Abstract
Angiotensin II causes endothelial dysfunction which is associated with cardiovascular risk. We investigated the hypothesis that angiotensin II increases microvascular reactive oxygen species and asymmetric dimethylarginine and switches endothelial function from vasodilator to vasoconstrictor pathways. Acetylcholine-induced endothelium dependent responses of mesenteric resistance arterioles were assessed in a myograph and vascular nitric oxide and reactive oxygen species by fluorescent probes in groups (n=6) of male rats infused for 14 days with angiotensin II (200 ng/kg/min) or given a sham infusion. Additional groups of angiotensin or sham-infused rats were given oral tempol (2 mmol · l−1). Angiotensin II infusion increased mean blood pressure (119±5 vs 89±7 mmHg; P<0.005) and plasma malondialdehyde (0.57±0.02 vs 0.37±0.05μmol · l−1; P<0.035) and decreased maximal endothelium dependent relaxation (18±5 vs 54±6%, P<0.005) and hyperpolarizing (19±3 vs 29±3%, P<0.05) responses and nitric oxide activity (0.9±0.1 vs 1.6±0.2 Units, P<0.01) yet enhanced endothelium dependent contraction responses (23±5 vs 5±5%, P<0.05) and reactive oxygen species production (0.82±0.05 vs 0.15±0.03 units; P<0.01). Angiotensin II decreased the expression of dimethylarginine dimethylaminohydrolase-2 and increased asymmetric dimethylarginine in vessels (450±50 vs 260±35 pmol/mg protein, P<0.01) but not plasma. Tempol prevented any significant changes with angiotensin II. In conclusion, angiotensin redirected endothelial responses from relaxation to contraction, reduced vascular nitric oxide and increased asymmetric dimethylarginine. These effects were dependent on reactive oxygen species and could, therefore, be targeted with effective antioxidant therapy.
Keywords: Reactive oxygen species, nitric oxide, hypertension, endothelial dysfunction, dimethylarginine dimethylaminohydrolase
Introduction
Normal vessels display endothelium-dependent relaxation responses to acetylcholine (Ach). The endothelium-dependent relaxation factor (EDRF) is mediated by endothelial nitric oxide synthase (NOS-3). The mediators of the endothelium-dependent hyperpolarizing factor (EDHF) vary by species and vascular bed, but can include hydrogen peroxide (H2O2) 1, epoxyeicosatrienoic acids (EETs) 2 and electro-mechanical coupling 3. These cause activation of calcium-dependent potassium channels (KCa) on VSMCs that can be blocked by a combination of apamin and charybdotoxin 2. Some vessels also display a cyclooxygenase (COX)-dependent relaxation response attributable to prostacyclin (PGI2) although this was not apparent in rat afferent arterioles 2. An endothelium-dependent contracting factor (EDCF) occurs in models with increased reactive oxygen species (ROS). The EDCF in renal afferent arterioles from rabbits infused with Ang II entailed a vasoconstrictor prostaglandin generated by COX that activated thromboxane prostanoid receptors (TP-Rs) on VSMCs 4.
Ang II generates ROS in blood vessels that can bioinactivate NO 4. However, NOS-dependent EDRF responses of resistance arterioles are not consistently reduced 4,5 by angiotensin infusion. Moreover, ROS can generate an EDCF 4,6. However, it is not clear whether prolonged inhibition of ROS can prevent the defects in endothelium-dependent relaxations and prevent the EDCF responses of resistance vessels from animals infused with Ang II.
Increasing evidence suggests that an endogenous inhibitor of nitric oxide synthase (NOS), asymmetric dimethylarginine (ADMA), and its metabolism by dimethylarginine dimethylaminohydrolase (DDAH) 7 may regulate vascular NO. Angiotensin II infusion can increase circulating levels of ADMA 8,9. However, it is unclear whether increased ADMA levels in plasma or tissues can be prevented by an effective antioxidant. We recently reported that culture of VSMCs with Ang II increased cellular, but not medium, levels of ADMA 10. To resolve these issues, we assessed the three major endothelium-dependent pathways and related these responses to vascular NO and ROS activities and plasma and tissue ADMA concentrations in mesenteric resistance arteries in rats during a slow pressor infusion of angiotensin II. Other groups of sham and angiotensin II-infused rats received the antioxidant drug tempol throughout 11.
