Abstract
Multimodal treatment has improved the outcome of many solid tumors, and in some cases the use of radiosensitizers has significantly contributed to this gain. Activation of the extracellular signaling kinase pathway (MEK/ERK) generally results in stimulation of cell growth and confers a survival advantage playing the major role in human cancer. The potential involvement of this pathway in cellular radiosensitivity remains unclear. We previously reported that the disruption of c-Myc through MEK/ERK inhibition blocks the expression of the transformed phenotype, affects in vitro and in vivo growth, angiogenic signaling, and induces myogenic differentiation in the embryonal rhabdomyosarcoma (ERMS) cell lines (RD). The present study was designed to examine whether the ERK pathway affects intrinsic radiosensitivity of rhabdomyosarcoma cancer cells. Exponentially growing human ERMS, RD, xenograft-derived RD-M1 and TE671 cell lines were used. The specific MEK/ERK inhibitor, U0126, reduced the clonogenic potential of the three cell lines, and was effected by radiation. U0126 inhibited phospho/active ERK1/2 and reduced DNAPKcs suggesting that ERKs and DNA-PKcs cooperate in radioprotection of rhabdomyosarcoma cells. The TE671 cell line-xenotransplanted in mice showed a reduction in tumor mass and increase in the time of tumor progression with U0126 treatment associated with reduced DNAPKcs, an effect enhanced by radiotherapy. Thus, our results show that MEK/ERK inhibition enhances radiosensitivity of rhabdomyosarcoma cells suggesting a rational approach in combination with radiotherapy.
Keywords: Radiotherapy, Rabdomyosarcoma, U1026, MEK/ERK, c-Myc
INTRODUCTION
Rhabdomyosarcoma (RMS) is a rare malignancy. Nonetheless, it is a common childhood cancer, constituting more than 50% of all soft tissue sarcomas. In contrast, RMS is exceedingly infrequent in adults: soft tissue sarcomas make up less than 1% of all adult malignancies, and RMS accounts for 3% of all soft tissue sarcomas (1). Treatment for this malignancy requires a multimodality approach combining surgery with radiotherapy (RT) and/or chemotherapy. Although overall outcomes have improved considerably, the outcome for patients with high-risk disease remains relatively poor, which points to a clear need for new therapeutic strategies. Most RMSs are not amenable to complete surgical resection, and for the majority (70%) of patients recurrence occurs within the first two years after treatment (1-2). In this scenario RT is a major tool in the treatment of RMS. It can eradicate residual tumor cells, especially when the surgical eradication is not complete or limited by the anatomic position such as in the RMS of head and neck and the pelvis region (3). However, local recurrence remains a significant clinical obstacle and represents a common pattern for treatment failure for RMS. One of the major goals of local control for tumors in patients already treated with surgery and chemotherapy is to enhance the sensitivity of RMS tumor cells to the cytotoxicity of ionizing radiation. The substantial experimental body of evidence demonstrates that radiation resistance is associated with the abnormal expression of activated oncogenes, including Ras (4-5) and c-Myc (6). The Ras/Raf/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) cascade regulates proliferation, differentiation, survival, motility, and tissue formation (7-16). Mutated forms of Ras are found in 30% of human cancers (9-11) including RMS (12) and mutations produce proteins that remain locked in a constitutively active state, thereby relaying uncontrolled signals (13). The Ras-MAPK pathway when constitutively activated mediates resistance to ionizing radiation (14) through EGF induction and EGF-receptor mediated activation of prosurvival PI3K-AKT pathway which in turn activates DNA protein kinase catalytic subunit (DNA-PKcs) (15). The Myc/Max transcription factors family play a role in human cancer (16). In conditional transgenic models, Myc induced tumors can regress upon reduction in Myc transgene expression (17). The Ras/Raf/MEK/ERKs pathway induces c-Myc stability and GSK-3β reduces its stability (18-19). Accordingly, Ras/MEK/ERK activation / phosphatidyinositol 3-kinase/AKT-mediated GSK-3β inactivation leads to c-Myc accumulation (20). Interestingly, the radiation resistant phenotype of cells transformed by mutated Ras is enhanced by the c-Myc oncogene (6, 21). The Ras/Raf/MEK/ERKs pathway has been considered as a target for the radiosensitazion of cancer cells (22) but no data have been reported in RMS as radiosensitizer agents.
DNA double-strand break is critical in DNA lesions induced by radiation. In mammalian cells the repair of these lesions occurs by non-homologous end joining (NHEJ) requiring Ku70/Ku86 and the recruitment of the catalytic subunit of DNA-dependent PK (DNA-PKcs), which phosphorylates and regulate proteins involved in ligation processes. DNA-PKcs determine radioresponsiveness of human glioblastoma cell lines (23-24). DNA-PKcs also play roles in cell cycle checkpoint control, cell death and protein stabilization such as p53 and c-Myc (25-26). DNA-PKcs are necessary for genomic stability whereas abnormal levels in cancer cell may contribute to cell proliferation, radioprotection and change in c-Myc levels, eventually contributing to oncogenic phenotype. The relationship between DNA-PKcs level and chemosensitivity and radioresponse has been documented (27-29). Moreover, the human cell lines which are deficient in DNA-PKcs are radiosensitive because of the inefficient DNA DSB repair (30).
