Abstract
Exocytosis, consisting of the merger of vesicle and plasma membrane, is a common mechanism used by different types of nucleated cells to release their vesicular contents. Taste cells possess vesicles containing various neurotransmitters to communicate with adjacent taste cells and afferent nerve fibers. However, whether these vesicles engage in exocytosis on a stimulus is not known. Since vesicle membrane merger with the plasma membrane is reflected in plasma membrane area fluctuations, we measured membrane capacitance (Cm), a parameter linearly related to membrane surface area. To investigate whether taste cells undergo regulated exocytosis, we used the compensated tight-seal whole-cell recording technique to monitor depolarization-induced changes in Cm in the different types of taste cells. To identify taste cell types, mice expressing green fluorescent protein from the TRPM5 promoter or from the GAD67 promoter were used to discriminate type II and type III taste cells, respectively. Moreover, the cell types were also identified by monitoring their voltage–current properties. The results demonstrate that only type III taste cells show significant depolarization-induced increases in Cm, which were correlated to the voltage-activated calcium currents. The results suggest that type III, but neither type II nor type I cells exhibit depolarization-induced regulated exocytosis to release transmitter and activate gustatory afferent nerve fibers.
Introduction
Taste buds are the transducing elements of gustatory sensation. Each taste bud houses between 50 and 100 taste cells, which extend from the basal lamina to the surface of the epithelium, where their apical processes protrude through a taste pore and encounter taste stimuli in the oral cavity. The basolateral regions of taste cells communicate sensory information to other taste cells and to gustatory nerve fibers, which course throughout the taste bud. Considerable progress has been made in identifying the taste receptor proteins and downstream signaling effectors involved in taste transduction (Chandrashekar et al., 2006). However, much less is understood about how taste information is communicated from taste cells to afferent nerve fibers.
Taste buds comprise three types of cells, based on morphological, immunocytochemical, and functional criteria. Type I cells, called “glial-like” cells, express enzymes for inactivation and uptake of neurotransmitters (Lawton et al., 2000; Bartel et al., 2006) and are generally presumed to have a support function. Type I cells possess only voltage-gated outward currents (Medler et al., 2003; Romanov and Kolesnikov, 2006) and do not form synaptic contacts with afferent nerve fibers. Type II taste cells, also called “receptor” cells, possess the taste receptors and signaling effectors for bitter, sweet, and umami stimuli (Zhang et al., 2003; Clapp et al., 2004). These cells possess voltage-gated Na+ and K+ currents (Medler et al., 2003; Romanov and Kolesnikov, 2006) and generate action potentials to taste stimuli (Yoshida et al., 2006). Type II cells release ATP as a transmitter to activate purinergic receptors on afferent nerve fibers (Finger et al., 2005). These cells associate with nerve fibers, but lack voltage-gated Ca2+ channels and the typical presynaptic specializations (Clapp et al., 2006; DeFazio et al., 2006). ATP is released through gap junction hemichannels (Y. J. Huang et al., 2007; Romanov et al., 2007; Dando and Roper, 2009). Recent data suggest, however, that type II cells also express vesicular transporters for ATP (Iwatsuki et al., 2009), suggesting ATP may also be released by vesicular mechanisms. Type III taste cells, called “synaptic” cells, form conventional synapses with afferent nerve fibers (Yang et al., 2000; Yee et al., 2001). These cells are highly excitable with voltage-gated Na+, K+, and Ca2+ currents, and release serotonin and noradrenalin on membrane depolarization (Y. A. Huang et al., 2008) or ATP released from type II taste cells (Y. J. Huang et al., 2005; Y. A. Huang et al., 2009). Type III taste cells express glutamic acid decarboxylase isoform GAD67, suggesting GABA also may be released as a neurotransmitter (DeFazio et al., 2006).
Fusion of vesicle membrane with the plasma membrane causes an increase in membrane surface area that can be measured with whole-cell patch clamp as changes in membrane capacitance (Cm) (Neher and Marty, 1982). We report the first high-resolution Cm measurements of regulated exocytosis in taste cells. Using transgenic mice expressing green fluorescent protein (GFP) from cell type-specific promoters, we report that type III taste cells exhibit depolarization-induced increases in Cm, which are correlated with voltage-activated calcium currents, suggesting that transmitter release from these cells is mediated by Ca2+-dependent vesicular mechanisms.