These experiments were designed to test the hypothesis that ROS, generated in microvascular resistance vessels during prolonged Ang II-induced hypertension, impairs EDRF, EDHF and NO activity and increases microvascular ADMA and EDCF. We selected tempol, which is a redox cycling nitroxide, to reduce ROS and enhance vascular NO 11. These experiments are significant because restoration of endothelial function and NO, and abrogation of EDCF, ROS and ADMA, would reverse some of the earliest manifestations of hypertension and vascular disease 12.
Methods
Male Sprague Dawley rats (200–220; Taconics Lab, Germantown, NY) were maintained on tap water and standard chow (Na+ 0.4g · 100g−1) under constant humidity and temperature and 12 hour light-dark cycles. The protocols were approved by the Institutional Animal Care and Use Committee of Georgetown University. Details of methods appear in supplement (Please see http://hyper.ahajournals.org)
Animal model
Three sets each of 4 groups of Sprague-Dawley rats were prepared (6 per group were used). Rats were anesthetized with sodium pentobarbital (50 mg · kg−1) for subcutaneous insertion of a sham mini pump (sham) or an osmotic mini pump (model 202; Alzato Palo Alto CA) at the dorsum of the neck. Group 1 had a sham infusion. Group 2 received Ang II (Peninsula Lab, San Carlos, CA) at 200ng · kg−1 · min−1 for 14 days. Group 3 received a sham mini pump and oral tempol (2 mmol · l−1 of water). Group 4 received Ang II mini pump and tempol. On the experimental day, rats were anesthetized with inhaled isoflurane (3% balance with oxygen). The carotid artery was cannulated for measurement of mean arterial pressure (MAP; model DPM-1B, Biotek, VT, USA). Blood was withdrawn and the plasma was stored at −80°C. Thereafter, rats were euthanized by exsanguination, the abdomen was opened and mesenteric arteries were isolated and studied in a myograph or snap frozen and stored at −80°C for analysis of ADMA or protein.
EDRF/NO, EDHF and EDCF responses of mesenteric resistance arterioles
The mesenteric vessels were isolated, mounted in a Mulvany-Halpern myograft (JP Trading, Science Park Aarhus, Denmark) and studied as described in detail previously 13, described in the supplement and summarized in Figure S1 (see supplement).
Fluorescence detection of acetylcholine–induced nitric oxide and ROS in mesenteric resistance arterioles
These followed methods 13 described in details in the supplement.
Protein expression studies
The protein expression in lysates of isolated mesenteric resistance arterioles were studied as described 13.
Plasma malondialdehyde
Plasma malondialdehyde was measured by High Performance Capillary Electrophoresis-Micellar Electrokinetic Chromatography (see supplement).
L-arginine, asymmetric - and symmetric - dimethylarginine concentrations in plasma and tissue
For details, see supplement to page 3.
Statistics
Values are expressed as mean ± SEM. The concentration-response curves to Ach were analyzed using nonlinear regression of sigmoidal concentration-response curves (GraphPad Prism), which were used to calculate the EC50. Other values were analyzed by ANOVA followed by a post hoc test for multiple comparisons (GraphPad Prism). A value of P<0.05 was considered statistically significant.
Results
Mean arterial pressure under anesthesia and plasma malondialdehyde were increased by Ang II, but this was prevented in rats receiving tempol. There were no changes in plasma arginine, ADMA or SDMA (Table 1).
Table 1.
