Abstract
Cigarette smoking is a major source of human exposure to acrolein, a widespread environmental pollutant and toxicant that is also formed endogenously through metabolism of amino acids and polyamines, and lipid peroxidation. Acrolein reacts with DNA, producing two pairs of regioisomeric 1, N2-propanodeoxyguanosine adducts: (6R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one (α-OH-Acr-dGuo) and (8R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one (γ-OH-Acr-dGuo). Previous studies indicate that these adducts might be involved in producing mutations in the p53 tumor suppressor gene, as observed in lung tumors in smokers, but there are only limited published data comparing acrolein-DNA adducts in smokers and non-smokers. In this study, we developed a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method to analyze Acr-dGuo adducts in human leukocyte DNA. Potential for artifactual formation was found in two steps of the assay: DNA isolation and DNA hydrolysis. This was eliminated by employing a Ficoll-Hypaque double density gradient to obtain leukocytes free of erythrocyte contamination, and by adding glutathione to scavenge acrolein present in H2O. The accuracy and precision of the method were confirmed. Acr-dGuo adducts were analyzed in leukocyte DNA from 25 smokers and 25 non-smokers. γ-OH-Acr-dGuo was the predominant isomer in all samples, while α-OH-Acr-dGuo was detected in only 3 subjects. There was no significant difference between the total Acr-dGuo levels in smokers (7.4 ± 3.4 adducts/109 nucleotides) and non-smokers (9.8 ± 5.5 adducts/109 nucleotides). Although our study is limited in size, these results, together with the results of previous analyses of acrolein-derived mercapturic acids in the urine of smokers and non-smokers, suggest that glutathione conjugation effectively removes acrolein from external exposures such as cigarette smoking, protecting leukocyte DNA from damage.
Keywords: Acrolein; 1,N2-propanodeoxyguanosine adducts; cigarette smoking; human leukocytes
Introduction
Acrolein is widely distributed in the human environment, occurring in automobile exhaust and industrial emissions (1). It is present in cigarette smoke in relatively high concentrations, about 18-98 μg/cigarette (2). Other sources of exposure to acrolein include heated cooking oil, endogenous metabolism of amino acids and polyamines, and lipid peroxidation of both ω-3 and ω-6 polyunsaturated fatty acids (3,4). It is also formed as a secondary metabolite of the chemotherapeutic agent cyclophosphamide (5).
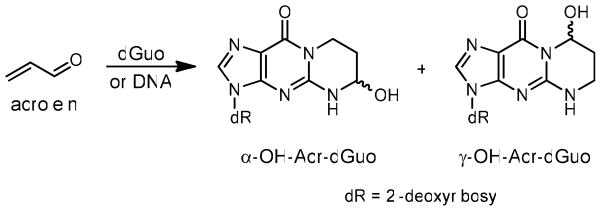
As the simplest α, β-unsaturated aldehyde, acrolein reacts with dGuo in DNA to form two pairs of regioisomeric 1, N2-propanodeoxyguanosine adducts: (6R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one (α-OH-Acr-dGuo) and (8 R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one (γ-OH-Acr-dGuo) (Scheme 1) (6). α-OH-Acr-dGuo in double stranded DNA was more mutagenic than γ-OH-Acr-dGuo in human cells, and induced predominantly G → T transversions (7,8). Using 32P-postlabeling, γ-OH-Acr-dGuo has been detected as the major adduct in various tissues from humans and untreated animals (9–11). Its level was significantly higher in human oral tissue DNA from smokers than from non-smokers (9).
Scheme 1.
Formation of Acr-dGuo adducts in DNA.
In a study by Feng et al., acrolein-DNA adducts formed preferentially at locations in the p53 tumor suppressor gene which were similar to the mutational hotspots in this gene found in human lung tumors induced by cigarette smoking (12). In addition, acrolein treatment inhibited nucleotide excision repair. Earlier studies had shown that diol epoxide metabolites of polycyclic aromatic hydrocarbons (PAH) resulted in a similar spectrum of p53 mutations (13), but considering the fact that acrolein occurs in quantities about 1,000-fold higher than PAH in cigarette smoke, it was proposed to be a major etiological agent for cigarette smoking-related lung cancers. We and others have analyzed (3-hydroxypropyl)mercapturic acid (3-HPMA), a major metabolite of acrolein, in human urine (14,15). Levels of 3-HPMA were about 5-fold higher in smokers than in non-smokers, while a significant 80–85% decrease in levels of urinary 3-HPMA was observed after smoking cessation (13,16). These studies indicate that cigarette smoking is a significant source of acrolein exposure in humans. Taken together, these observations encouraged us to further investigate the possible role of acrolein in cigarette smoking-related cancers, by analyzing acrolein-DNA adducts in human tissue DNA. We have previously developed a sensitive LC-ESI-MS/MS method to quantitate Acr-dGuo adducts in human lung DNA, and both α-OH-Acr-dGuo and γ-OH-Acr-dGuo were detected (17). However, we had limited information on the smoking histories of the subjects in that study. In the present study, we extended this research by analyzing Acr-dGuo adducts in human leukocyte DNA, a readily available source of DNA. We examined the effects of cigarette smoking on the levels of Acr-dGuo in leukocytes from smokers and non-smokers. In developing this assay, we took into account the recent observations by the Chung group that Acr-dGuo adducts can form readily as artifacts during analysis of DNA (18).
