a history of at least one pathology-confirmed adenomatous colorectal polyp within the past 36 months. Exclusions from participation included contraindications to calcium or vitamin D supplementation or rectal biopsy procedures, and medical conditions, habits, or medication usage that potentially could interfere with the study. Participants were recruited from patients attending the Digestive Diseases Clinic at the Emory Clinic, Emory University.
Clinical trial protocol
Between April 2005 and January 2006, 522 potentially eligible patients were identified through initial screening of electronic medical records; of these, 244 (43%) were contacted, and of these 105 (47%) attended the eligibility visit to be interviewed, sign a consent form, complete questionnaires, and provide blood samples (20). Diet was assessed using a semi-quantitative Willett Food Frequency Questionnaire (21). Medical and pathology records were reviewed. Following a 30-dayplacebo run-in trial, 92 (88%) participants with no significant perceived side effects and who took at least 80% of their assigned tablets underwent a baseline rectal biopsy, and, with additional consent, were randomly assigned to the following four treatment groups: placebo (n = 23), 2.0 g elemental calcium supplementation (as calcium carbonate in equal doses twice daily; n = 23), 800 IU vitamin D3 supplementation (400IU twice daily; n = 23), and 2.0g elemental calcium plus 800 IU vitamin D3 supplementation (n = 23). Additional details and rationale for the doses and forms of calcium and vitamin D3 supplements were previously published (20). Participants were instructed to maintain their usual diet and not take any new nutritional supplements they were not taking at the time of entry into the study. All aspects of the trial were approved by the Institutional Review Board of Emory University.
During the 6-monthtreatment period, participants attended follow-up visits 2 and 6 months after randomization. At follow-up visits participants completed questionnaires and were interviewed about adherence and adverse events. At the 6-monthfollow-up, participants again under went a venipuncture and rectal biopsy. All visits for a given participant were scheduled for the same time of day to control for potential circadian variation. Dietary, lifestyle, and other factors hypothesized to modify biomarker expression in normal colon mucosa were assessed at baseline and at 6-monthsfollow-up. Participants were asked to abstain from aspirin use seven days prior to each biopsy. Participants were not required to be fasting for their visits and did not take a bowel cleansing preparation or enema.
Six approximately one millimeter thick biopsy specimens were taken from the normal appearing rectal mucosa 10 cm above the level of the external anal aperture through a short rigid sigmoidoscope using a jumbo cup flexible biopsy forceps mounted on a semi-rigid rod. No biopsies were taken within 4.0 cm of a polypoid lesion. Biopsies were placed onto a strip of bibulous paper and immediately placed in phosphate buffered saline(PBS), oriented, transferred to 10% normal buffered formalin for 24hours, and then transferred to 70% ethanol. Then, within a week, the biopsies were processed and embedded in paraffin blocks (2 blocks of 3 biopsies per participant, per biopsy visit).
Immunohistochemistry protocol
Five slides with 4 levels of 3 micron thick biopsy sections taken 40 microns apart were prepared for each antigen, yielding a total of 20 levels for each antigen. Antigen retrieval was performed by placing the slides in a preheated Pretreatment Module (Lab Vision Corp.) with 100x Citrate Buffer (pH 6.0; DAKO S1699, DAKO Corp.) and steaming them for 40 min. Following antigen retrieval, slides were immunohistochemically processed in a DAKO Automated Immunostainer (DAKO Corp.) using a labeled streptavidin-biotin method for CaR, VDR, CYP27B1, and CYP24A1 (Table 1, Figure 1, and supplementary material Figure 1 and References). No slides were counter stained. After processing, slides were cover slipped with a Leica CV5000 Cover slipper (Leica Microsystems, Inc.). Each staining batch contained positive and negative slides; normal kidney was the positive control tissue for CYP27B1 and CYP24A1, and normal colon tissue was the positive control for CaR and VDR. The negative and positive control slides were treated identically to the patients’ slides except that antibody diluent was used rather than primary antibody on the negative slides.
Table 1.
Summary of biomarker immunohistochemical protocols for: calcium receptor, vitamin D receptor, CYP27B1, and CYP24A1
| Antibody | Manufacturer | Catalog No. | Dilution | Detection kit* |
|---|---|---|---|---|
| A. CaR | Sigma-Aldrich Co., St. Louis, MO | C0493 | 1:200 | LSAB2 |
| B. VDR | Santa Cruz Inc., Santa Cruz, CA | 1531-1 | 1:7500 | LSAB2 |
| C. CYP27B1 | Santa Cruz Inc., Santa Cruz, CA | SC-49642 | 1:100 | LSAB+ |
| D. CYP24A1 | Santa Cruz Inc., Santa Cruz, CA | SC-32166 | 1:50 | LSAB+ |
DAKO Corp., Carpinteria, CA
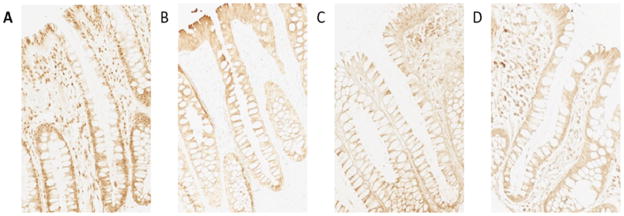
Figure 1. Biomarker immunohistochemical images of colon crypts (200×).
A. calcium receptor
B. vitamin D receptor
C. CYP27B
D. CYP24A1
Protocol for quantifying labeling densities of immunohistochemically detected biomarkers in normal colon crypts (“scoring”)
A detailed description of the protocol used to quantify biomarker labeling optical densities (“biomarker expression”) in normal colon crypts was previously described (20). Briefly, a scorable crypt was defined as an intact crypt extending from the muscularis mucosa to colon lumen (22). Prior to “scoring”, the negative and positive control slides were checked for staining adequacy. The major equipment and software for the image analysis procedures included: personal computer, light microscope (Olympus BX40, Olympus Corporation, Japan) with appropriate filters and attached digital light microscope camera (Polaroid DMC Digital Light Microscope Camera, Polaroid Corporation, USA), digital drawing board, ImagePro Plus image analysis software (Media Cybernetics, Inc., MD), our in-house developed plug-in software for colorectal crypt analysis, and Microsoft Access 2003 relational database software (Microsoft Corporation, WA).