Effects of angiotensin II infusion alone or with tempol on mean arterial pressure and plasma malondialdehyde, L-arginine, ADMA and SDMA
| Variable | Sham | Ang II | Tempol | Tempol + Ang II |
|---|---|---|---|---|
| MAP (mmHg) | 89 ± 7 | 119 ± 5† | 90.0 ± 3 | 98.2 ± 6 |
| Malondialdehyde (μmol · l−1) | 0.37 ± 0.05 | 0.57 ± 0.02* | 0.46 ± 0.05 | 0.45 ± 0.05 |
| L-arginine (μmol · l−1) | 173 ± 13 | 179 ± 55 | 137 ± 48 | 141 ± 66 |
| ADMA (nmol · l−1) | 510 ± 46 | 594 ± 127 | 610 ± 46 | 700 ± 20 |
| SDMA (nmol · l−1) | 280 ± 30 | 309 ± 31 | 300 ± 12 | 300 ± 9 |
Mean ± SEM values (n=6 per group). Effects of Ang II:
P<0.05;
P<0.005.
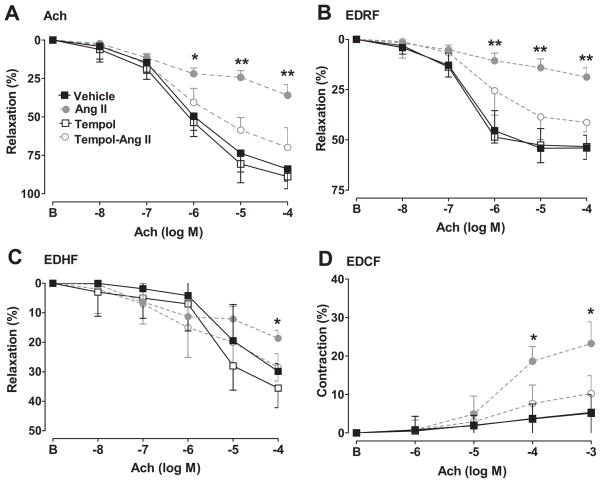
Figure S1 depicts how endothelium-dependent responses were assessed (see Fig S1 in supplement). Responses to Ach are shown in Figure 1 and EC50 and maximal values in Table 2. Compared to sham-infused rats, those infused with Ang II had a 57% reduction in maximal relaxation with Ach, a 67% reduction in EDRF but a more modest 34% reduction in EDRF. Ang II enhanced EDCF responses by 4 fold. Ang II infusion also reduced the sensitivity of the Ach and EDRF responses but increased the sensitivity of the EDCF responses and did not change the sensitivity of EDHF responses. There were no significant effects of Ang II infusion on these responses in rats administered tempol (Table 2).
Figure 1.
Mean ± SEM values for relaxation responses to acetylcholine in norepinephrine preconstricted mesenteric arterioles (panel A) and calculated EDRF (panel B) and EDHF (panel C) and EDCF responses in vessels under spontaneous tone (panel D). Comparing sham- and angiotensin-infused responses: *, P<0.05; **, P<0.01. There were no significant differences between sham- and angiotensin-infused responses in rats given tempol. B, before.
Table 2.
Maximal relaxation responses and EC50 values of vessels incubated with acetylcholine to Ach of mesenteric arteries from vehicle, Ang II, tempol, tempo with Ang II infusion rats
| Response | Maximum (%) | Maximum (%) | P | Log EC50 (mol/L) | Log EC50 (mol/L) | P |
|---|---|---|---|---|---|---|
| Vehicle | Ang II | |||||
| Ach | 83.9 ± 7.9 | 36.0 ± 7.0 | <0.01 | −6.17 ± 0.06 | −5.47 ± 0.04 | <0.005 |
| EDRF | 54.4 ± 4.6 | 18.0 ± 4.7 | <0.01 | −6.55 ± 0.06 | −5.78 ± 0.05 | <0.005 |
| EDHF | 29.4 ± 2.7 | 18.7 ± 2.7 | <0.05 | −5.18 ± 0.07 | −5.46 ± 0.16 | ns |
| EDCF | 5.2 ± 4.6 | 23.0 ± 5.6 | <0.05 | −4.11 ± 0.35 | −4.49 ± 0.03 | <0.005 |
| Tempol | Tempol-Ang II | |||||
| Ach | 88.9 ± 7.9 | 69.9 ± 8.9 | ns | −6.21 ± 0.05 | −6.16 ± 0.08 | ns |
| EDRF | 53.4 ± 5.7 | 41.4 ± 4.7 | ns | −6.76 ± 0.09 | −6.67 ± 0.01 | ns |
| EDHF | 35.4 ± 3.7 | 27.4 ± 4.7 | ns | −5.38 ± 0.15 | −5.15 ± 0.14 | ns |
| EDCF | 5.1 ± 6.2 | 10.4 ± 4.6 | ns | −4.16 ± 0.13 | −4.44 ± 0.07 | ns |
Data were obtained from experiments described in Figure 1.