Materials and Methods
Mice and taste cell isolation.
Breeding pairs of GAD67-GFP mice, obtained from The Jackson Laboratory (stock 007677), were bred and used to identify type III taste cells. Approximately 75% of type III taste cells express GAD67 (DeFazio et al., 2006). TrpM5-GFP mice were bred and used to identify type II taste cells, since TrpM5 is exclusively expressed in type II taste cells (Clapp et al., 2006). These mice were a generous gift from Dr. Robert Margolskee (Mt. Sinai Medical School, New York, NY; now at Monell Chemical Senses Center, Philadelphia, PA). TrpM5 is the Ca2+-activated monovalent-selective cation channel required for bitter, sweet, and umami taste transduction (Pérez et al., 2002; Zhang et al., 2003) and is a convenient marker for type II taste cells. In this study, we used only GAD67-GFP-positive taste cells to identify type III cells, since these cells represent only ∼15% of the cells in the taste bud. For type II cells, which make up ∼35% of the taste bud, we used both TrpM5-GFP-positive cells and unlabeled cells showing voltage-gated Na+ and K+ currents, but no voltage-gated Ca2+ current (Vandenbeuch and Kinnamon, 2008). Mice of both genders were used for these studies.
Circumvallate taste cells were isolated by a method adapted from Béhé et al. (1990). Briefly, adult mice were killed by CO2 inhalation and cervical dislocation. The tongue was removed and ∼0.2 ml of an enzyme mixture was injected between the epithelium and the underlying muscle. The enzyme mixture consists of 1 mg/ml collagenase B (Roche), 3 mg/ml dispase II (Roche), and 1 mg/ml trypsin inhibitor (Sigma-Aldrich) dissolved in 1 mg/ml Tyrode's. In some cases, 0.05 mg/ml elastase (Roche) was added to the enzyme mixture to improve the ability to obtain seals with the membrane. The tongue was bubbled in oxygenated Ca2+/Mg2+-free Tyrode's containing BAPTA (2 mm; Invitrogen) for 45 min, or until the epithelium containing the taste buds could be gently separated from the underlying connective tissue. Individual taste cells and isolated taste buds were removed by gentle suction applied by mouth and plated onto polylysine-coated coverslips affixed to perfusion chambers (RF-20; Warner Instruments). In general, isolated taste cells remained viable for up to several hours after isolation. However, during recording, Ca2+ currents in some cells tended to run down quickly, allowing us to test only a few solutions on each cell. Taste cells were viewed with an Olympus IX71 inverted microscope equipped with differential interference contrast optics and epifluorescence. All procedures were approved by the University of Colorado, Denver, Institutional Animal Care and Use Committee.
Solutions.
The bath contained Tyrode's saline solution, consisting of 10 mm HEPES/NaOH, pH 7.4, 10 mm d-glucose, 1 mm Na-pyruvate, 140 mm NaCl, 4 mm CaCl2, 1 mm MgCl2, and 5 mm KCl. Barium Tyrode's (for isolation of Ca2+ currents) contained 136 mm tetraethylammonium, 10 mm BaCl2, 1 mm MgCl2, 10 mm HEPES/NaOH, pH 7.4, 10 mm d-glucose, 1 mm Na-pyruvate, and 400 nm TTX. In some experiments, 0.5 mm CdCl2 was added to Tyrode's to block voltage-gated Ca2+ currents (Medler et al., 2003). The intracellular pipette solution consisted of 140 mm KCl, 1 mm CaCl2, 2 mm MgCl2, 10 mm HEPES/KOH, pH 7.2, 11 mm EGTA, 2 mm ATP, and 0.4 mm GTP. In most experiments the pipette tip (only) was backfilled with KF solution (110 mm KF, 30 mm KCl, 1 mm CaCl2, 2 mm MgCl2, 10 mm HEPES/KOH, pH 7.2, and 11 mm EGTA), to assist in seal formation. The prefilling of pipette solution by the KF solution did not affect either the voltage dependence of the currents or the membrane capacitance change compared with a KCl solution. All salts were of highest grade available (Sigma-Aldrich).