We previously showed in cultured ERMS-derived cell lines (RD) that the transformed phenotype could be reversed by disrupting c-Myc through the specific MEK/ERK inhibitor, U0126 (31), which prevented activation of MEK1/2 (32) and ERK pathways. U0126 induces growth arrest in in vitro and in vivo ERMS models as seen in other tumor types (9).
We investigated whether MEK/ERK inhibition, by U0126, affects the radiosensitivity of ERMS tumor in an in vitro and in vivo model of xenograft. Herein, U0126 suppressed colony formation of RD xenograft – derived cell line RD-M1 and TE671 embryonal rhabdomyosarcoma cell lines. U0126 increased the radiosensitivity of ERMS-xenografted nude mice. Both in vitro and in vivo models MEKs/ERKs inhibition down regulated c-Myc and DNA-PKcs. These results corroborate the idea that Ras/Raf/MEK/ERKs pathway may be a useful target for enhancement of radiotherapy effect. It is therefore sage to conclude that MEK inhibitors may have an important role in combination with radiotherapy for patients with embryonal rhabdomyosarcoma.
MATERIALS AND METHODS
Cell Cultures, Treatments and Radiation exposure
The human ERMS RD cell lines were obtained by American Type Culture Collection in 2004. The ERMS TE671 (HTL97021) cell line were obtained by Interlab Cell Line Collection in 2006. RD cell line was tested and authenticated by ATCC for the expression of myoglobin and myosin ATPase cellular products. TE671 cell line tested and authenticated by Interlab Cell Line Collection respectively for the expression of nicotinic acetylcholine receptor; acetylcholine receptor; peripheral type benzodiazepine receptor. Tumor-derived RD-M1 cell line were obtained from RD cells xenografted on nude mice as previously described (29) tested and authenticated by our laboratory for the expression of myoglobin and myosin ATPase cellular products. The cell lines are tested in our laboratory every one year for the expression of specific markers by western-blot analysis and were last tested in 2009. The cell lines were cultured as previously described (29). Treatment with 10 μmol/L MEK/ERK inhibitor U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene; Promega) was done for the times shown in the figures and started before radiation lasting for 24 hours. Radiation was delivered at room temperature using an x-6 MV photon linear accelerator. The total single dose of 4 Gy was delivered with a dose rate of 2Gy/min using a source-to-surface distance (SSD) of 100 cm. Doses of 200 kV X-rays (Yxlon Y.TU 320; Yxlon, Copenhagen, Denmark) filtered with 0.5 mm Cu. The absorbed dose was measured using a Duplex dosimeter (PTW, Freiburg, Germany). The dose-rate was approximately 1.3 Gy/min and applied doses ranged from 0 to 6 Gy.
Colony formation assay
For clonogenic survival assay exponentially growing RD, RDM1 and TE671 cells in 25-cm2 flasks were harvested by exposure to trypsin and counted. They were diluted serially to appropriate densities and plated in triplicate in 6 multi-well plates with 2mL of complete medium/each well in the presence or absence of 10μM U0126 or vehicle for 24 hours (final DMSO concentration of 0.1%; we confirmed that this DMSO concentration did not affect the proliferation of RD and TE671 and RD-M1 cell lines). After incubation for 24 hours, the cells were exposed at room temperature to various doses of radiation as already described. The cells were then washed with PBS, cultured in drug-free medium for 14 days , fixed with methanol:acetic acid (10:1, v/v), and stained with crystal violet. Colonies containing >50 cells were counted. The plating efficiency (PE) was defined as the number of colonies observed/ the number of cell plated; the surviving fraction (SF) was calculated as follows: colonies counted / cells seeded X (PE/100).
Immunoblot Analysis
Western blotting was conducted as described previously (33). Briefly, cells from cultures were lysed in 2% SDS containing phosphatase and protease inhibitors (Roche) sonicated for 30 seconds. Proteins of whole-cell lysates were assessed using the Lowry method (34), and equal amounts were separated on SDS-PAGE. Tumors were crushed in nitrogen, and the powder was collected and resuspended in 2% SDS containing phosphatase and protease inhibitors, sonicated for 30 s, and clarified by centrifugation. Aliquots of tissue extracts were used for total protein evaluation. SDSPAGE analysis was done as for cultured cells. The proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell, Bioscience) by electroblotting. The total protein level balance was confirmed by staining the membranes with Ponceau S (Sigma). Immunoblottings were done as described previously (35) with the following antibodies: anti-c-Myc (N-262), anti-phospho-ERK1/2 (E-4), anti-ERK2 (C-14), anti-phospho-GSK3, anti-Cyclin D1 and GAPDH ( all from Santa Cruz Biotechnology), and DNA-PKcs (from Abcam). Peroxidase-conjugate anti-mouse or anti-rabbit IgG (Amersham GE Healthcare) were used for enhanced chemiluminescence detection.
In vivo Experiments, U0126 and Radiation Treatment
Female CD1 athymic nude mice (Charles River, Milan, Italy) were maintained under the guidelines established by our Institution (University of L'Aquila, Medical School and Science and Technology School Board Regulations, in compliance with the Italian government regulation n.116 January 27 1992 for the use of laboratory animals). Before any invasive manipulation, mice were anesthetized with a mixture of ketamine (25 mg/ml)/xylazine (5 mg/ml). For xenotransplants exponentially growing TE671 cells were detached by trypsin-EDTA, washed twice in PBS, and resuspended in saline solution at cell densities of 1×106/200 l. Xenotransplants were done in 45-day-old female nude CD1 mice by s.c. injection in the leg using a 21-gauge needle on a tuberculin syringe. Treatments started when tumors reached a volume of 0.2 to 0.5 cm3.
l. Xenotransplants were done in 45-day-old female nude CD1 mice by s.c. injection in the leg using a 21-gauge needle on a tuberculin syringe. Treatments started when tumors reached a volume of 0.2 to 0.5 cm3.