Experimental Section
Chemicals and Enzymes
Acr-dGuo and [13C10,15N5]Acr-dGuo were prepared as described (17). Ethanol was obtained from AAPER Alcohol and Chemical Co. (Shelbyville, KY). 2-Propanol was purchased from Fisher Scientific (Fair Lawn, NJ). Puregene DNA purification solutions were obtained from Qiagen (Valencia, CA). Calf thymus DNA and micrococcal nuclease (from Staphylococcus aureus) were obtained from Worthington Biochemical Co. (Lakewood, NJ). Phosphodiesterase II (from bovine spleen) was purchased from US Biological (Swampscott, MA). Alkaline phosphatase (from calf intestine) was obtained from Roche Diagnostics Corporation (Indianapolis, IN). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Separation of Leukocytes from Whole Blood and DNA Isolation from Human Leukocytes
Separation of leukocytes from whole blood was performed as previously described with some modifications (19). Briefly, 10 mL of whole blood was diluted with 20 mL of dextran-saline solution (30 g of dextran and 9 g of NaCl in 1 L of H2O), and allowed to stand for 45 min until the erythrocytes settled. The leukocyte-rich supernatant was centrifuged at 300 g for 10 min. The cells were re-suspended in 6 mL of dextran-saline solution, and layered over 2 mL of a discontinuous gradient of Ficoll-Hypaque. This discontinuous gradient was prepared by layering two Ficoll-Hypaque solutions. The top layer consisted of 1 mL commercially prepared Histopaque, giving a relative density of 1.077 kg/L at 25 °C. The bottom layer was a mixture of 0.67 mL Ficoll 400 (90 g/L) and 0.33 mL of 50% Hypaque (sodium diatrizoate), giving a relative density of 1.119 kg/L. After centrifugation at 600 g for 30 min, lymphocytes and neutrophils were harvested from the dextran-saline/Histopaque interface and the Histopaque/Ficoll-Hypaque interface, respectively. Lymphocytes and neutrophils were combined and washed with phosphate-buffered saline 3 times. DNA was isolated from the leukocytes immediately after this procedure.
DNA isolation was performed according to the DNA purification from buffy coat protocol (Qiagen) with some modifications. Briefly, leukocytes were incubated with 0.5 mL of red blood cell lysis solution and were collected by centrifugation. The cells were then treated with 6 mL of cell lysis solution, and were allowed to lyse with gentle shaking overnight. This was followed by treatment with 10 μL of RNase A (4 mg/mL) for 2 h to remove RNA. Then 2 mL of protein precipitation solution was added to the cell lysate, and the mixture was centrifuged to remove protein. DNA was precipitated from the supernatant by addition of 6 mL of 2-propanol. The precipitated DNA pellet was sequentially washed with 2 mL of 70% ice-cold ethanol in H2O and then 100% ice-cold ethanol. DNA was dried in a stream of N2 and stored at −20°C until analysis.
To investigate artifactual formation of Acr-dGuo during the leukocyte and DNA isolation, whole blood was split into two portions. In one portion, Ficoll gradient reagents and DNA isolation reagents all contained 0.5 mM of GSH, while in the other portion, no GSH was added to the Ficoll gradient or DNA isolation reagents. Acr-dGuo levels were analyzed in these two DNA samples and compared.