Further data for mesenteric resistance arterioles are shown in Table 3. Ach-induced DAFFM-NO fluorescence was reduced by 41% in vessels from Ang-infused rats, whereas acetylcholine-induced Tempo-9AC-ROS fluorescence was increased five-fold (Table 3). Both of these changes were prevented in rats given tempol. Tissue concentrations of L-arginine and SDMA in mesenteric vessels were unaffected by Ang II infusion, but ADMA was increased significantly by 73%. This was prevented by tempol, which led to a significant increase in tissue L-arginine in rats infused with Ang II. The expression of DDAH-2 protein was reduced by Ang II infusion but was prevented in rats given tempol.
Table 3.
Effects of angiotensin II infusion alone or during tempol administration on mesenteric resistance arterioles
| Variable | Sham | Ang II | Tempol | Tempol + Ang II |
|---|---|---|---|---|
| NO activity with Ach (F/Fo DAF) | 1.6 ± 0.2 | 1.0 ± 0.1‡ | 1.7 ± 0.2 | 1.3 ± 0.1 |
| ROS activity with Ach (F/Fo tempo 9AC) | 0.15 ± 0.03 | 0.82 ± 0.05‡ | 0.22 ± 0.05 | 0.42 ± 0.11 |
| L-arginine (nmol · mg protein −1) | 71 ± 25 | 95 ± 23 | 33 ± 4 | 57 ± 8* |
| ADMA (pmol · mg protein −1) | 260 ± 35 | 450 ± 50† | 228 ± 36 | 312 ± 36 |
| SDMA (pmol · mg protein −1) | 80 ± 50 | 127 ± 76 | 56 ± 9 | 103 ± 28 |
| DDAH-2 protein (relative to β-actin) | 1.4 ± 0.2 | 0.7 ± 0.1‡ | 1.2 ± 0.3 | 1.1 ± 0.2 |
Mean ± SEM values (n=6 per group) for mesenteric resistance arterioles. Effects of angiotensin II:
P<0.05;
P<0.01;
P<0.005.
We evaluated whether a reduced response to NO, H2O2 or EETs could account for the reduced EDRF or EDHF responses of vessels from Ang-infused rats. The maximal relaxation to the NO donor, sodium nitroprusside in vessels from sham-infused rats (10−4 M; 97 ± 8%) was not significantly different (94 ± 6%) in vessels from Ang II-infused rats. H2O2 elicited a modest relaxation of 9 ± 3% at 10−5 mol · l−1 and a marked relaxation at 10−4 mol · l−1. However, these responses were similar in vessels from sham- and Ang-infused rats (Fig S2A in supplement). EETs did not elicit a significant relaxation even at 10−5 mol · l−1 (Fig S2B in supplement). Therefore, differences in responsiveness to NO, H2O2 or EETs could not explain the reduced relaxation responses from Ang-infused rats.
To assess the role of vasodilator prostaglandins (PGs) in EDRF responses, vessels from sham-infused rats were incubated with 5 × 10−5 mol · l−1 of indomethacin (Indo; to block COX-1 and -2). Relaxation responses to 10−4 M acetylcholine after vehicle (84 ± 8%) were unchanged after inhibition of cyclooxygenase with 5 × 10−5 mol · l−1 of Indo (79 ± 6%) but were abolished by endothelium removal (1 ± 3%). Therefore, Indo was not used in subsequent relaxation studies.