Electrophysiology and data analysis.
Taste cells, whose input resistance typically exceeds 1 GΩ (Bigiani, 2001; Medler et al., 2003), were patch clamped and their membrane capacitance (Cm) was measured by using the compensated tight-seal whole-cell recording technique (Neher and Marty, 1982; Marty and Neher, 1983; Zorec et al., 1991). Measurements were performed with a dual-phase lock-in patch-clamp amplifier with the 1 GΩ resistor in the head-stage (SWAM IIC; Celica). A 1591 Hz sine wave (11.1 mV rms) was superimposed on a command potential (−70 mV) in whole-cell recording. We coated fire-polished thick-wall pipettes (3–7 MΩ) with dental periphery wax and used a low level of bath solution (400 μl per coverslip) to reduce the slow drift of the real (Re) and imaginary (Im) part of the admittance signals. The imaginary signal is linearly related to changes in membrane capacitance, whereas the real part of the admittance signal is contributed mainly by membrane conductance and access conductance (Neher and Marty, 1982; Zorec et al., 1991). During the measurements, the phase setting of the lock-in amplifier was adjusted to nullify the changes in the Re signal in response to a 100 fF calibration steps. For the frequencies of the sine wave stimulation used (up to ∼20 kHz), the phase determination by the capacitance dithering provides a suitable calibration value (Debus and Lindau, 2000). The criteria of correct phase setting were as described previously (Neher and Marty, 1982; Zorec et al., 1991; Henkel et al., 2000). Signals from the lock-in amplifier (Re and Im part of admittance signals were low-pass filtered at 100 to 1 kHz, −3 dB, two-pole Bessel), together with the DC current (low-pass filtered at 10 Hz, −3 dB, two-pole Bessel), unfiltered current and voltage were digitized at 10 kHz (DIGIDATA 1322A 16-bit data acquisition system and the CLAMPFIT 9.2 software suite; Molecular Devices). Signals were additionally filtered by the filter options available with the CLAMPFIT 9.2 software.
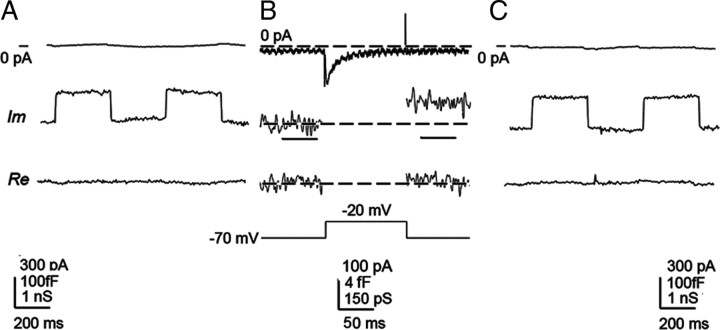
When a fluorescent cell was identified under the microscope, we obtained a gigaseal whole-cell recording and read the resting membrane capacitance from the dials of the patch-clamp amplifier. After the phase adjustment (Fig. 1A), a series of 100 ms depolarizing voltage pulses in 10 mV increments was applied from the holding potential (−70 mV). This voltage series was repeated 10 times with a 100 ms interval, and responses were averaged to reduce the noise level. For Figure 5C, a single voltage series was used.
Figure 1.
Protocol for capacitance measurements. A, Membrane capacitance (Cm) measurements were performed by initially monitoring membrane current (top trace), filtered at 10 Hz (low pass, −3 dB, Bessel), at a holding potential −70 mV, to which a sine wave of 1591 Hz (11.1 mV rms) was summed. Simultaneously, we monitored imaginary (Im) and real (Re) part of admittance signals (200 Hz low pass, Gaussian filter), which were used to set the phase by the calibrated 100 fF capacitance step. B, The recording of the voltage-induced current (3 kHz, low pass, −3 dB, 2-pole, Bessel) stimulated by a voltage step from −70 to −20 mV in a type III taste cell. Note that, after the pulse application, there was a small increase in the Im part of the signal (low pass, 200 Hz, Gaussian filter), reporting a 3.26 ± 1.7 fF increase in Cm; there was, however, no change in the Re part of the signal (200 Hz, low pass, Gaussian filter). The voltage-induced change in Cm was significantly different from the Cm value before the pulse (p < 0.001, Student's t test). The horizontal bars beneath the middle Im trace show epochs of 50 ms, during which the amplitude of the change in Cm was determined. C, To test whether the application of a series of voltage pulses induced a phase shift in the measuring system, we routinely monitored the phase angle setting at the end of the recording, where signals are shown at the same settings as in A.