Analysis of DNA for Acr-dGuo
This was performed as previously described (17) with some modifications. DNA (50 – 200 μg) was dissolved in 900 μL of 10 mM sodium succinate/5 mM CaCl2 buffer (pH 7.0) containing 0.5 mM glutathione (GSH), to which [13C10,15N5]Acr-dGuo was added as internal standard. The mixture was heated at 100 °C for 30 min and cooled to room temperature. Enzymatic hydrolysis was performed by incubation with 75 units of micrococcal nuclease and 0.45 unit of phosphodiesterase II at 37 °C for 6 h. Then, 150 units of alkaline phosphatase (from calf intestine) were added, and the mixture was incubated at 37 °C overnight. A 10 μL aliquot was removed for dGuo quantitation, and the remaining hydrolysate was purified using a solid-phase extraction (SPE) cartridge [Strata-X, 33 μm, 30 mg/1 mL (Phenomenex, Torrance, CA)]. After the sample was applied, the cartridge was washed with 1 mL 0.1 mM GSH and 1 mL 5% CH3OH in H2O containing 0.1mM GSH, and the analyte was eluted with 1 mL 70% CH3OH in H2O containing 0.1 mM GSH. The eluants, after addition of 100 nmol GSH, were evaporated to dryness, and dissolved in 20 μL H2O for LC-ESI-MS/MS analysis. A buffer control which lacked DNA was prepared each time and processed in the same way to evaluate possible contamination; while 0.2 mg calf thymus DNA served as a positive control.
LC-ESI-MS/MS analysis was carried out with an Agilent 1100 capillary flow HPLC (Agilent Technologies, Palo Alto, CA) equipped with a 250 mm × 0.5 mm 5 μm particle size Luna C18 column (Phenomenex, Torrance, CA) and coupled to a Discovery Max triple quadrupole mass spectrometer (Thermo Scientific, San Jose, CA). The solvent elution program was a gradient from 5 to 25% CH3OH in 15 mM ammonium acetate buffer in 20 min at a flow rate of 10 μL/min at 30 °C. The ESI source was set in the positive ion mode as follows: voltage, 3.7 kV; current, 3 μA; and heated ion transfer tube, 275 °C. The adducts were analyzed by MS/MS using selected reaction monitoring (SRM). Ion transitions monitored were m/z 324 → m/z 208 ([M+H]+ → [BH]+) for Acr-dGuo and m/z 339 → m/z 218 for [13C10,15N5]Acr-dGuo with a collision energy of 12 eV. Other MS parameters were optimized to achieve maximum signal intensity.
Calibration curves were constructed before each analysis using standard solutions of Acr-dGuo and [13C10,15N5]Acr-dGuo. A constant amount of [13C10,15N5]Acr-dGuo (10 fmol) was mixed with differing amounts of Acr-dGuo (0.5 – 100 fmol) and analyzed by LC-ESI-MS/MS-SRM. Quantitation of dGuo was performed on a Waters Associates (Milford, MA) HPLC system with a UV detector operated at 254 nm. A 4.6 mm × 250 mm Luna 5 μm C18(2) column (Phenomenex) was used. The elution program was a gradient from 5% to 22% CH3OH in H2O in 15 min at a flow rate of 0.7 mL/min. Adduct levels were expressed as adducts per 109 nucleotides.
Human Blood Samples
The study was approved by the University of Minnesota Research Subject’s Protection Programs Institutional Review Board Human Subjects Committee. Blood samples from 25 smokers and 25 non-smokers were obtained from ongoing studies for the development of tobacco related biomarkers in the University of Minnesota Tobacco Use Research Center. All the subjects were 18 years or older, not pregnant or breastfeeding, consumed less than 21 alcoholic drinks per week, and were in good physical and mental health. Additional criteria for smokers include smoking at least 10 cigarettes per day (CPD), having been a smoker for at least 5 years with no change greater than 50% in CPD or brand in the last year, and not using any other tobacco products in the last 6 months. Smoking status was confirmed by measuring exhaled CO. Non-smokers were required to have smoked less than 100 cigarettes in their lifetime and were not using any tobacco products regularly. Lymphocytes and neutrophils were separated from 20 – 30 mL of freshly collected blood as described above and DNA was isolated.
Statistical Analysis
Two sample t-tests were performed to compare the Acr-dGuo levels between smokers and non-smokers. Statistical significance was set at p < 0.05.
Results
We previously developed a LC-ESI-MS/MS method for the analysis of Acr-dGuo adducts in lung tissue DNA (17). This method was modified in the present study based on the observations by the Chung group that artifactual formation of Acr-dGuo adducts during analysis of DNA is possible (18). When a negative control of four commercially procured deoxyribonucleosides was analyzed using our published method, 18 fmol of α-OH-Acr-dGuo and 20 fmol of γ-OH-Acr-dGuo were detected in the sample, while no adduct was detected in a negative control containing buffer only. Adduct detection in this experiment was likely due either to the reaction of dGuo with trace amounts of acrolein present in the H2O used in the assay and/or to contamination of the commercial deoxyribonucleosides, as observed by the Chung group (18). Therefore, during enzymatic hydrolysis of DNA to free deoxyribonucleosides, artificial formation of Acr-dGuo could occur, potentially leading to over estimation of Acr-dGuo in DNA samples. Based on the previous report (18), we used GSH to scavenge acrolein that may be present in the H2O used in the assay. When 0.5 mM GSH was added to the sodium succinate/CaCl2 buffer used for DNA hydrolysis, Acr-dGuo levels in calf thymus DNA decreased by approximately 50% for α-OH-Acr-dGuo and 20% for γ-OH-Acr-dGuo (Table 1). To ensure a complete block of artifact formation by GSH, a concentration dependent study was performed. The results shown in Table 1 indicate that blocking of artifact formation was complete when 0.5 mM GSH was added to the buffer for enzymatic hydrolysis. In addition, we added 0.1 mM GSH to the SPE eluting solutions, and 100 nmol GSH to the fraction from SPE containing Acr-dGuo adducts. These precautions were taken to exclude artifact formation.