The mediation of EDHF was studied in vessels from sham-infused rats with the use of specific inhibitors (Table 4A). EDHF response to 10−4 M acetylcholine with vehicle was 29 ± 3%. This was not significantly blunted by catalase to metabolize H2O2 or by 14,15-epoxyeicosa-5-(Z)-enoic acid (14,15EEZE) to block EETs 14 in concentrations shown to be effective in isolated vessels 2,15 but was abolished by endothelium removal. Thus, the EDHF response depended on the endothelium but were not mediated significantly by H2O2 or EETs.
Table 4A.
Maximal endothelium-dependent hyperpolarization responses in mesenteric resistance arterioles from sham-infused rats
| Added to the bath | Relaxation (%) | P value vs vehicle |
|---|---|---|
| Vehicle | 29 ± 3 | |
| Catalase (125 units · ml−1) | 26 ± 6 | ns |
| 14,15-EEZE (10−5M) | 32 ± 5 | ns |
| Endothelium removal | 1 ± 5 | P<0.005 |
Mean ± SEM values (n=6 per group). All vessels were preconstricted with 10−5 mol · l−1 norepinephrine, pretreated with 10−4 mol · l−1 l-nitroarginine to inhibit nitric oxide synthase and relaxed with 10−4 mol · l−1 acetylcholine. ns = not significant
The EDCF response of vessels from Ang-infused rats (Table 4B) was not reduced significantly by incubation with parecoxib to block COX-2 or with OKY-046 to block thromboxane synthase. However, the response was inhibited similarly by SC-560 to block COX-1 or SQ-29,548 to block thromboxane prostanoid receptors (TP-Rs) and was abolished by endothelium removal. These drug concentrations were fully effective in isolated vessels 4. Thus, EDCF responses depended on the endothelium and COX-1 products that activated TP-Rs.
Table 4B.
Maximum endothelium-dependent contraction factor responses in mesenteric resistance arterioles from angiotensin II-infused rats
| Added to bath | Contraction (%) | P value vs vehicle |
|---|---|---|
| Vehicle | 23 ± 5 | |
| SC-560 (10−7M) | 7 ± 5 | P<0.005 |
| Parecoxib (10−5M) | 19 ± 4 | ns |
| OKY-046 NA (10−5M) | 13 ± 3 | ns |
| SQ-29,584 (10−5M) | 6 ± 4 | P<0.005 |
| Endothelium removal | 1 ± 4 | P<0.005 |
Mean ± SEM values (n=6 per group). Vessels were studied under spontaneous tone. They were pretreated with 10−4 mol · l−1 l-nitroarginine, 10−6 mol · l−1 apamin and 10−5 mol · l−1 charybdotoxin to block relaxation responses and constricted with 10−4 mol · l−1 acetylcholine. ns = not significant
Concentrations of L-arginine, ADMA and SDMA in homogenates of aorta, kidney and liver are shown in Table S1 in supplement. Tissue levels of L-arginine and SDMA were unaffected by Ang II infusion but levels of ADMA in all three tissues were increased. These effects were prevented in rats given tempol.
Discussion
The main new findings are that a prolonged slow pressor infusion of Ang II into rats changed endothelial function of mesenteric resistance vessels by diminishing vasodilator and increasing vasoconstrictor pathways. This was accompanied by a reduced endothelial release of microvascular NO, and increased ROS and ADMA. Angiotensin infusion downregulated the vascular expression of DDAH-2. These effects of Ang II were prevented by two weeks of oral tempol administration. Plasma ADMA did not predict vascular, renal or hepatic tissue levels of ADMA.
We confirmed previous findings that oral tempol prevented the increased blood pressure with a slow pressor infusion of Ang II 11,16. Vascular ROS with Ang II are derived from NADPH oxidase activity 17 and a reduction in extracellular superoxide dismutase expression 18. Tempol prevented superoxide anion (O2·−) generation by Ang II in VSMCs 19 and inhibited lipid peroxidation and microvascular ROS in response to Ang II in the present study. We selected a slow pressor rate of Ang II infusion in this study since high rates of Ang II infusion caused renal damage that reduced DDAH activity 8.