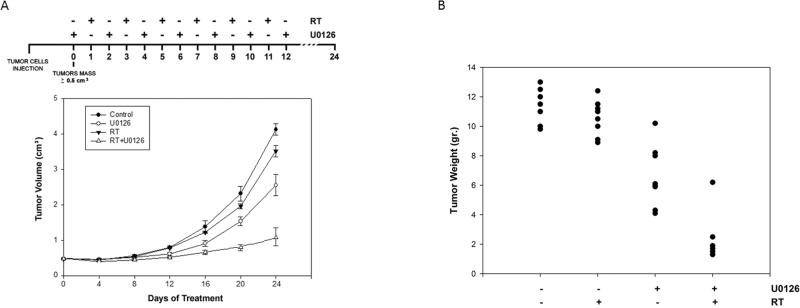
U0126 solution was prepared in DMSO as a stock solution of 10μmol/L and the amount of drug to be injected into a set of mice was diluted with carrier solution (40% DMSO in physiologic solution). The U0126 dose here used has been already tested as a dose non toxic to mice and efficient in inducing down regulation of ERK1/2 in the tumors (36). U0126 was administered 3 times/week, the day before of RT treatment. This protocol was chosen because a full inhibition of ERK activation is guarantee in vivo after 24 hours and was documented after this time (36). Mice were irradiated at room temperature using an Elekta 6-MV photon linear accelerator. Five fractions of 2 Gy were delivered over 5 consecutive days for a total dose of 10 Gy. A dose rate of of 1.5 Gy/min will be used with a source-to-surface distance (SSD) of 100 cm. Prior to irradiation mice were anesthetized and were protected from off-target radiation by a 3 mm lead shield. Before tumor inoculation mice were randomly assigned to 4 experimental groups. Each group was composed of 8 mice. One control group received intraperitoneal (i.p.) injection of 200 μl carrier solution; one group received i.p. injection of 200 μl U0126 solution at the dose of 25μmol/Kg; one group received RT (6 fractions of 2 Gy delivered 3 times/week to a total dose of 12 Gy); one group received 200 μl U0126 solution at the dose of 25μmol/Kg coupled with RT (6 fractions of 2 Gy delivered 3 times/week to a total dose of 12 Gy) delivered 24 hrs after the beginning of treatment with U0126 (Figure 4). Experiments were stopped 12 days after the last RT treatment and mice were sacrificed by carbon dioxide inhalation. Tumours was directly frozen in liquid nitrogen for protein analysis and biochemical evaluation.
Figure 4.
(A) Growth curve of tumors volumes from xenografted TE671 cell lines, untreated ( ), U0126-treated (
), U0126-treated ( ), irradiated (RT ▼-▼), U0126-pretreated and irradiated (RT+U Δ-Δ). Tumor volumes were evaluated as describes in methods and represent the mean ±.SEM of 8 mice. The upper panel shows the sequential treatments of xenografted mice started when tumor reached a volume of approximate 0,5 cm3. U0126 was administered 1 day before each irradiation. (B) Tumor weights in mice untreated or treated with U0126, radiotherapy or combined treatment.
), irradiated (RT ▼-▼), U0126-pretreated and irradiated (RT+U Δ-Δ). Tumor volumes were evaluated as describes in methods and represent the mean ±.SEM of 8 mice. The upper panel shows the sequential treatments of xenografted mice started when tumor reached a volume of approximate 0,5 cm3. U0126 was administered 1 day before each irradiation. (B) Tumor weights in mice untreated or treated with U0126, radiotherapy or combined treatment.
Evaluation of treatment response in vivo
The effects on tumour growth of different treatments were evaluated as follows: (1) measuring tumour volume during and at the end of experiment. Tumor volume was assessed every 4 days measurement with a Vernier calliper (length × width). The volume of the tumor was expressed in mm3 according to the formula 4/3π r3; (2) measuring tumor weight at the end of experiment; (3) Time to progression (TTP), defining tumor progression (TP) an increase of greater than 100% of tumor volume respect to baseline.
Statistical methods
Continuous variables were summarized as mean and standard deviation (SD) or as median and 95% CI for the median. For continuous variables, statistical comparisons between control and treated groups were established by carrying out the Kruskal-Wallis Tests (a parametric one-way analysis of variance for independent groups) or the Mann-Whitney test (in the case of two independent groups). Dichotomous variables were summarized by absolute and/or relative frequencies. For Dichotomous variables, statistical comparisons between control and treated groups were established by carrying out the exact Fisher's test. For multiple comparisons the level of significance was corrected by multiplying the P value by the number of comparisons performed (n) according to Bonferroni correction. TTP was analyzed by Kaplan-Meier curves and Gehan's generalized Wilcoxon test. When more than two survival curves were compared the Logrank test 10 for trend was used. This tests the probability that there is a trend in survival scores across the groups. All tests were two-sided and were determined by Monte Carlo significance. P values <0.05 were considered statistically significant.