Table 1.
Effects on Acr-dGuo adduct levels of GSH added during enzymatic hydrolysis of calf thymus DNA.
| [GSH], mM | Acr-dGuo (adducts/109 nucleotides)a |
|
|---|---|---|
| α-OH | γ-OH | |
| 0 | 43 ± 6 | 85 ± 10 |
| 0.1 | 24 ± 3 | 72 ± 6 |
| 0.5 | 21 ± 4 | 66 ± 7 |
| 1.0 | 24 ± 2 | 74 ± 4 |
Mean ± SD, (N=3)
We also observed that erythrocyte contamination of leukocytes resulted in artifactual formation of Acr-dGuo (data not shown). Therefore, a Ficoll-Hyapque gradient was used to obtain lymphocytes and neutrophils free of erythrocyte contamination. The leukocytes were further treated with red cell lysis buffer to remove any remaining erythrocytes before leukocyte lysis. When 0.5 mM GSH was added to the reagents for Ficoll-Hypaque gradient isolation of leukocytes and subsequent DNA isolation, Acr-dGuo adduct levels were similar to those in DNA isolated without addition of GSH. This indicated that there was no artifactual formation of the Acr-dGuo adducts in the process of leukocyte and DNA isolation when a Ficoll-Hypaque gradient was used. An outline of the method for analysis of Acr-dGuo adducts in human leukocytes is shown in Scheme 2.
Scheme 2.
Summary of LC-ESI-MS/MS Method for Acr-dGuo Analysis.
Accuracy was confirmed by analyzing human leukocyte DNA enriched with differing amounts of Acr-dGuo standards. Each sample was analyzed in triplicate. The results are summarized in Figure 1. After subtracting the amount in the leukocyte DNA (2 adducts/109 nucleotides of γ-OH-Acr-dGuo), the measured values were in good agreement with expected values. Precision was determined by analyzing calf thymus DNA in triplicate on three different days (Table 2). Levels measured on different days were all similar. The limit of detection was estimated at about 1–2 adducts/109 nucleotides, corresponding to a signal to noise ratio of 3, starting with 200 μg of DNA.
Figure 1.
Relationship of added to detected Acr-dGuo in human leukocyte DNA. Various amounts of α-OH- and γ-OH-Acr-dGuo (10, 25, 50, and 100 fmol) were added to human leukocyte DNA (80 μg) and analyzed in triplicate. Background levels in human leukocyte DNA (2 adducts/109 nucleotides for γ-OH-Acr-dGuo) were subtracted from each amount detected. (A) α-OH-Acr-dGuo; (B) γ-OH-Acr-dGuo.
Table 2.
Precision of the LC-ESI-MS/MS method for analysis of Acr-dGuoa.
| Acr-dGuo level in calf thymus DNA (adducts/109 nucleotides) |
Interday average | Interday %RSD | |||
|---|---|---|---|---|---|
| Day 1 (mean ± SD) | Day 2 (mean ± SD) | Day 3 (mean ± SD) | |||
| α-OH-Acr-dGuo | 22 ± 1 | 21 ± 4 | 20 ± 3 | 21 ± 1 | 5.7 |
| γ-OH-Acr-dGuo | 67 ± 3 | 66 ± 7 | 69 ± 4 | 67 ± 2 | 2.3 |
Calf thymus DNA samples (150–450 μg) were analyzed in triplicate on each day.
LC-ESI-MS/MS chromatograms obtained upon analysis of a human leukocyte DNA sample are shown in Figure 2A–D. Quantitation of all samples was achieved at a collision energy of 12 eV, with SRM transitions of m/z 324 → m/z 208 for the analyte (Figure 2A) and m/z 339 → m/z 218 for the internal standard (Figure 2B). One peak corresponding to the retention time of γ-OH-Acr-dGuo was observed, and it coeluted with the internal standard peak in the transition m/z 339 → m/z 218. α-OH-Acr-dGuo was not detected in this sample. To confirm the identity of γ-OH-Acr-dGuo, the sample was also analyzed at a collision energy of 32eV. Under these conditions, γ-OH-Acr-dGuo loses deoxyribose and a CH3CHO moiety, producing a major fragment at m/z 164 (17). Peaks corresponding to the retention time of γ-OH-Acr-dGuo were observed in the SRM of m/z 324 → m/z 164 (analyte, Figure 2C) and m/z 339 → m/z 174 (internal standard, Figure 2D), thus confirming the identity of γ-OH-Acr-dGuo. However, only the transitions m/z 324 → 208 and m/z 339 → m/z 218 were used for quantitation.