A reduction in EDRF in microvessels of rabbits infused with Ang II was improved by bath addition of tempol 20. A reduced EDRF has been related to bioinactivation of NO by ROS 11. This is consistent with our findings that tempol administration prevented the reduction in microvascular EDRF and NO, and the increase in ROS, in rats infused with Ang II.
The maximal EDHF response was also reduced by prolonged Ang II infusion but the effect was smaller than on EDRF and the EC50 response was not changed. Mediation of EDHF varies by vessel and species 21 but can include H2O2 1 and EETs 2. However, the impaired EDHF responses in this study in mesenteric arterioles of rats infused with Ang II were independent of H2O2 or EET generation or action. Thus, neither H2O2 nor EETs caused significant vasorelaxation except at very high H2O2 concentrations, and the addition of catalase to metabolize H2O2 or the EET antagonist, 14,15-EEZE to the bath did not alter EDHF responses. The mediator of EDHF was not identified in this study.
EDHF is upregulated in blood vessels of NOS-3 knockout mice 22. We confirm that Ang II infused rats have impaired EDRF responses in mesenteric arterioles 23. Kang et al reported that Ang II reduced the sensitivity of mesenteric vessels to Ach, as in the present study, but reduced the maximal response only after blockade of NOS. They proposed that NOS was upregulated in Ang II-infused rats to compensate for a defect in K+-channel mediated relaxation 5. However, in our study, the maximal EDRF response and the Ach-induced NO activity were reduced in vessels from Ang II infused rats. The difference may relate to the lower rate of Ang II infusion.
An endothelium dependent contraction is apparent in many models of hypertension 4,24. EDCF responses entailed an endothelial COX-dependent production of a factor under the influence of ROS 6 that activates TP-Rs on VSMCs 4. The contractile responses of vessels from Ang II infused rats in the present study were prevented by blockade of COX-1, TP-Rs or endothelium removal but blockade of TxA2 synthase had no significant effect. The present study did not identify the COX-1 product responsible, but prostaglandin endoperoxides have been implicated. The vascular signalling of Ang II contractile response via TP-Rs in this study may underlie the finding that TP-R knockout mice have an impaired pressor and renal vasoconstrictor response to infused Ang II 25.
The kidney 26 and the liver 27 clear plasma ADMA and the liver clears L-arginine. Avid uptake by these organs may account for the many-fold higher arginine concentrations in the liver and ADMA concentrations in the kidney, relative to the other organs sampled. DDAH-2 is the principal isoform expressed in the rat mesenteric resistance arterioles 13 and DDAH-1 in the renal proximal tubules 28. The down regulation by Ang II of DDAH-2 in cultured endothelial cells 29 and in mesenteric resistance arterioles in this study, and of DDAH-1 in the kidney cortex in a prior study 28, could therefore have reduced the metabolism of ADMA in these organs and contributed to the increased tissue levels of ADMA. Indeed, tempol prevented the angiotensin-induced reduction in DDAH-2 expression in the mesenteric vessels, which could have contributed to the prevention of an Ang II-induced increase in vascular ADMA in angiotensin-infused rats receiving tempol. DDAH activity was not assessed in these studies. However, Tain and Baylis reported that directly increasing ROS in the kidney cortex decreased DDAH activity 30. Since tempol prevented the Ang II-induced increased plasma malondialdehyde and vascular ROS activity, this relates the decreased tissue DDAH-2 expression and increased tissue ADMA to oxidative stress. However, tissue ADMA also is increased by up regulation of protein arginine methyltransferases (PRMTs) and by down regulation of cationic amino acid transporters (CATs) which could have contributed to the increased tissue ADMA in this study 7. We reported recently that incubation of VSMCs with Ang II increased NADPH oxidase and reduced DDAH and CAT activity and increased cellular, but not medium, levels of ADMA 10. The increase in cellular ADMA was prevented by tempol or blockade of angiotensin type 1 receptors. Further studies in VSMCs transfected with p22phox to increase NADPH oxidase directly demonstrated reduced DDAH-1 and -2 expression and activity, increased PRMT-3 expression and activity and decreased CAT-2A expression and activity and increased cellular ADMA concentrations. Thus, ROS can direct cellular metabolic pathways to increase cellular ADMA concentrations, but a reduction in CAT activity may restrain ADMA export and limit accumulation of ADMA in the extracellular fluid or plasma.