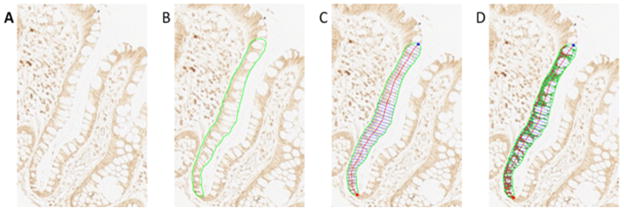
Evaluation of biomarker expression consisted of the same technician cleaning all slides, selecting the two of the three biopsies with the most scorable crypts per biopsy, creating background correction images for each slide scored, capturing 16-bit grayscale images of crypts at 200× magnification, and tracing the border of the “hemicrypt” (one half of the crypt). The program then divided the outlined hemicrypt into equally spaced segments that corresponded to the average width of colonocytes, and measured the optical density of the labeling across the entire hemicrypt and within each segment, adjusting for the background. The technician then repeated this process for the adjacent hemicrypt, and proceeded to the next crypt, level, biopsy, and/or slide. The goal was to score 16 to 20 hemicrypts per biopsy visit for each biomarker (Figure 2).
Figure 2. Quantitative image analysis.
A. finding scorable crypts
B. tracing the hemicrypt
C. automated sectioning of the trace
D. automated quantification of CYP24A1 labeling optical density
Reliability control was performed by selecting samples of previously analyzed slides to be re-analyzed by the technician. The technician was blinded to the selection. Intra-reader reliability for CaR, VDR, CYP27B1, and CYP24A1 was between 0.95 – 0.99 throughout.
Protocol for measuring serum 25-OH-vitamin D and 1,25-(OH)2-vitamin D levels
Serum25-OH-vitamin D and 1,25-(OH)2-vitamin D were measured by Dr. Bruce W. Hollis at the Medical University of South Carolina using a RIA method as previously described (23, 24). Serum samples for baseline and follow-up visits for all subjects were assayed together, ordered randomly, and labeled to mask treatment group, follow-up visit, and quality control replicates. The average intra-assay coefficient of variation was 2.3% and 6.2% for serum25-OH-vitamin D and for 1,25-(OH)2-vitamin D, respectively.
Statistical analysis
Treatment groups were assessed for comparability of characteristics at baseline and at final follow-up by the Fisher’s exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. Slide scoring reliability was analyzed using intra-class correlation coefficients.
The mean labeling optical density expression of each biomarker on each study participant, at baseline and 6-month follow-up, was calculated by summing the biomarker’s expression for all analyzed crypts and dividing by the total number of analyzed crypts. Biomarker expression was transformed to adjust for possible staining batch effects by dividing an individual’s mean biomarker expression by their batch mean biomarker expression (20). Measures of crypt biomarker distribution selected a priori were the upper 40% of the crypts (differentiation zone) and the lower 60% of the crypts (proliferation zone), to represent distinct functional zones of colon crypts. In a sensitivity analysis, we also transformed biomarker expression by subtracting from an individual’s mean biomarker expression their batch mean expression; the results did not materially differ from those reported.
Primary analyses were based on assigned treatment at the time of randomization, regardless of adherence status (intent-to-treat analysis). Treatment effects were evaluated by assessing the differences in the transformed biomarker expression from baseline to the 6-month follow-up between participants in the active treatment groups and those in the placebo group by a repeated-measures linear MIXED effects model. The model included the intercept, follow-up visit effects (baseline and follow-up), and interactions between treatment groups and the follow-up visit effect. Because optical density is measured in arbitrary units, to provide perspective on the magnitude of the treatment effects, we also calculated the relative effect. The relative effect was calculated as the (treatment group at follow-up/treatment group at baseline)/(placebo group at follow-up/placebo group at baseline). The relative effect provides a conservative estimate of the proportional change in the treatment group relative to that in the placebo group, and its interpretation is somewhat analogous to that of an odds ratio (e.g., a relative effect of 2.0 would mean that the proportional change in the treatment group was two times that in the placebo group).
The distributions of batch standardized CaR, VDR, CYP27B1, and CYP24A1 labeling optical densities along the full length of the crypts were graphically plotted and evaluated using the LOESS procedure. First, each hemicrypt was standardized to 50 sections. Then, the average of each section across all crypts was predicted by the LOESS model separately for each patient and then for each treatment group by visit. The results were graphically plotted along with the smoothing lines. Although the plots illustrate the distribution of expression, they do not provide a complete analysis of treatment effects because they do not account for changes in the placebo group.
Statistical analyses were performed using SAS 9.2 statistical software (SAS Institute Inc.). A P value ≤ 0.05 (two-sided) was considered statistically significant.
Results
Characteristics of study participants
Baseline characteristics of study participants did not significantly differ by treatment group (Table 2). The mean age of study participants was 61 years, 70% were men, 71% were white, and 20% had a family history of colorectal cancer in a first degree relative. Most participants were non-smokers, college graduates, and overweight.
Table 2.