Figure 2.
LC-ESI-MS/MS chromatograms obtained upon analysis of human leukocyte DNA for α-OH-Acr-dGuo and γ-OH-Acr-dGuo. SRM was carried out at (A) m/z 324 → m/z 208 for analyte and (B) m/z 339 → m/z 218 for internal standard in a typical leukocyte DNA sample analyzed at 12 eV (conditions used for quantitation of all samples from smokers and non-smokers). SRM was carried out at (C) m/z 324 → m/z 164 for analyte and (D) m/z 339 → m/z 174 for internal standard in a sample from a non-smoker using a collision energy of 32 eV to confirm identity of γ-OH-Acr-dGuo.
We analyzed leukocyte DNA from 25 smokers and 25 non-smokers, who were recruited from ongoing studies for the development of tobacco related biomarkers in the University of Minnesota Tobacco Use Research Center. DNA was isolated from leukocytes and analyzed for Acr-dGuo adducts. γ-OH-Acr-dGuo was the predominant isomer and was detected in all samples (Table 3). α-OH-Acr-dGuo was observed in only one non-smoker’s DNA and in two smokers’ DNA samples. Total adduct levels (α-OH-Acr-dGuo + γ-OH-Acr-dGuo) were 9.8 ± 5.5 adducts/109 nucleotides in non-smokers and 7.4 ± 3.4 adducts/109 nucleotides in smokers. There was no significant difference in total Acr-dGuo levels between smokers and non-smokers (p > 0.05). However, the level of γ-OH-Acr-dGuo was significantly higher in non-smokers than in smokers (p = 0.03). A negative control which consisted of buffer only was analyzed each time to exclude contamination, while a calf thymus DNA sample was included as a positive control. Levels of Acr-dGuo in positive controls were 26 ± 1 adducts/109 nucleotides for α-OH-Acr-dGuo and 77 ± 1 adducts/109 nucleotides for γ-OH-Acr-dGuo (N = 3).
Table 3.
Levels of Acr-dGuo in human leukocyte DNA from smokers and nonsmokers.
| NON-SMOKERS | SMOKERS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Gender | Age | Amount of DNA (mg)a | Acr-dGuo levelb (adducts/109 nucleotides) |
# | Gender | Age | CPDc | Amount of DNA (mg) | Acr-dGuo level (adducts/109 nucleotides) |
||||
| α-OH | γ-OH | total | α-OH | γ-OH | total | |||||||||
| 1 | F | 34 | 0.15 | NDe | 15.2 | 15.2 | 26 | F | 38 | 15 | 0.20 | ND | 5.6 | 5.6 |
| 2 | F | 44 | 0.07 | ND | 10.9 | 10.9 | 27 | F | 23 | 9 | 0.15 | ND | 10.6 | 10.6 |
| 3 | F | 51 | 0.09 | ND | 18.1 | 18.1 | 28 | M | 20 | 10 | 0.07 | ND | 8.6 | 8.6 |
| 4 | F | 36 | 0.07 | ND | 11.7 | 11.7 | 29 | F | 50 | 20 | 0.10 | 7.9 | 10.5 | 18.4 |
| 5 | F | 26 | 0.08 | ND | 13.0 | 13.0 | 30 | F | 23 | 15 | 0.08 | 2.9 | 10.0 | 12.8 |
| 6 | F | 52 | 0.06 | ND | 13.2 | 13.2 | 31 | F | 56 | 20 | 0.26 | ND | 4.3 | 4.3 |
| 7 | M | 25 | 0.08 | ND | 15.3 | 15.3 | 32 | M | 50 | 34 | 0.12 | ND | 6.9 | 6.9 |
| 8 | M | 35 | 0.17 | ND | 26.1 | 26.1 | 33 | F | 41 | 30 | 0.09 | ND | 7.4 | 7.4 |
| 9 | F | 52 | 0.07 | ND | 10.5 | 10.5 | 34 | F | 38 | 15 | 0.16 | ND | 9.9 | 9.9 |
| 10 | F | 27 | 0.10 | ND | 12.3 | 12.3 | 35 | F | 53 | 38 | 0.07 | ND | 7.0 | 7.0 |
| 11 | F | 23 | 0.09 | ND | 9.6 | 9.6 | 36 | F | 38 | 12 | 0.06 | ND | 11.9 | 11.9 |