The 50% reduction in DDAH-2 expression in mesenteric vessels with Ang II in this study was similar to that produced by in vivo gene silencing 13 which impaired EDRF responses and NO bioactivity in mesenteric arterioles substantially. Moreover, Torondel et al 31 reported that overexpression of DDAH-1 or -2 in vascular endothelial cells reduced ADMA concentrations, enhanced in vitro vascular NO production and conferred resistance to administration of ADMA in DDAH heterozygote mice. The 70% increase in ADMA and 33% reduction in arginine:ADMA ratio in mesenteric vessels of angiotensin infused rats should contribute to the reduced EDRF responses, since this would reduce the substrate:inhibitor ratio for NOS. Ang II increased the mesenteric vascular arginine levels in rats given tempol, which also could have contributed to improved EDRF responses in tempol-treated rats, but the reason for this was unclear. Moreover, the increase in superoxide in the blood vessels during Ang II infusion should have enhanced NO bioinactivation 11. The finding that the Ang II-induced functional defects and reduced NO and DDAH-2 expression and increased tissue levels of ADMA and ROS were all prevented by tempol, implies that an increase in ROS was an upstream event that impaired endothelial responses by coordinating these pathways for endothelial dysfunction.
Perspective
Infusions of Ang II have variable effects on plasma ADMA. Thus, prolonged Ang II infusions into mice doubled plasma ADMA in one study 9 whereas in others Ang II did not change plasma ADMA 8,32, as in the present study, except at high rates of Ang II infusion that caused renal damage and reduced renal DDAH expression 8. We find that tissue ADMA can increase during a slow pressor Ang II infusion despite unchanged plasma levels.
Endothelial dysfunction or plasma markers of ROS 11 or ADMA 33 predict future cardiovascular events or death in high risk patients. The present study shows that tissue ADMA may not always be reflected by plasma levels, but that plasma malondialdehyde was a valid predictor of vascular ROS. A recent review concluded that the mechanisms that regulate the balances between NO and EDCF, and the processes that transform the endothelium from a protective organ to a source of vasoconstriction, proaggregatory and promitogenic responses in human hypertension are important but remain to be determined 24. The present finding that tempol given to Ang II infused rats prevented the defective endothelial relaxation responses and NO bioactivity and the enhanced ROS, endothelial contractions and ADMA suggest that vascular ROS is an important component of this transformation of endothelial function. Thus, maneuvers to reduce ROS could have therapeutic potential 11.
Supplementary Material
Acknowledgments
We thank Ms Sigrid de Jong for expert technical assistance and Ms Emily Wing Kam Chan for preparing and editing the manuscript.
Sources of funding
The work described in this review was supported by research grants to Christopher S. Wilcox from the NIDDK (DK-049870 and DK-036079) and from the NHLBI (HL-68686) and by a training grant to Zaiming Luo (T32-DK-059274) and by funds from the George E. Schreiner Chair of Nephrology and by grants to John R. Falck from the NIH (GM31278) and the Robert A. Welch Foundation.
Footnotes
Disclosures
None.