The effects of the treatments were examined as previously described by Prewett et al. (37). The effect on tumor growth was measured by taking the mean tumor volume on day 24 for the different treatment groups: controls, treatment with RT (treatment a), treatment with U0126 (treatment b) and treatment with RT + U0126 (treatment a + b). For tumor volume, fractional tumor volume (FTV) for each treatment group was calculated as the ratio between the mean tumor volumes of treated and untreated tumors. For TTP, fractional TTP (FTTP) for each treatment group was calculated as the ratio between the median TTP of untreated and treated tumors. This was done for treatment a, for treatment b and for treatment a + b. The expected FTV or FTTP for the << a + b >> combination was defined as FTVa-observed X FTVb-observed or as FTTPa-observed X FTTP-observed. The ratio FTV a + b-expected/ FTV a + b-observed or FTTP a + b-expected/FTTP a + b-observed was the combination Index (CI). If CI > 1, there are supra-additive effects and if CI < 1 infra-additive ones. Strictly additive effects are observed if CI = 1. All statistical analyses were performed using the SPSS® statistical analysis software package, version 10.0.
RESULTS
Radiation decreases clonogenic survival of embryonal rhabdomyosarcoma cell lines
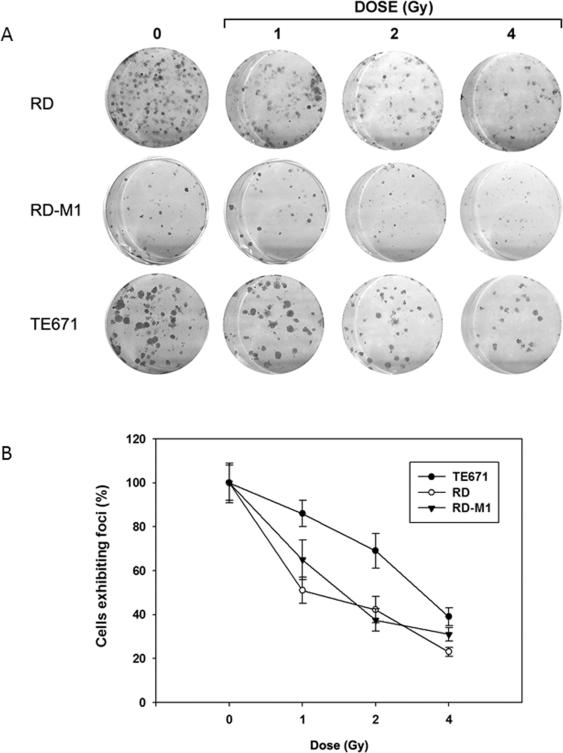
In order to explore the differential effect of radiation on RD, RD-M1 and TE671 cell lines, clonogenic survival was determined upon treatment with increasing radiation doses (0-6 Gy) (Fig. 1). Increasing doses of radiation significantly (p<0.05) decreased the number of colonies formation compared with control (Fig. 1A). This was associated with a reduction of clonogenicity in all tested cell lines (Fig. 1B). RD and RD-M1 cell lines showed the greatest sensitivity to RT (Fig. 1 A-B).
Figure 1.
Clonogenic assay of embryonal rhabdomyosarcoma cell lines, RD, RD-M1 and TE671 at different Gy doses. A) picture of cristal violet stained cultures 15 days after irradiation at indicated doses (1, 2, 4 Gy); B) dose-dependent colonies formation, each point of the three curve is the average ± SEM of three samples. Similar results were obtained in three experiments.
MEK/ERK inhibition synergistically increases the radiosensitivity of rhabdomyosarcoma cell lines
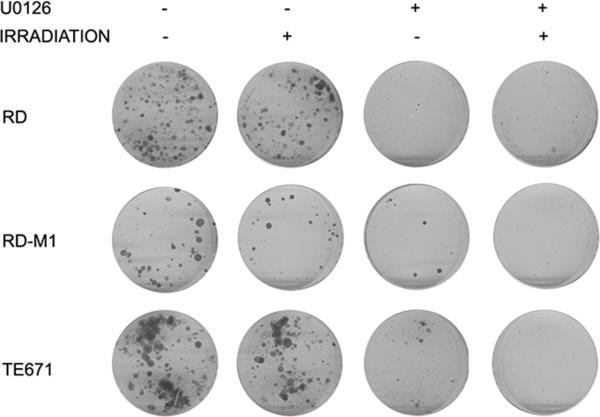
As previously reported (31,36) MEK/ERK inhibition reverses the transformed phenotype of embryonal rhabdomyosarcoma cells. Clonogenic survival was determined in tumor cells cultures upon treatment with a potent MEK/ERK inhibitor U0126 1 hour before radiation of RD, RDM1 and TE671 (Fig. 2 and Table 1). The rationale of treating the cells before radiation was to assess whether the inactivation of MEK/ ERK pathway positively enhanced radiosensitization of the tumor cell lines (Fig. 2A).
Figure 2.
Clonogenic assay of the three embryonal cell lines with (+) or without (-) U0126 and 4 Gy irradiation treatments. A) picture of crystal violet stained cultures 15 days after treatments, U0126 and 4 Gy irradiation or in combination.
Table 1.