| 12 | M | NA | 0.08 | ND | 9.6 | 9.6 | 37 | M | 31 | 20 | 0.06 | ND | 11.2 | 11.2 |
| 13 | M | 57 | 0.23 | ND | 5.3 | 5.3 | 38 | F | 31 | 12 | 0.14 | ND | 5.1 | 5.1 |
| 14 | F | 28 | 1.75 | ND | 5.6 | 5.6 | 39 | F | 21 | 10 | 0.15 | ND | 4.6 | 4.6 |
| 15 | M | 44 | 0.11 | ND | 6.2 | 6.2 | 40 | F | 50 | 20–30 | 0.24 | ND | 3.0 | 3.0 |
| 16 | F | 33 | 0.18 | ND | 4.5 | 4.5 | 41 | F | 56 | 20 | 0.11 | ND | 4.6 | 4.6 |
| 17 | F | 28 | 0.20 | ND | 4.6 | 4.6 | 42 | F | 34 | 15 | 0.34 | ND | 6.5 | 6.5 |
| 18 | F | 53 | 0.29 | ND | 3.4 | 3.4 | 43 | M | 49 | 30 | 0.17 | ND | 6.3 | 6.3 |
| 19 | F | 58 | 0.26 | 1.1 | 5.4 | 6.4 | 44 | M | 38 | 20 | 0.25 | ND | 5.3 | 5.3 |
| 20 | M | 25 | 0.04 | ND | 16.0 | 16.0 | 45 | F | 50 | 30 | 0.15 | ND | 6.4 | 6.4 |
| 21 | F | 46 | 0.19 | ND | 7.1 | 7.1 | 46 | M | 52 | 17 | 0.10 | ND | 8.3 | 8.3 |
| 22 | F | 53 | 0.16 | ND | 4.5 | 4.5 | 47 | M | 50 | 20 | 0.16 | ND | 4.8 | 4.8 |
| 23 | F | 27 | 0.19 | ND | 4.5 | 4.5 | 48 | F | 25 | 12 | 0.12 | ND | 5.9 | 5.9 |
| 24 | F | 31 | 0.23 | ND | 5.9 | 5.9 | 49 | M | 58 | 30 | 0.12 | ND | 5.7 | 5.7 |
| 25 | F | 36 | 0.19 | ND | 4.9 | 4.9 | 50 | M | 42 | 20 | 0.15 | ND | 4.7 | 4.7 |
| Mean ± S.D. | 9.7 ± 5.5 | 9.8 ± 5.5 | 7.0 ± 2.5 | 7.4 ± 3.4 | ||||||||||
Calculated based on dGuo
Each value represents a single measurement. dGuo was determined by HPLC-UV.
CPD, cigarettes per day
NA, not available
ND, not detected. Limit of detection is 2 adducts/109 nucleotides dGuo.
Discussion
We developed a sensitive and reliable method for the analysis of Acr-dGuo adducts in human leukocyte DNA. It was necessary to address the problem of artifact formation in two steps of the assay. First, during DNA isolation from leukocytes, the presence of contaminating erythrocytes increased the levels of Acr-dGuo adducts. Although the mechanism is not clear, it is possible that erythrocytes could release acrolein during cell lysis, and this acrolein could subsequently react with leukocyte DNA. We excluded artifact formation in this step by using a discontinuous Ficoll gradient to obtain lymphocytes and neutrophils free of erythrocyte contamination. Second, artifact formation could also occur during the DNA hydrolysis step. Acr-dGuo adduct levels clearly decreased when DNA was hydrolyzed in the presence of GSH, which can quantitatively react with acrolein to form a stable thioether, 3-oxopropyl glutathione (20). The inhibition was complete with the addition of 0.5 mM GSH, since adding more GSH to the enzymatic hydrolysis reaction did not further reduce the amount of Acr-dGuo. As demonstrated by the Chung group, the source of acrolein that causes artifactual formation of Acr-dGuo in the enzymatic hydrolysis step is likely the H2O used in the assay. In their study, they trapped acrolein in H2O with GSH and identified the conjugate by mass spectrometry (18). After eliminating the sources of artifact formation, our method is highly accurate and precise, with a limit of detection in the low femtomole range. This sensitivity is necessary for analysis of human leukocyte DNA.