Selected baseline characteristics of the study participants* (n=92)
| Characteristics | Treatment Group |
P-value** | |||
|---|---|---|---|---|---|
| Placebo (n=23) | Calcium (n=23) | Vitamin D (n=23) | Calcium + Vit. D (n=23) | ||
| Demographics, medical history, habits, anthropometrics | |||||
| Age, years | 58.5 (8.2) | 61.9 (8.2) | 60.2 (8.1) | 62.1 (7.5) | 0.39 |
| Men (%) | 70 | 70 | 70 | 70 | 1.00 |
| White (%) | 74 | 83 | 65 | 61 | 0.39 |
| College graduate (%) | 65 | 61 | 57 | 44 | 0.53 |
| History of colorectal cancer in 1° relative (%) | 17 | 30 | 17 | 13 | 0.60 |
| Take NSAID¥ regularly§ (%) | 22 | 13 | 9 | 22 | 0.60 |
| If woman (n = 28), taking estrogens (%) | 4 | 9 | 4 | 4 | 1.00 |
| Current smoker (%) | 9 | 4 | 0 | 0 | 0.61 |
| Take multivitamin (%) | 30 | 30 | 26 | 39 | 0.86 |
| Body mass index (BMI), kg/m2 | 30.6 (7.2) | 29.4 (5.5) | 28.9 (5.56) | 31.6 (6.0) | 0.44 |
| Mean dietary intakes*** | |||||
| Total energy intake, kcal/d | 1,596 (528) | 1,788 (691) | 1,848 (821) | 1,845 (752) | 0.59 |
| Total§§ calcium, mg/d | 625 | 678 | 753 | 733 | 0.75 |
| Total§§ vitamin D, IU/d | 279 | 326 | 348 | 401 | 0.50 |
| Total fat, gm/d | 66 | 66 | 61 | 65 | 0.59 |
| Dietary fiber, gm/d | 15 | 16 | 16 | 15 | 0.97 |
| Alcohol intake, gm/d | 8 | 10 | 13 | 9 | 0.84 |
| Total serum vitamin D | |||||
| 25-OH-vitamin D, ng/mL | 20.4 (7.6) | 25.7 (7.6) | 21.0 (8.3) | 20.9 (9.7) | 0.12 |
Data are given as means (SD) unless otherwise specified.
By Fisher’s exact χ2 test for categorical variables, and ANOVA for continuous variables.
Nonsteroidal anti-inflammatory drug.
At least once a week.
All nutrients energy adjusted using residual method (49).
Diet plus supplements.
Adherence to visit attendance averaged 92% and did not significantly differ among the four treatment groups. On average, at least 80% of pills were taken by 93% of participants at the first follow-up visit and by 84% of participants at the final follow-up visit. No adverse events were attributed to study procedures or treatments. Seven participants (8%) were lost to follow-up. Dropouts included one person from the vitamin D3 supplementation group and two from each of the other three groups.
Baseline serum 25-OH-vitamin D and 1,25-(OH)2-vitamin D levels did not differ between the four treatment groups. At the conclusion of the study, serum 25-OH-vitamin D levels had increased 60% (p<0.0001) and 56% (p<0.0001) in the vitamin D3 and calcium/vitamin D3 groups, respectively, relative to placebo; however, mean serum 25-OH-vitamin D concentrations remained below 32 ng/ml in all treatment groups (20). There was no evidence of treatment effect modification by obesity status (body mass index ≥ 30).
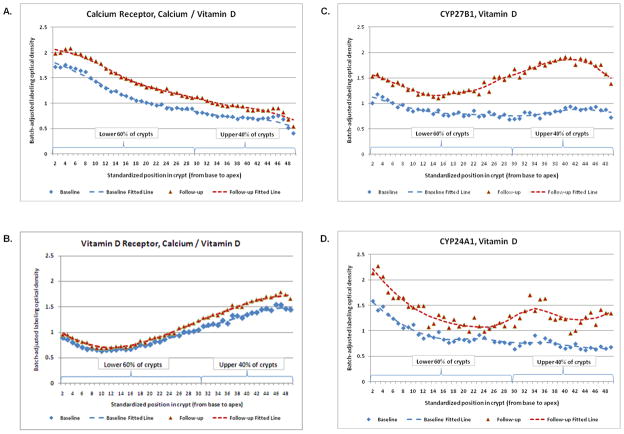
Calcium receptor
CaR expression along the full lengths of colorectal crypts appeared highest at the base of the crypts and gradually decreased towards the crypt apex (Figure 3A). After 6 months of treatment, CaR expression statistically significantly increased along the full length of crypts by 27% (p=0.03), 39% (p=0.01), and 24% (p=0.05) in the calcium, vitamin D3, and calcium/vitamin D3 groups, respectively, relative to the placebo group. In the upper 40% of crypts the CaR expression increased 21% (p=0.13), 38% (p=0.02), and 26% (p=0.07) in the calcium, vitamin D3, and calcium/vitamin D3 groups, respectively, relative to the placebo group. In the lower 60% of crypts CaR expression increased, 17% (p=0.14), 26% (p=0.06), and 17% (p=0.15) in the calcium, vitamin D3, and calcium/vitamin D3 groups, respectively, relative to the placebo group(Table 3A).
Figure 3. Representative examples of labeling expression distribution* of the (A) calcium receptor†, the (B) vitamin D receptor†, (C) CYP27B1‡, and (D) CYP24A1‡ along normal colorectal crypts by treatment group at baseline and 6-month follow-up.
* Distribution plots represent labeling optical density in colon crypts
† Representative example from the calcium/vitamin D3 treatment group
‡ Representative example from the vitamin D3 treatment group
Table 3.