Surviving fraction after treatments
| Cell Line | Plating efficiency (PE) | RT Surviving fraction (SF) | U0126 Surviving fraction (SF) | U0126 + RT Surviving fraction (SF) |
|---|---|---|---|---|
| RD | 0.8 | 0.67 | 0.17 | 0.08 |
| RD-M1 | 0.67 | 0.42 | 0.3 | 0.14 |
| TE671 | 0.84 | 0.83 | 0.24 | 0.11 |
As shown in Tab.1, the more radioresistant tumor cell line was TE671 with SF of 0.83. Although the antitumor effect of U0126 was evident in all cell lines the U0126 synergistically increased the radiosensitivity in all three cell lines (Table 2).
Table 2.
Synergism or additivity of combined treatment
| Cell Line | RT Observed effect | U0126 Observed effect | U0126 + RT Observed effect | U0126 + RT Expected effect | Combination index (CI) |
|---|---|---|---|---|---|
| RD | 0.84 | 0.22 | 0.10 | 0.18 | Synergism (1.8) |
| RD-M1 | 0.63 | 0.45 | 0.21 | 0.28 | Synergism (1.3) |
| TE671 | 0.76 | 0.29 | 0.13 | 0.22 | Synergism (1.7) |
MEK/ERK inhibition inhibits growth and radioresistant signals in RD and TE671 cells
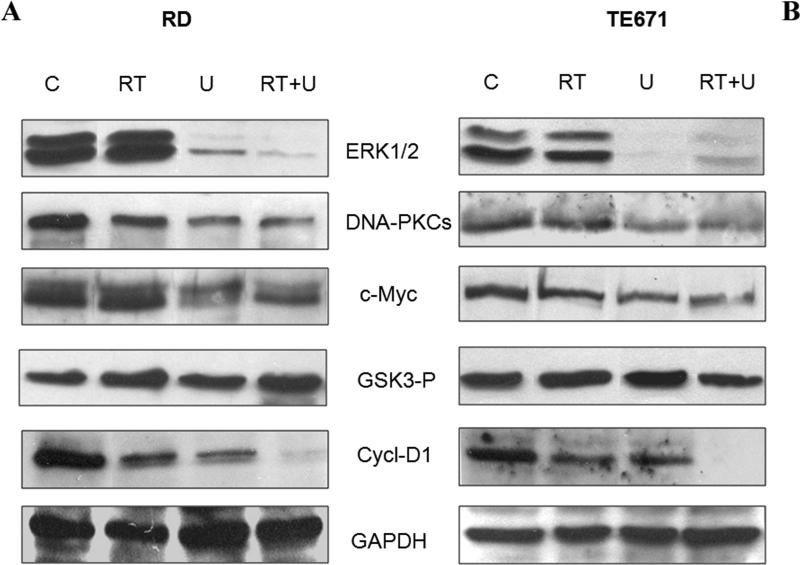
DNA-PKcs is up regulated in many tumors and is important in the radiation response. DNAPKcs abundance was assessed by western blot in response to U0126 (Fig 3A and B). The U0126 treatment alone and in combination with radiation reduced (Fig 3A and B, see also supplementary data) DNA-PKcs in parallel with c-Myc inhibition. The combined treatment of U0126 with radiation inhibited Cyclin D1 expression more than either treatment alone. U0126 alone inhibited phospho-active ERKs but not GSK3-β.
Figure 3.
Expression of protein kinases (ERK1/2-P04, DNA-PKcs, GSK3-beta-PO4), c-Myc and Cyclin-D1 in untreated (C), irradiated (RT), U0126-treated (U0126) and U0126-pretreated and irradiated (RT+U) RD cells (A) and TE671 (B). GAPDH was blotted as loading control.
U0126 with radiation results in synergistic antitumor effects in vivo
For in vivo experiments, TE671 cell line was chosen due to its intrinsic radioresistance in comparisons with the other tumor cells (Fig. 1). When tumor volume reached 0,5-1.0 cm3 (T0), U0126 was i.p. administered and followed by radiation one day after (Fig. 4). Tumor volumes were measured every 4 days for a period of 24 days in untreated (control), U0126-treated (U0126), irradiated (RT) and U0126/Irradiated (RT+U0126) tumors (Fig. 4A). Tumor masses from irradiated mice grew rapidly from the twelfth day of treatments but at lesser extent than control mice (Fig. 4A). U0126-treated mice grew at significantly less extent (45% of inhibition at end point) (36). The treatment with U0126 before RT decreases growth further with an 80% inhibition. Computed CI value, in terms of tumor volume as outcome measure, was determined on day 24 after cell implantation and indicated synergistic effect for the combination of RT with U0126 (CI=1.92). Tumor weights in mice treated with U0126 and radiotherapy decreased significantly ranging from 60 to 80% respect to controls (Fig. 4B). Treatments with U0126 or RT alone were not as effective as in combination (Fig. 4B).