Reference List
- 1.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Borrego-Conde L, Falck JR, Sharma KK, Wilcox CS, Umans JG. Contributions of NO, EDHF and EETs to endothelium-dependent relaxation in rabbit renal afferent arterioles. Kidney Int. 2003;63:2187–2193. doi: 10.1046/j.1523-1755.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 3.Figueroa XF, Isakson BE, Duling BR. Vascular gap junctions in hypertension. Hypertens. 2006;48:804–811. doi: 10.1161/01.HYP.0000242483.03361.da. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane-prostanoid receptors and endothelium. Circ Res. 2004;94:1436–1442. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- 5.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase--dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertens. 2007;49:893–901. doi: 10.1161/01.HYP.0000259669.40991.1e. [DOI] [PubMed] [Google Scholar]
- 6.Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448–460. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasser JM, Moningka NC, Cunningham MW, Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R740–R746. doi: 10.1152/ajpregu.90875.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa K, Wakino S, Tatematsu S, Yoshioka K, Homma K, Sugano N, Kimoto M, Hayashi K, Itoh H. Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dimethylaminohydrolase 2. Circ Res. 2007;101:e2–e10. doi: 10.1161/CIRCRESAHA.107.156901. [DOI] [PubMed] [Google Scholar]
- 10.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertens. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharm Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Strandgaard S, Iversen J, Wilcox CS. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;296:R195–R200. doi: 10.1152/ajpregu.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Gill P, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol. 2007;293:H2085–H2092. doi: 10.1152/ajpheart.00968.2006. [DOI] [PubMed] [Google Scholar]
- 16.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 17.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circ. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 18.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill P, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertens. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 19.Luo Z, Chen Y, Chen S, Welch WJ, Andresen BT, Jose PA, Wilcox CS. Comparison of inhibitors of superoxide generation in vascular smooth muscle cells. Br J Pharmacol. 2009;157:935–943. doi: 10.1111/j.1476-5381.2009.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego L, Umans J, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to Ang II of afferent arterioles from rabbits infused with Ang II. J Am Soc Nephrol. 2003;14:2783–2789. doi: 10.1097/01.asn.0000090747.59919.d2. [DOI] [PubMed] [Google Scholar]
- 21.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 22.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, Kaley G. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol. 2000;278:H762–H768. doi: 10.1152/ajpheart.2000.278.3.H762. [DOI] [PubMed] [Google Scholar]
- 23.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats. Role of peroxisome proliferator-activated receptor-γ. Circ. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 24.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada N, Dennehy K, Solis G, Modlinger P, Hamel R, Kawada JT, Aslam S, MOriyama T, Imai E, Welch WJ, Wilcox CS. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am J Physiol. 2004;287:F753–F759. doi: 10.1152/ajprenal.00423.2003. [DOI] [PubMed] [Google Scholar]
- 26.Nijveldt RJ, van Leeuwen PAM, van Guldener C, Stehouwer CDA, Rauwerda JA, Teerlink T. Net renal extraction of asymmetrical (ADMA) and symmetrical (SMDA) dimethylarginine in fasting humans. Nephrol Dial Tranplant. 2002;17:1999–2002. doi: 10.1093/ndt/17.11.1999. [DOI] [PubMed] [Google Scholar]
- 27.Nijveldt RJ, Teerlink T, Siroen MPC, van Lambalgen AA, Rauwerda JA, Van Leeuwen PA. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA) Clin Nutr. 2003;22:17–22. doi: 10.1054/clnu.2002.0612. [DOI] [PubMed] [Google Scholar]
- 28.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of DDAH and PRMT isoforms in the diabetic rat kidney; effects of angiotensin II receptor blockers. Diabetes. 2008;57:172–180. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- 29.Chen MF, Xie XM, Yang TL, Wang YJ, Zhang XH, Luo BL, Li YJ. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res. 2007;44:391–402. doi: 10.1159/000103284. [DOI] [PubMed] [Google Scholar]
- 30.Tain YL, Baylis C. Determination of dimethylarginine dimethylaminohydrolase activity in the kidney. Kidney Int. 2007;72:886–889. doi: 10.1038/sj.ki.5002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torondel B, Nandi M, Kelly P, Wojciak-Stothard B, Fleming I, Leiper J. Adenoviral-mediated overexpression of DDAH improves vascular tone regulation. Vasc Med. 2010;15:205–213. doi: 10.1177/1358863X09360264. [DOI] [PubMed] [Google Scholar]
- 32.Jacobi J, Maas R, Cordasic N, Koch K, Schmieder RE, Boger RH, Hilgers KF. Role of asymmetric dimethylarginine for angiotensin II-induced target organ damage in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1058–H1066. doi: 10.1152/ajpheart.01103.2007. [DOI] [PubMed] [Google Scholar]
- 33.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int. 2006;70:26–33. doi: 10.1038/sj.ki.5000417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.