Expression of calcium receptor and vitamin D receptor in the normal-appearing colorectal mucosa during the clinical trial
| Treatment Group | Baseline |
6-mo follow-up |
Absolute Rx effect |
Relative Effect¥ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std Err | P | n | Mean | Std Err | P | Rx effect* | Std Err | P** | ||
| A. CaR§ | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 20 | 1.11 | 0.07 | 19 | 1.00 | 0.07 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 1.01 | 0.06 | 0.28 | 20 | 1.16 | 0.07 | 0.09 | 0.26 | 0.12 | 0.03 | 1.27 |
| Vitamin D | 23 | 0.87 | 0.06 | 0.01 | 19 | 1.09 | 0.07 | 0.37 | 0.33 | 0.12 | 0.01 | 1.39 |
| Ca + Vit D | 22 | 1.04 | 0.06 | 0.45 | 20 | 1.16 | 0.07 | 0.10 | 0.23 | 0.12 | 0.05 | 1.24 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 20 | 0.34 | 0.02 | 19 | 0.32 | 0.02 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 0.31 | 0.02 | 0.37 | 20 | 0.35 | 0.02 | 0.33 | 0.06 | 0.04 | 0.13 | 1.21 |
| Vitamin D | 23 | 0.25 | 0.02 | 0.01 | 19 | 0.33 | 0.02 | 0.89 | 0.09 | 0.04 | 0.02 | 1.38 |
| Ca + Vit D | 22 | 0.29 | 0.02 | 0.13 | 20 | 0.35 | 0.02 | 0.45 | 0.07 | 0.04 | 0.07 | 1.26 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 20 | 0.76 | 0.05 | 19 | 0.74 | 0.05 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 0.73 | 0.04 | 0.63 | 20 | 0.84 | 0.05 | 0.16 | 0.12 | 0.08 | 0.14 | 1.17 |
| Vitamin D | 23 | 0.63 | 0.04 | 0.04 | 19 | 0.77 | 0.05 | 0.66 | 0.16 | 0.08 | 0.06 | 1.26 |
| Ca + Vit D | 22 | 0.73 | 0.04 | 0.57 | 20 | 0.83 | 0.05 | 0.18 | 0.12 | 0.08 | 0.15 | 1.17 |
| B. VDR*** | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 21 | 1.06 | 0.06 | 18 | 1.00 | 0.07 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.95 | 0.06 | 0.19 | 21 | 0.93 | 0.06 | 0.46 | 0.05 | 0.12 | 0.67 | 1.05 |
| Vitamin D | 22 | 0.93 | 0.06 | 0.14 | 17 | 0.97 | 0.07 | 0.70 | 0.09 | 0.12 | 0.43 | 1.10 |
| Ca + Vit D | 21 | 1.06 | 0.06 | 0.92 | 18 | 1.18 | 0.07 | 0.07 | 0.18 | 0.12 | 0.13 | 1.19 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 21 | 0.58 | 0.03 | 18 | 0.59 | 0.04 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.52 | 0.03 | 0.19 | 21 | 0.56 | 0.03 | 0.47 | 0.03 | 0.06 | 0.66 | 1.05 |
| Vitamin D | 22 | 0.49 | 0.03 | 0.04 | 17 | 0.55 | 0.04 | 0.38 | 0.05 | 0.06 | 0.41 | 1.11 |
| Ca + Vit D | 21 | 0.54 | 0.03 | 0.42 | 18 | 0.66 | 0.04 | 0.19 | 0.10 | 0.06 | 0.09 | 1.19 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 21 | 0.47 | 0.04 | 18 | 0.41 | 0.04 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.46 | 0.04 | 0.81 | 21 | 0.41 | 0.04 | 0.92 | 0.01 | 0.07 | 0.92 | 1.01 |
| Vitamin D | 22 | 0.45 | 0.04 | 0.58 | 17 | 0.44 | 0.04 | 0.58 | 0.06 | 0.08 | 0.44 | 1.14 |
| Ca + Vit D | 21 | 0.45 | 0.04 | 0.69 | 18 | 0.52 | 0.04 | 0.06 | 0.13 | 0.08 | 0.10 | 1.31 |
Rx effect = [(treatment group follow-up) − (treatment group baseline)] − [(placebo group follow-up) − (placebo group baseline)].
P value for difference between each active treatment group and placebo group from repeated-measures MIXED model.
Relative effect = [(treatment group follow-up)/(treatment group baseline)]/[(placebo follow-up)/(placebo baseline)]; interpretation similar to that for an odds ratio (e.g., a relative effect of 1.7 indicates a 70% proportional increase in the treatment group relative to that in the placebo group).
CaR = Calcium receptor
VDR = Vitamin D receptor
Vitamin D receptor
VDR expression along the full lengths of colorectal crypts appeared lowest at the base of crypts and gradually increased towards the crypt apex (Figure 3B). After 6 months of treatment, VDR expression increased in the calcium/vitamin D3 group 19% (p=0.13) along the full length of crypts, 19% (p=0.09) in the upper 40% of crypts, and 31% (p=0.10) in the lower 60% of crypts, relative to the placebo group. In the calcium and vitamin D3 groups there were also estimated increases in VDR expression, but they were of lower magnitude and not statistically significant (Table 3B).
CYP27B1
CYP27B1 expression along the full lengths of colorectal crypts at baseline tended to decrease from the base to the mid-third of the crypt where it remained level and then tended to increase slightly from there to the crypt apex (Figure 3C). After 6 months of treatment, CYP27B1 expression increased in the vitamin D3 group 159% (p=0.06) along the full length of crypts, 111% (p=0.04) in the upper 40% of crypts, and 110% (p=0.04) in the lower 60% of crypts, relative to the placebo group. In the calcium and the calcium/vitamin D3 groups, there were also estimated increases in CYP27B1 expression, similar in both treatment groups, but of much lower magnitude than in the vitamin D3 alone group and not statistically significant (Table 4A).
Table 4.
Expression of CYP27B1 andCYP24A1 in the normal-appearing colorectal mucosa during the clinical trial.