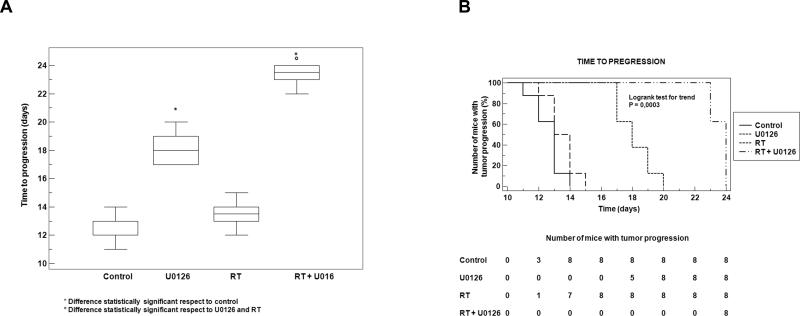
U0126 synergizes with radiation in delaying time to progression (TTP)
The number of mice with tumor progression significantly differed across the groups and this was confirmed by the Kaplan-Meier curves (Fig. 5A) and the median values of TTP (Fig. 5B). In the control group tumor progression occurred within 14 days after the beginning of treatment (Fig. 5A) with a median TTP of 13 days (95% CI 11.3 to 13.7). Upon RT treatment a negligible improvement in the TTP was documented compared to controls (p=0.10). In the RT group the tumor progression occurred within 15 days after RT with a median TTP of 13.5 days (95% CI 12.3 to 14.7). The treatment with U0126 significantly improved the TTP compared to controls (p=0.0002) or RT (p=0.0002). In the group treated by U0126 tumor progression occurred from the 17th day after the beginning of treatment and completed within the day 20. The median TTP after this treatment was 18 days (95% CI 17.0 to 19.7). The most evident improvement was documented when U0126 was coupled with RT. This combined treatment significantly improved the TTP compared to RT (p<0.0001) or U0126 (p<0.0001) with a clear synergistic effect (CI=1.27). For this group the median TTP was 24 days (95% CI 22.0 to 24.0) and the tumor progression was documented only from the 22th day after the beginning of treatments.
Figure 5.
(A) U0126 with RT combined treatment affects time to progression in vivo xenografted tumors. (B) Box plot diagram comparing median time (days) of tumor progression (TTP) among treatment groups. Box represents interquartile range (IQR), bar median value and whiskers represent data points within IQR.
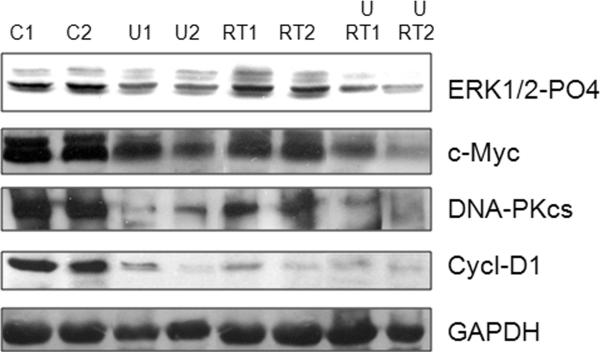
DNA-PKcs and c-Myc tumor expression is reduced by MEK/ERK inhibition and radiation in vivo
Tumors from different settings at end point were analyzed for the expression levels of phospho-ERKs, cMyc, DNAPKcs and Cyclin-D1. c-Myc, DNA-PKcs and Cyclin-D1 expression levels were decreased upon U0126 treatment either alone and in combination with RT radiotherapy (Fig. 6). ERK inhibition was evaluated in tumors that were taken 16 hrs after the last U0126 administration. These results support the hypothesis that in vivo radiosensitivity can be recovered following growth and DNA-repair signals inhibition.
Figure 6.
Expression of protein Kinases (ERK1/2 and DNA-PKcs), cMyc and Cyclin-D1 in tumors untreated (C1,C2), U0126-tretaed (U1, U2), irradiated (RT1, RT2) and U0126-pretreated and irradiated (U+RT1, U+RT2). At the end point mice were sacrificed, tumor excised and extracted as described in methods. GAPDH was blotted as loading control.
DISCUSSION
The Ras-MAPK pathway is important in radioresistance and activated oncogenic Ras mediates resistance to ionizing radiation (14). Active Ras-protein induces MAPK- and PI3K/AKT-pathway via autocrine mechanisms such as the production of EGFR ligands (4-5, 38). It has been recently reported that radiation-mediated EGFR activation induces cell survival also supported by DNADSB repair through direct interaction with DNA-PKcs. In this regard, the effect of Ras/MEKs/ERKs inhibition and the downstream target pathways in radiation response has not yet studied in rhabdomyosarcoma tumors.
We previously reported that MEK/ERK pathway inhibition reverses the transformed phenotype of RMS in in vivo and in vitro system (31, 36). Herein, MEK/ERK inhibition enhances radiosensitivity of ERMS cell lines. RT diminished the colonies formation of three embryonal rhabdomyosarcoma cell lines and pre-treatment with the MEK/ERK inhibitor, U0126, synergistically enhances the RT response also in TE671 line which behaved more radioresistant.
U0126 treatment down regulated phospho-active ERKs Cyclin-D1 and c-Myc. RT alone sensibly reduced DNA-PKcs and Cyclin-D1 expression levels, the latter being even more affected by U0126 and strongly down regulated in combined U0126 and irradiation therapy. The role of Cyclin D1 in cell survival has been documented. Cyclin D1-/- mouse embryonic fibroblast have enhanced response invoked by γ-irradiation (39), consistent with earlier experiments showing that Cyclin D1 expression inhibited UV-induced apoptosis in human trophoblastic cells (40). A positive correlation between Cyclin D1expression and tumor radioresistance has been proposed earlier (41). Cyclin-D1 has been implicated in radioresistance that can be inhibited by short interfering RNA (42). In line with these findings, U0126 pre-treatment, reduced Cyclin D1 enhancing decrease in clonogenic survival. U0126-mediated decrease of c-Myc is consistent with the role of ERKs and DNA-PKcs in mediating c-Myc stabilization and expression (26, 43). Inhibition of DNA-PKcs suppressed tumor cells growth and enhanced cervical cancer cells sensitivity to chemotherapeutic agents (44). Consistently, in rhabdomyosarcoma the synergistic effect of MEK/ERK inhibitor on radiation results from the targeting of DNA-PKcs and Cyclin D1 compromising DNA-repair and growth mechanisms respectively, thus preventing oncogenic phenotype expression .