We have previously detected Acr-dGuo adducts in human lung DNA, which is a relevant but not very practical source of DNA (17). Leukocyte DNA, analyzed in this study, is suitable for diagnostic and clinical investigations of the relationship of acrolein exposure to Acr-dGuo adducts and disease. Early studies by Chung and co-workers used a 32P-postlabeling method and detected Acr-dGuo in various human tissues including liver, mammary, leukocytes and oral tissues (9–11). The widespread occurrence of Acr-dGuo adducts indicates common exposure of humans to acrolein, exogenously from the environment or endogenously from amino acid metabolism and lipid peroxidation. γ-OH-Acr-dGuo is the major isomer detected in leukocyte DNA. Although it is the less mutagenic isomer, it is able to form interchain or DNA-protein crosslinks (21–25). The levels of γ-OH-Acr-dGuo in human leukocyte DNA measured by Chung, et al. using 32P-postlabeling were between 0.6 – 5 adducts/109 nucleotides (10). We observed slightly higher levels of Acr-dGuo in human leukocytes. The low recovery of the 32P-postlabeling assay may account for the difference, and Chung’s study included samples from only 3 subjects.
A limitation of using leukocyte DNA to assess acrolein exposure is the relatively short life span of some leukocytes. The leukocyte fraction we obtained from the Ficoll-Hypaque gradient contained about 67% polymorphonuclear neutrophils and 33% lymphocytes. The lifetime of neutrophils is normally 4 to 8 h circulating in the blood and another 4 to 5 days in tissues. Lymphoctes have a longer life span, ranging from weeks to months (26). Therefore, adduct measurement in leukocyte DNA might not provide sufficient information on long term exposure to acrolein.
Acrolein is metabolized by GSH conjugation, with eventual excretion of the mercapturic acid 3-HPMA (1,27). Levels of urinary 3-HPMA are typically five times higher in smokers than non-smokers (15). After smoking cessation, urinary 3-HPMA decreased by as much as 85% (14,16). These results clearly indicate that there is significant exposure to acrolein through cigarette smoking, consistent with the relatively high levels of this aldehyde in smoke (2). However, we didn’t observe a significant difference in total Acr-dGuo levels in leukocyte DNA of smokers versus nonsmokers. This suggests that GSH conjugation is an effective mechanism for detoxification of acrolein from cigarette smoke. GSH conjugation is protecting DNA from damage by acrolein from external exposure. Consistent with this conclusion, Nath et al demonstrated a 2-fold increase of hepatic Acr-dGuo adducts in GSH-depleted rats (28). This effect is thought to result from a decrease in GSH conjugation of enals and/or an increase of lipid peroxidation in GSH-depleted rats. The inter-individual variation of Acr-dGuo levels in leukocyte DNA which we did observe is most likely due to differences in endogenous formation of acrolein, perhaps due to amino acid and polyamine metabolism, and lipid peroxidation (3). Since we don’t have any information on the background exposure to acrolein in our subjects, and our study only included a relatively small number of subjects, inter-individual variation of Acr-dGuo levels may account for the slightly higher levels observed in non-smokers.
Our results from leukocyte DNA do not support the hypothesis that acrolein plays a major role in cigarette smoke-induced lung cancer, but they also do not disprove it. This hypothesis was formulated based on the observation that acrolein reacts at hotspots in the p53 gene similar to those commonly mutated in lung tumors in smokers. The mutational spectrum in the p53 gene is significantly different in lung tumors from non-smokers. If the mutations in the p53 gene in smokers were due to Acr-dGuo adducts, we would have expected to observe a difference in their levels between smokers and non-smokers. We did not observe a difference and furthermore it does appear that acrolein is being effectively detoxified by GSH conjugation in smokers. However, Acr-dGuo adduct levels in leukocyte DNA may not reflect the amounts in lung DNA. Further studies are required to address this question.
In summary, we have developed a LC-ESI-MS/MS method to analyze Acr-dGuo adducts in human leukocyte DNA. To exclude artifact formation, we used a Ficoll gradient to obtain pure lymphocytes and neutrophils, and DNA was hydrolyzed in the presence of GSH. γ-OH-Acr-dGuo was the major isomer detected. No difference was observed in levels of leukocyte Acr-dGuo adducts between smokers and nonsmokers, which, together with other data, suggests efficient clearance of acrolein by GSH conjugation. Further studies are required to assess the role of acrolein as a lung cancer agent in cigarette smoke.