| Treatment Group | Baseline |
6-mo follow-up |
Absolute Rx effect |
Relative Effect¥ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std Err | P | n | Mean | Std Err | P | Rx effect* | Std Err | P** | ||
| A. CYP27B1§§ | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 20 | 1.07 | 0.18 | 20 | 1.00 | 0.09 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.89 | 0.10 | 0.38 | 16 | 1.11 | 0.36 | 0.76 | 0.29 | 0.42 | 0.49 | 1.34 |
| Vitamin D | 22 | 0.85 | 0.12 | 0.31 | 18 | 2.06 | 0.61 | 0.10 | 1.28 | 0.66 | 0.06 | 2.59 |
| Ca + Vit D | 22 | 1.20 | 0.15 | 0.56 | 19 | 1.46 | 0.41 | 0.29 | 0.32 | 0.48 | 0.50 | 1.30 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 20 | 0.40 | 20 | 0.45 | 0.10 | 0.10 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.38 | 0.10 | 0.86 | 16 | 0.51 | 0.11 | 0.70 | 0.08 | 0.21 | 0.69 | 1.21 |
| Vitamin D | 22 | 0.35 | 0.10 | 0.73 | 18 | 0.84 | 0.11 | 0.01 | 0.44 | 0.20 | 0.04 | 2.11 |
| Ca + Vit D | 22 | 0.46 | 0.10 | 0.67 | 19 | 0.57 | 0.10 | 0.43 | 0.05 | 0.20 | 0.79 | 1.09 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 20 | 0.65 | 0.13 | 20 | 0.58 | 0.13 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.52 | 0.13 | 0.47 | 16 | 0.60 | 0.14 | 0.92 | 0.15 | 0.25 | 0.55 | 1.30 |
| Vitamin D | 22 | 0.51 | 0.13 | 0.44 | 18 | 0.96 | 0.14 | 0.05 | 0.52 | 0.25 | 0.04 | 2.10 |
| Ca + Vit D | 22 | 0.67 | 0.13 | 0.95 | 19 | 0.72 | 0.13 | 0.43 | 0.14 | 0.25 | 0.59 | 1.23 |
| B. CYP24A1† | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 21 | 1.04 | 0.21 | 18 | 1.00 | 0.23 | 0.00 | . | . | 1.00 | ||
| Calcium | 20 | 1.05 | 0.21 | 0.96 | 20 | 0.80 | 0.21 | 0.52 | −0.22 | 0.43 | 0.62 | 0.79 |
| Vitamin D | 21 | 0.85 | 0.21 | 0.52 | 19 | 1.34 | 0.22 | 0.28 | 0.53 | 0.43 | 0.22 | 1.65 |
| Ca + Vit D | 22 | 1.06 | 0.20 | 0.94 | 17 | 1.54 | 0.23 | 0.10 | 0.52 | 0.43 | 0.24 | 1.51 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 21 | 0.37 | 0.09 | 18 | 0.40 | 0.10 | 0.00 | . | . | 1.00 | ||
| Calcium | 20 | 0.42 | 0.09 | 0.70 | 20 | 0.27 | 0.09 | 0.35 | −0.18 | 0.19 | 0.35 | 0.59 |
| Vitamin D | 21 | 0.31 | 0.09 | 0.68 | 19 | 0.51 | 0.10 | 0.40 | 0.17 | 0.19 | 0.37 | 1.51 |
| Ca + Vit D | 22 | 0.40 | 0.09 | 0.81 | 17 | 0.59 | 0.10 | 0.17 | 0.16 | 0.19 | 0.39 | 1.38 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 21 | 0.69 | 0.13 | 18 | 0.63 | 0.14 | 0.00 | . | . | 1.00 | ||
| Calcium | 20 | 0.66 | 0.13 | 0.89 | 20 | 0.58 | 0.13 | 0.79 | −0.03 | 0.25 | 0.92 | 0.96 |
| Vitamin D | 21 | 0.61 | 0.13 | 0.67 | 19 | 0.78 | 0.14 | 0.46 | 0.22 | 0.25 | 0.39 | 1.38 |
| Ca + Vit D | 22 | 0.74 | 0.13 | 0.78 | 17 | 0.89 | 0.14 | 0.21 | 0.21 | 0.26 | 0.43 | 1.31 |
Rx effect = [(treatment group follow-up) − (treatment group baseline)] − [(placebo group follow-up) − (placebo group baseline)].
P value for difference between each active treatment group and placebo group from repeated-measures MIXED model.
Relative effect = [(treatment group follow-up)/(treatment group baseline)]/[(placebo follow-up)/(placebo baseline)]; interpretation similar to that for an odds ratio (e.g., a relative effect of 1.7 indicates a 70% proportional increase in the treatment group relative to that in the placebo group).
CYP27B1 = Cytochrome P450 family 27B1
CYP24A1 = Cytochrome P450 family 24A1
CYP24A1
CYP24A1 expression along the full lengths of colorectal crypts appeared highest at the base of crypts and sharply declined towards the upper 40% of the crypts, followed by a leveling off of expression throughout the upper parts of crypts (Figure 3D). After 6 months of treatment CYP24A1 expression in the calcium group tended to decrease, particularly in the upper 40% of the crypts, whereas in the vitamin D3 groups it tended to increase throughout the crypts (Table 4B); however, despite the large estimated treatment effects none of these findings was statistically significant.
Discussion
The results of this pilot randomized, controlled clinical trial provide the first evidence that supplemental calcium and vitamin D3, alone or in combination, modify CaR, VDR, CYP27B1, and CYP24A1 expression in the normal colorectal mucosa of sporadic adenoma patients. Following six months of treatment, 2.0 g of calcium daily significantly increased CaR expression, but did not substantially change VDR expression. Although not statistically significant, the results also suggested that calcium substantially increased CYP27B1 and decreased CYP24A1 expression. Treatment with 800 IU of vitamin D3 daily significantly increased expression of the CaR (slightly more than did calcium) andCYP27B1. Although not statistically significant, the results also suggested that vitamin D3 increased VDR and CYP24A1 expression. The combined calcium and vitamin D3 treatment statistically significantly increased CaR expression to about the same degree as calcium treatment alone, and thus less than vitamin D3 alone. Although not statistically significant, the results also suggested that the combined treatment increased expression of the VDR (more than did calcium or vitamin D3 alone), CYP27B1 (about the same as did calcium alone, but less than vitamin D3 alone), and CYP24A1 (about the same as did vitamin D3 alone). The findings from this study are relevant because there is substantial epidemiological (11, 25, 26) and basic science (19, 27)evidence that calcium and vitamin D3 may reduce risk of incident or recurrent colorectal neoplasms, and that any protective effects of calcium and vitamin D3 may, at least in part, operate through the CaR, the VDR, and the vitamin D metabolizing enzymes CYP27B1 and CYP24A1.
Expression of the CaR was reported in normal appearing mucosa in humans (28–32) and rats (33), and in differentiated carcinomas (28–32); however, sporadic to no expression was reported in undifferentiated carcinomas (28–32). It was proposed that in normal colon crypts there is a calcium gradient with low calcium concentrations at the base of the crypts and high concentrations at the crypt apex (34). Decreased calcium concentrations were reported to increase cellular proliferation (4, 32, 35, 36), while increased calcium concentrations were reported to decrease proliferation and promote differentiation (4, 28–30, 32, 36). Loss of function of the CaR disrupts the signaling actions from increased calcium concentrations, such as activating E-cadherin and down regulating β-catenin/TCF signaling (28).