Growth of tumors masses from TE671 that, in in vitro system, are more radioresistant than other ERMS cell lines, were inhibited by U0126 by 47% after U0126 alone whereas the radiation itself by 13% at the end points. Combined RT and MEK inhibition reduced tumor mass of 75%. Consistently, data analysis on tumor time progression provided the evidence of a synergistic effect of the MEK inhibitor when combined with radiotherapy. Immuno-biochemical evaluation of tumors demonstrated concordant findings to the in vitro analysis. Phospho-ERK was inhibited by U0126 either alone or in combination with RT. The DNA-PKcs and c-Myc levels were decreased after U0126 but not by RT. Cyclin-D1 was reduced by U0126 or RT and further inhibited by combined therapy. The role of Cyclin-D1 in radioprotection or radiosensitivity is still an open question although it has been reported that the sensitivity of the Cyclin D1–overexpressing MCF7 human breast cancer cell line to ionizing radiation was higher than that of cells that did not over-express Cyclin-D1 (45). In our system, Cyclin-D1 decrement by both U0126 and radiation in vitro and in vivo system might be indicative of the success of combined therapy in rhabdomyosarcoma.
This manuscript shows for the first time that more successfully radiotherapy in embryonal rhabdomyosarcoma tumors can be achieved when combination with signal transduction-based chemotherapy is applied.
Supplementary Material
Acknowledgments
Financial Support. This work was supported in part by National Institutes of Health (NIH) Grants R01CA70896, R01CA75503, R01CA107382 (to Richard G. Pestell). The Kimmel Cancer Center was supported by the NIH Cancer Center Core Grant P30CA56036 (to Richard G. Pestell). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from Pennsylvania Department of Health (to Richard G. Pestell). The Department specifically disclaims responsibility for an analysis, interpretation or conclusion. This project is funded in part from MIUR to BMZ and from University of L'Aquila (Bianca M Zani). This project is funded in part from “Cassa Edile di Roma e Provincia”(Bianca M Zani).
Abbreviations list
- ERMS
Embryonal Rhabdomyosarcoma
- RMS
Rhabdomyosarcoma
- MEK
Mitogen-activated Protein Kinase
- ERK
extracellular signal-regulated kinase
- DNA-PKcs
DNA-dependent Protein Kinase catalytic subunit
- GSK3-β
Glycogen-Synthase kinase 3- β
- DNA DSB
DNA Double Strand Break
- NHEJ
Non-Homologous End Joining
Footnotes
Conflict of Interest: I (we) declare that there is not conflict of interest.
REFERENCE LIST
- 1.Ferrari A, Dileo P, Casanova M, et al. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;98:571–580. doi: 10.1002/cncr.11550. [DOI] [PubMed] [Google Scholar]
- 2.Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 3.Paulino AC. Role of radiation therapy in parameningeal rhabdomyosarcoma. Cancer Invest. 1999;17:223–230. doi: 10.3109/07357909909021426. [DOI] [PubMed] [Google Scholar]
- 4.McKenna WG, Weiss MC, Bakanauskas VJ, et al. The role of the H-ras oncogene in radiation resistance and metastasis. Int J Radiat Oncol Biol Phys. 1990;18:849–59. doi: 10.1016/0360-3016(90)90407-b. [DOI] [PubMed] [Google Scholar]
- 5.Jones HA, Hahn SM, Bernhard E, et al. Ras inhibitors and radiation therapy. Semin Radiat Oncol. 2001 Oct;11(4):328–37. doi: 10.1053/srao.2001.26020. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CS, Sawyers CL, Mcbride WH. Oncogene Expression and Cellular Radiation Resistance: A Modulatory Role for c-myc. Mol Diagn. 1998;3:21–27. doi: 10.154/MODI00300021. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signaling pathway. Br J Cancer. 2004;90:283–288. doi: 10.1038/sj.bjc.6601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Kohno M, Pouyssegur J. Pharmacological inhibitors of the ERK signaling pathway: application as anticancer drugs. Prog Cell Cycle Res. 2003;5:219–224. [PubMed] [Google Scholar]
- 10.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–420. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, Liu Z, Condouris S, et al. BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol Metab. 2007;92:2264–2271. doi: 10.1210/jc.2006-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinelli S, McDowell HP, Vigne SD, et al. RAS signaling dysregulation in human embryonal Rhabdomyosarcoma. Genes Chromosomes. Cancer. 2009;48:975–982. doi: 10.1002/gcc.20702. [DOI] [PubMed] [Google Scholar]
- 13.Marshall CJ. The ras oncogenes. J Cell Sci. 1998;(Suppl 10):157–169. doi: 10.1242/jcs.1988.supplement_10.12. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Bakanauskas VJ, Cerniglia GJ, et al. The Ras radiation resistance pathway. Cancer Res. 2001a;61:4278–4282. [PubMed] [Google Scholar]
- 15.Toulany M, Baumann M, Rodemann HP. Stimulated PI3K-AKT signaling mediated through ligand or radiation-induced EGFR depends indirectly, but not directly, on constitutive K-Ras activity. Mol Cancer Res. 2007;5:863–872. doi: 10.1158/1541-7786.MCR-06-0297. [DOI] [PubMed] [Google Scholar]