Acknowledgments
This study was supported by grant no. ES-11297 from the National Institute of Environmental Health Sciences. We thank Peter Villalta and Brock Matter of the Analytical Biochemistry Shared Resource, Masonic Cancer Center, for assistance with mass spectrometry. This resource is supported in part by grant no. CA-77598 from the National Cancer Institue. We thank Bob Carlson for editorial assistance. We also thank Elizabeth Thompson for help with sample collection from smokers and non-smokers.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 63. IARC; Lyon, France: 1995. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; pp. 393–407. [PMC free article] [PubMed] [Google Scholar]
- 2.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan J, Chung FL. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem Res Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- 5.Brock N, Hilgard P, Phol J, Ormstad K, Orrenius S. Pharmacolkinetics and mechanism of action of detoxifying low-molecular-weight thiols. J Cancer Res Clin Oncol. 1984;108:87–97. doi: 10.1007/BF00390979. [DOI] [PubMed] [Google Scholar]
- 6.Chung FL, Young R, Hecht SS. Formation of cyclic 1, N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 7.Yang IY, Chan G, Miller H, Huang Y, Torres MC, Johnson F, Moriya M. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 8.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1, N(2)-deoxyguanosine adducts of the alpha, beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath RG, Ocando JE, Guttenplan JB, Chung FL. 1, N2-Propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998;58:581–584. [PubMed] [Google Scholar]
- 10.Nath RG, Ocando JE, Chung FL. Detection of 1, N2-propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues. Cancer Res. 1996;56:452–456. [PubMed] [Google Scholar]
- 11.Nath RG, Chung FL. Detection of exocyclic 1, N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc Natl Acad Sci USA. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Hu W, Chen JX, Pao A, Li H, Rom W, Hung MC, Tang MS. Preferential DNA damage and poor repair determine ras gene mutational hotspot in human cancer. J Natl Cancer Inst. 2002;94:1527–1536. doi: 10.1093/jnci/94.20.1527. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 14.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived 3-hydroxypropylmercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS, Yuan J-M, Hatsukami DK. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Villalta PW, Wang M, Hecht SS. Detection and quantitation of acrolein-derived 1, N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emami A, Dyba M, Cheema AK, Pan J, Nath RG, Chung FL. Detection of the acrolein-derived cyclic DNA adduct by a quantitative 32P-postlabeling/solid-phase extraction/HPLC method: blocking its artifact formation with glutathione. Anal Biochem. 2008;374:163–172. doi: 10.1016/j.ab.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitehouse RC, Prasad AS, Rabbani PI, Cossack ZT. Zinc in plasma, neutrophils, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin Chem. 1982;28:475–480. [PubMed] [Google Scholar]
- 20.Tacka KA, Dabrowiak JC, Goodisman J, Souid AK. Kinetic analysis of the reactions of 4-hydroperoxycyclophosphamide and acrolein with glutathione, mesna, and WR-1065. Drug Metab Dispos. 2002;30:875–882. doi: 10.1124/dmd.30.8.875. [DOI] [PubMed] [Google Scholar]
- 21.de los SC, Zaliznyak T, Johnson F. NMR characterization of a DNA duplex containing the major acrolein- derived deoxyguanosine adduct gamma -OH-1,-N2-propano-2′-deoxyguanosine. J Biol Chem. 2001;276:9077–9082. doi: 10.1074/jbc.M009028200. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez AM, Minko IG, Kurtz AJ, Kanuri M, Moriya M, Lloyd RS. Comparative evaluation of the bioreactivity and mutagenic spectra of acrolein-derived alpha-HOPdG and gamma-HOPdG regioisomeric deoxyguanosine adducts. Chem Res Toxicol. 2003;16:1019–1028. doi: 10.1021/tx034066u. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz AJ, Lloyd RS. 1, N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J Biol Chem. 2003;278:5970–5976. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- 24.VanderVeen LA, Harris TM, Jen-Jacobson L, Marnett LJ. Formation of DNA-protein cross-links between gamma-hydroxypropanodeoxyguanosine and EcoRI. Chem Res Toxicol. 2008;21:1733–1738. doi: 10.1021/tx800092g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozekov ID, Turesky RJ, Alas GR, Harris CM, Harris TM, Rizzo CJ. Formation of deoxyguanosine cross-links from calf thymus DNA treated with acrolein and 4-hydroxy-2-nonenal. Chem Res Toxicol. 2010 doi: 10.1021/tx100179g. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyton AC, Hall JE. Textbook of Medical Physiology. 10. Saunders; Philadelphia: 2000. [Google Scholar]
- 27.Parent RA, Paust DE, Schrimpf MK, Talaat RE, Doane RA, Caravello HE, Lee SJ, Sharp DE. Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. II. Identification of urinary and fecal metabolites. Toxicol Sci. 1998;43:110–120. doi: 10.1006/toxs.1998.2462. [DOI] [PubMed] [Google Scholar]
- 28.Nath RG, Ocando JE, Richie J, Chung FL. Effect of glutathione depletion on exocyclic adduct levels in the liver DNA of F344 rats. Chemical Research in Toxicology. 1997;10:1250–1253. doi: 10.1021/tx9701079. [DOI] [PubMed] [Google Scholar]