There are no reported human randomized clinical trials on the effects of calcium and vitamin D on CaR expression in normal colon mucosa; however, these effects have been evaluated in colon cancer cell lines. In cell culture, incubation with calcium and/or 1,25(OH)2D3 increased CaR expression (30, 36); calcium and 1,25(OH)2D3 combined increased CaR expression more than did calcium or 1,25(OH)2D3 alone(30). We therefore hypothesized that supplementation with calcium and vitamin D3 alone and combined would increase CaR expression in the normal colon mucosa. Our hypothesis was further supported by the presence of vitamin D response elements in both the P1 and P2 promoters of the CaR gene (37). As hypothesized, we observed increased CaR expression in the calcium, vitamin D, and the combination calcium/vitamin D3 supplementation groups. Vitamin D3 supplementation alone appeared to have had the largest treatment effect, while calcium alone and in combination with vitamin D3 appeared to yield lesser, approximately equal treatment effects. We expected that calcium plus vitamin D3 supplementation would have the greatest treatment effect. There are several plausible explanations for why this was not observed, the first being that our findings may have been due to chance. At least one experiment in rodents reported that calcium and vitamin D individually suppressed tumorigenesis, but the combination of the two was ineffective (38). However, some studies reported stronger combined effects of calcium and vitamin D3 in reducing adenoma and colorectal cancer risk (39, 40). Some of the strongest evidence comes from a large clinical trial of colorectal adenoma recurrence that suggested that calcium supplementation was primarily effective among those with 25(OH)D concentrations greater than the median in the study population (29.1 ng/ml) (11). In our trial only the vitamin D3 supplementation group reached 25(OH)D concentrations greater than 29.1 ng/ml (20), suggesting the possibility of a threshold effect.
There is little consensus on the distribution of the CaR in the normal colon crypt (28, 30, 31, 33). Our study, the largest to investigate this thus far, used disease free colon tissue rather than normal appearing sections of colon mucosa adjacent to neoplastic tissue, and, to the best of our knowledge, is the first to use image analysis software to analyze CaR distribution in the normal colon mucosa. We found CaR expression to be highest at the base of the crypts, amongst the most proliferative cells, and to gradually decline in the differentiation zone towards the crypt apex. These results suggest that CaR expression may undergo negative feed-back regulation by luminal calcium.
An autocrine/paracrine function for vitamin D in the normal colon mucosa is suggested by the expression of the VDR and the vitamin D metabolizing enzymes CYP27B1 and CYP24A1 in the colon mucosa (15). The ability of colon cells to produce the active vitamin D metabolite 1,25(OH)2D was recently demonstrated (41–43). Evidence from animal models suggests that the colonic vitamin D system is regulated separately from the renal vitamin D system (5). It was reported that renal synthesis of 1,25(OH)2D does not produce 1,25(OH)2D in the nanomolar concentrations needed to halt proliferation and induce apoptosis, suggesting that colonic metabolism of 1,25(OH)2D is needed to explain vitamin D’s possible anti-colon carcinogenesis effects (19). The expression of the VDR and CYP27B1 was reported to increase in the early stages of cancer development, but to be greatly reduced in undifferentiated cancers; this suggests that the VDR and CYP27B1act as tumor suppressors, presumably by maximizing 1,25(OH)2D signaling to normalize cell cycle regulation (16–18). CYP24A1 expression was reported to be increased in undifferentiated cancers, presumably mitigating the effects of 1,25(OH)2D signaling (17, 41). Our observation of increased VDR and CYP27B1 expression in the active treatment groups is in agreement with previous reports, which suggests that the anti-proliferative and pro-apoptotic effects of supplemental vitamin D3 may in part be due to increased VDR and CPY27B1 expression. We also observed an insignificant increase in CYP24A1 expression in the vitamin D and calcium plus vitamin D treatment groups, which may minimize the effects of increased VDR and CYP27B1 expression. However, the apparent decrease of CYP24A1 and increase in CYP27B1 in the calcium treatment group may lead to increased vitamin D signaling despite no increase in VDR expression; however, these estimated treatment effects were not statistically significant. The distribution of the VDR and CYP27B1 in the upper 40% of crypts provides additional evidence of the importance of vitamin D signaling for promoting differentiation and apoptosis. CYP24A1 also appeared to be expressed most strongly in the bottom half of the colon crypts, presumably lessening the effects of 1,25(OH)D and thus favoring proliferation.
There are only a few cell culture and animal studies, and no large randomized human trials on the effects of calcium and/or vitamin D on the expression of the VDR, CYP27B1, or CYP24A1 in the normal human colon mucosa. The effects of 25(OH)D3 or 1,25,(OH)2D3 on VDR and CYP27B1 expression in colon cancer cells appear to vary depending upon the differentiation status of the cells; however, CYP24A1 expression consistently increases in response to 25(OH)D3 or 1,25,(OH)2D3 treatment (42, 44, 45). Two separate studies in mice found that low calcium diets increased CYP24A1 mRNA expression, especially in male mice and in the right colon. The low calcium diet also increased VDR and CYP27B1 mRNA expression in the proximal colon and reduced VDR expression in the distal colon; however, this was only observed in female mice. No significant change in VDR or CYP27B1 expression was observed in either the left or right colon in male mice fed a low calcium diet (46, 47).
We previously reported that calcium and vitamin D3 supplementation in this same trial favorably modified expression of markers of proliferation (8), differentiation (8), apoptosis (20), and oxidative DNA damage (48)in the normal human colorectal mucosa. Previous reports in cancer cell lines (49)and human studies (48)suggest that some of the chemopreventative effects of supplemental calcium and vitamin D3 may depend on the expression of the CaR and the VDR. Our results provide in vivo human evidence that supplemental calcium and vitamin D3 modify the expression of the CaR, the VDR, CYP27B1, and CYP24A1 in the normal colorectal epithelium, which may then promote calcium and vitamin D anti-neoplastic signaling pathways.
Our study had several limitations. First, it was a pilot study with a relatively small sample size, increasing the role of chance observations and limiting our ability to perform stratified analyses. Also, we only examined the rectal mucosa and therefore treatment effects in other parts of the colon remain unknown. Another limitation is that we measured protein expression but not protein activity, and, therefore, could not correlate changes in expression with changes in protein activity. Finally, these markers are not proven biomarkers of risk and we do not yet know whether changes in CaR, VDR, CYP27B1, or CYP24A1 affect risk for colorectal neoplasms in humans.