- 16.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 17.Jain M, Arvanitis C, Chu K, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 18.Sears R, Nuckolls F, Haura E, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh E, Cunningham M, Arnold H, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 20.Bachireddy P, Bendapudi PK, Felsher DW. Getting at MYC through RAS. Clin Cancer Res. 2005;11:4278–4281. doi: 10.1158/1078-0432.CCR-05-0534. [DOI] [PubMed] [Google Scholar]
- 21.McKenna WG, Weiss MC, Endlich B, et al. Synergistic effect of the v-myc oncogene with H-ras on radioresistance. Cancer Res. 1990;50:97–102. [PubMed] [Google Scholar]
- 22.Shannon AM, Telfer BA, Smith PD, et al. The mitogen-activated protein/extracellular signalregulated kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) enhances the radiation responsiveness of lung and colorectal tumor xenografts. Clin Cancer Res. 2009;15:6619–6629. doi: 10.1158/1078-0432.CCR-08-2958. [DOI] [PubMed] [Google Scholar]
- 23.lalunis-Turner MJ, Zia PK, Barron GM, et al. Radiation-induced DNA damage and repair in cells of a radiosensitive human malignant glioma cell line. Radiat Res. 1995;144:288–293. [PubMed] [Google Scholar]
- 24.Lees-Miller SP, Godbout R, Chan DW, et al. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 25.Lees-Miller SP, Sakaguchi K, Ullrich SJ, et al. Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An J, Yang DY, Xu QZ, et al. DNA-dependent protein kinase catalytic subunit modulates the stability of c-Myc oncoprotein. Mol Cancer. 2008;7:32. doi: 10.1186/1476-4598-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian X, Chen G, Xing H, et al. The relationship between the down-regulation of DNA-PKcs or Ku70 and the chemosensitization in human cervical carcinoma cell line HeLa. Oncol Rep. 2007 Oct;18(4):927–32. [PubMed] [Google Scholar]
- 28.Gerweck LE, Vijayappa S, Kurimasa A, et al. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006 Sep 1;66(17):8352–5. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 29.Yan D, Ng WL, Zhang X, et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2010 Jul 1;5:e11397. doi: 10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001 Mar;2(3):196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 31.Marampon F, Ciccarelli C, Zani BM. Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol Cancer. 2006;5:31. doi: 10.1186/1476-4598-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Favata MF Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogenactivated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 33.Marampon F, Casimiro MC, Fu M, et al. Nerve Growth factor regulation of Cyclin D1 in PC12 cells through a p21RAS extracellular signal-regulated kinase pathway requires cooperative interactions between Sp1 and nuclear factor-kappaB. Mol Biol Cell. 2008;19:2566–2578. doi: 10.1091/mbc.E06-12-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LOWRY OH, ROSEBROUGH NJ, FARR AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Pelosi M, Marampon F, Zani BM, et al. ROCK2 and its alternatively spliced isoform ROCK2m positively control the maturation of the myogenic program. Mol Cell Biol. 2007;27:6163–6176. doi: 10.1128/MCB.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marampon F, Bossi G, Ciccarelli C, et al. MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol Cancer Ther. 2009;8:543–551. doi: 10.1158/1535-7163.MCT-08-0570. [DOI] [PubMed] [Google Scholar]
- 37.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002 May;8(5):994–1003. [PubMed] [Google Scholar]
- 38.Toulany M, Kasten-Pisula U, Brammer I, et al. Blockage of epidermal growth factor receptor-phosphatidylinositol 3-kinase-AKT signaling increases radiosensitivity of K-RAS mutated human tumor cells in vitro by affecting DNA repair. Clin Cancer Res. 2006;12:4119–4126. doi: 10.1158/1078-0432.CCR-05-2454. [DOI] [PubMed] [Google Scholar]
- 39.Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. doi: 10.1016/s0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 40.Albanese C, D'Amico M, Reutens AT, et al. Activation of the Cyclin D1 gene by the E1Aassociated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;26:34186–95. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- 41.Milas L, Akimoto T, Hunter NR, et al. Relationship between Cyclin D1 expression and poor radioresponse of murine carcinomas. Int J Radiat Oncol Biol Phys. 2002;52:514–521. doi: 10.1016/s0360-3016(01)02693-1. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed KM, Fan M, Nantajit D, et al. Cyclin D1 in low-dose radiation-induced adaptive resistance. Oncogene. 2008;27:6738–6748. doi: 10.1038/onc.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An J, Xu QZ, Sui JL, et al. Downregulation of c-myc protein by siRNA-mediated silencing of DNA-PKcs in HeLa cells. Int J Cancer. 2005;117:531–537. doi: 10.1002/ijc.21093. [DOI] [PubMed] [Google Scholar]
- 44.Tian X, Chen G, Xing H, et al. The relationship between the down-regulation of DNA-PKcs or Ku70 and the chemosensitization in human cervical carcinoma cell line HeLa. Oncol Rep. 2007;18:927–932. [PubMed] [Google Scholar]
- 45.Coco Martin JM, Balkenende A, Verschoor T, et al. Cyclin D1 overexpression enhances radiation-induced apoptosis and radiosensitivity in a breast tumor cell line. Cancer Res. 1999;59:1134–1140. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.