The strengths of this study include 1) that it is, to our knowledge, the first randomized, double-blind, placebo-controlled clinical trial to test the effects of supplemental calcium and vitamin D3, alone and in combination, on components of the calcium and vitamin D metabolizing system in the normal colorectal epithelium in sporadic adenoma patients, 2) the high protocol adherence by study participants, and 3) the automated immunostaining and newly-designed image analysis software to quantify the crypt distribution of the expression of the CaR, the VDR, CYP27B1, and CYP24A1, resulting in high biomarker measurement reliability.
In summary, the results of this pilot clinical trial indicate that calcium and vitamin D3 supplementation, alone and in combination, may increase expression of the CaR, the VDR, and CYP27B1, and that vitamin D3 supplementation, alone and in combination with calcium supplementation, may increase CYP24A1 expression in the normal colorectal mucosa of sporadic colorectal adenoma patients. The anti-carcinogenesis effects of supplemental calcium and vitamin D3 may in part depend on the ability of these agents to favorably modulate the expression of the CaR, the VDR, CYP27B1, and CYP24A1 in the colorectal mucosa. These results, taken together with previous reports (8, 20, 48), suggest that calcium and vitamin D3 supplementation modulate the colorectal mucosa molecular phenotype to inhibit proliferation and promote cellular differentiation and apoptosis. Our results also suggest that the CaR, the VDR, CYP27B1, and CYP24A1 may be treatable biomarkers of risk for colorectal neoplasms and warrant further investigation. Finally, our results support further investigation of calcium and vitaminD3 as chemopreventive agents against colorectal neoplasms.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute, National Institutes of Health (R01 CA104637 and R03 CA121873 to R.M.B.); Georgia Cancer Coalition Distinguished Scholar award (to R.M.B.); the Franklin Foundation. The National Cancer Institute, the Georgia Cancer Coalition, and the Franklin Foundation had no influence on the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA A Cancer Journal for Clinicians. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Potter JD. Colorectal Cancer: Molecules and Populations. J Natl Cancer Inst. 1999;91:916–32. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 3.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29:3687–98. [PubMed] [Google Scholar]
- 4.Kallay E, Bonner E, Wrba F, Thakker RV, Peterlik M, Cross HS. Molecular and functional characterization of the extracellular calcium-sensing receptor in human colon cancer cells. Oncol Res. 2003;13:551–9. doi: 10.3727/000000003108748072. [DOI] [PubMed] [Google Scholar]
- 5.Kallay E, Bises G, Bajna E, Bieglmayer C, Gerdenitsch W, Steffan I, et al. Colon-specific regulation of vitamin D hydroxylases--a possible approach for tumor prevention. Carcinogenesis. 2005;26:1581–9. doi: 10.1093/carcin/bgi124. [DOI] [PubMed] [Google Scholar]
- 6.McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004;23:6349–64. doi: 10.1038/sj.onc.1207716. [DOI] [PubMed] [Google Scholar]
- 7.Jiménez-Lara AM. Colorectal cancer: Potential therapeutic benefits of Vitamin D. The International Journal of Biochemistry & Cell Biology. 2007;39:672–7. doi: 10.1016/j.biocel.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Fedirko V, Bostick RM, Flanders WD, Long Q, Sidelnikov E, Shaukat A, et al. Effects of Vitamin D and Calcium on Proliferation and Differentiation In Normal Colon Mucosa: a Randomized Clinical Trial. Cancer Epidemiology Biomarkers & Prevention. 2009;18:2933–41. doi: 10.1158/1055-9965.EPI-09-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol. 2001;154:369–88. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostick RM, Goodman M, Sidelnikov E. Genetics of Colorectal Cancer. New York: Springer Science + Business Media, LLC; 2009. [Google Scholar]
- 11.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, et al. Vitamin D, Calcium Supplementation, and Colorectal Adenomas: Results of a Randomized Trial. J Natl Cancer Inst. 2003;95:1765–71. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 12.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal Vitamin D Status for Colorectal Cancer Prevention: A Quantitative Meta Analysis. American Journal of Preventive Medicine. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-Hydroxyvitamin D3 Concentrations and Incident Sporadic Colorectal Adenoma Risk: A Pooled Case-Control Study. Am J Epidemiol. 2010:kwq157. doi: 10.1093/aje/kwq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitfield JF. The calcium-sensing receptor--a driver of colon cell differentiation. Curr Pharm Biotechnol. 2009;10:311–6. doi: 10.2174/138920109787847510. [DOI] [PubMed] [Google Scholar]
- 15.Cross HS, Kallay E. Regulation of the colonic vitamin D system for prevention of tumor progression: an update. Future Oncology. 2009;5:493–507. doi: 10.2217/fon.09.22. [DOI] [PubMed] [Google Scholar]
- 16.Cross HS, Bareis P, Hofer H, Bischof MG, Bajna E, Kriwanek S, et al. 25-Hydroxyvitamin D3-1α-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66:287–92. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 17.Cross HS, Bises G, Lechner D, Manhardt T, Kállay E. The Vitamin D endocrine system of the gut--Its possible role in colorectal cancer prevention. The Journal of Steroid Biochemistry and Molecular Biology. 2005;97:121–8. doi: 10.1016/j.jsbmb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Bises G, Kallay E, Weiland T, Wrba F, Wenzl E, Bonner E, et al. 25-Hydroxyvitamin D3-1α-hydroxylase Expression in Normal and Malignant Human Colon. J Histochem Cytochem. 2004;52:985–9. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- 19.Cross HS, Nittke T, Peterlik M. Modulation of Vitamin D Synthesis and Catabolism in Colorectal Mucosa: A New Target for Cancer Prevention. Anticancer Research. 2009;29:3705–12. [PubMed] [Google Scholar]
- 20.Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, et al. Effects of Vitamin D and Calcium Supplementation on Markers of Apoptosis in Normal Colon Mucosa: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cancer Prev Res. 2009;2:213–23. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative fodd frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 22.Bostick RM, Fosdick L, Lillemoe TJ, Overn P, Wood JR, Grambsch P, et al. Methodological findings and considerations in measuring colorectal epithelial cell proliferation in humans. Cancer Epidemiology Biomarkers & Prevention. 1997;6:931–42. [PubMed] [Google Scholar]
- 23.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 24.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42:586–92. [PubMed] [Google Scholar]
- 25.Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes and Control. 1997;8:615–25. doi: 10.1023/a:1018450531136. [DOI] [PubMed] [Google Scholar]
- 26.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium Intake and Risk of Colon Cancer in Women and Men. J Natl Cancer Inst. 2002;94:437–46. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 27.Whitfield JF. Calcium, calcium-sensing receptor and colon cancer. Cancer Lett. 2009;275:9–16. doi: 10.1016/j.canlet.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Bhagavathula N, Hanosh AW, Nerusu KC, Appelman H, Chakrabarty S, Varani J. Regulation of E-cadherin and beta-catenin by Ca2+in colon carcinomain colon carcinoma is dependent on calcium-sensing receptor expression and function. International Journal of Cancer. 2007;121:1455–62. doi: 10.1002/ijc.22858. [DOI] [PubMed] [Google Scholar]
- 29.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular Calcium and Calcium Sensing Receptor Function in Human Colon Carcinomas: Promotion of E-Cadherin Expression and Suppression of β-Catenin/TCF Activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 30.Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium Sensing Receptor in Human Colon Carcinoma: Interaction with Ca2+ and 1,25-Dihydroxyvitamin D3. Cancer Res. 2005;65:493–8. [PubMed] [Google Scholar]
- 31.Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical Localization of the Extracellular Calcium-Sensing Receptor in Normal and Malignant Human Large Intestinal Mucosa. J Histochem Cytochem. 2000;48:595–602. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- 32.Kallay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect Prev. 2000;24:127–36. [PubMed] [Google Scholar]
- 33.Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240–50. doi: 10.1152/ajpgi.00500.2001. [DOI] [PubMed] [Google Scholar]
- 34.Brenner BM, Russell N, Albrecht S, Davies RJ. The effect of dietary vitamin D3 on the intracellular calcium gradient in mammalian colonic crypts. Cancer Letters. 1998;127:43–53. doi: 10.1016/s0304-3835(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 35.Kállay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, et al. Calcium-Dependent c-mycProto-Oncogene Expression and Proliferation of CACO-2 Cells: A Role for a Luminal Extracellular Calcium-Sensing Receptor. Biochemical and Biophysical Research Communications. 1997;232:80–3. doi: 10.1006/bbrc.1997.6225. [DOI] [PubMed] [Google Scholar]
- 36.Bhagavathula N, Kelley EA, Reddy M, Nerusu KC, Leonard C, Fay K, et al. Up regulation of calcium-sensing receptor and mitogen-activated protein kinase signalling in the regulation of growth and differentiation in colon carcinoma. Br J Cancer. 2005;93:1364–71. doi: 10.1038/sj.bjc.6602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canaff L, Hendy GN. Human Calcium-sensing Receptor Gene. Journal of Biological Chemistry. 2002;277:30337–50. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 38.Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988;9:187–90. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- 39.Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and Vitamin D Intakes in Relation to Risk of Distal Colorectal Adenoma in Women. Am J Epidemiol. 2007:kwm026. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 40.Zheng W, Anderson KE, Kushi LH, Sellers TA, Greenstein J, Hong CP, et al. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiology Biomarkers & Prevention. 1998;7:221–5. [PubMed] [Google Scholar]
- 41.Bareis P, Bises G, Bischof MG, Cross HS, Peterlik M. 25-Hydroxy-Vitamin D Metabolism in Human Colon Cancer Cells during Tumor Progression. Biochemical and Biophysical Research Communications. 2001;285:1012–7. doi: 10.1006/bbrc.2001.5289. [DOI] [PubMed] [Google Scholar]
- 42.Lechner D, Kállay E, Cross HS. 1α,25-Dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Molecular and Cellular Endocrinology. 2007;263:55–64. doi: 10.1016/j.mce.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Cross HS, Peterlik M, Reddy GS, Schuster I. Vitamin D metabolism in human colon adenocarcinoma-derived Caco-2 cells: Expression of 25-hydroxyvitamin D3-1α-hydroxylase activity and regulation of side chain metabolism. The Journal of Steroid Biochemistry and Molecular Biology. 1997;62:21–8. doi: 10.1016/s0960-0760(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 44.Bareis P, Kállay E, Bischof MG, Bises G, Hofer H, Pötzi C, et al. Clonal Differences in Expression of 25-Hydroxyvitamin D3-1α-hydroxylase, of 25-Hydroxyvitamin D3-24-hydroxylase, and of the Vitamin D Receptor in Human Colon Carcinoma Cells: Effects of Epidermal Growth Factor and 1α,25-Dihydroxyvitamin D3. Experimental Cell Research. 2002;276:320–7. doi: 10.1006/excr.2002.5528. [DOI] [PubMed] [Google Scholar]
- 45.Murillo G, Matusiak D, Benya RV, Mehta RG. Chemopreventive efficacy of 25-hydroxyvitamin D3 in colon cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2007;103:763–7. doi: 10.1016/j.jsbmb.2006.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nittke T, Selig S, Kallay E, Cross HS. Nutritional calcium modulates colonic expression of vitamin D receptor and pregnane X receptor target genes. Molecular Nutrition & Food Research. 2008;52:S45–S51. doi: 10.1002/mnfr.200700200. [DOI] [PubMed] [Google Scholar]
- 47.Nittke T, Kallay E, Manhardt T, Cross HS. Parallel Elevation of Colonic 1,25-Dihydroxyvitamin D3 Levels and Apoptosis in Female Mice on a Calcium-deficient Diet. Anticancer Research. 2009;29:3727–32. [PubMed] [Google Scholar]
- 48.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:280–91. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: Downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. International Journal of Cancer. 2010;126:631–9. doi: 10.1002/ijc.24762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.