Figure 5.
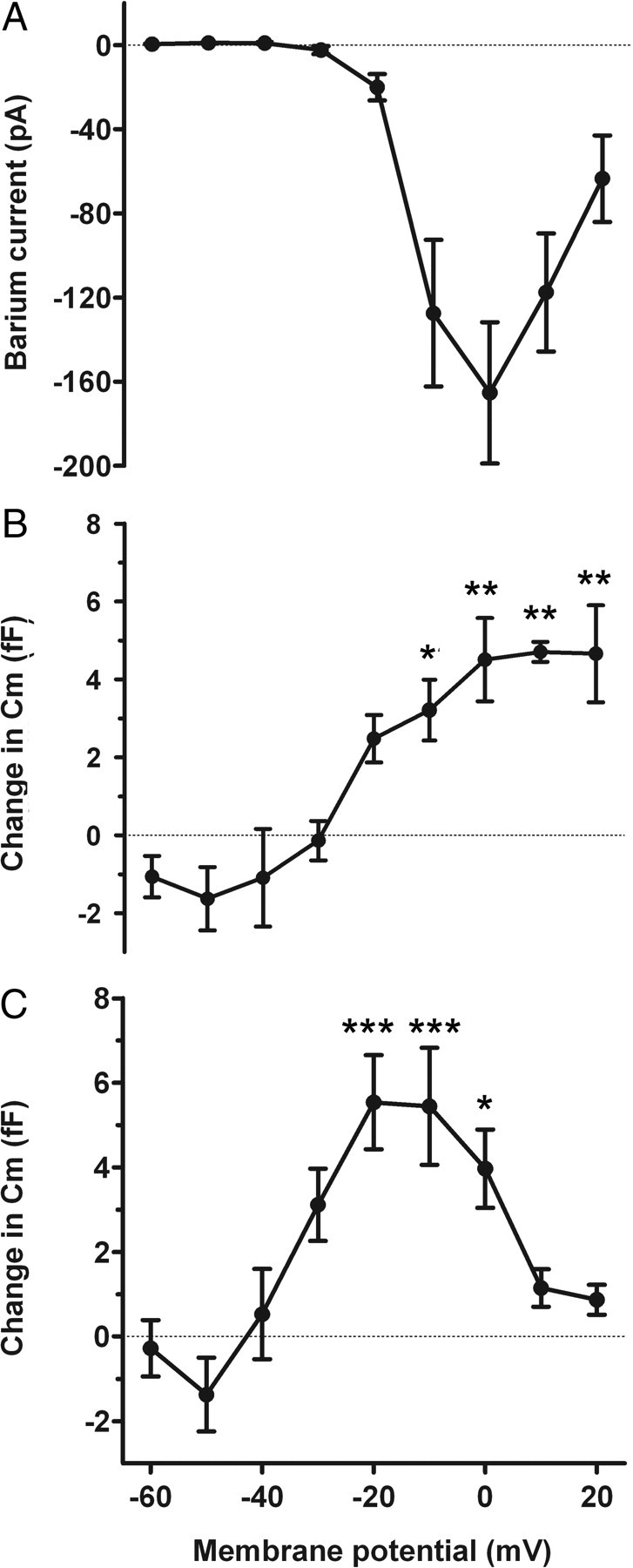
Relationship between the voltage pulse-induced calcium currents and the Cm change. A, Ba2+ currents (determined in the presence of extracellular Ba2+) at different membrane potentials. B, Changes in membrane capacitance (Cm) recorded before and after the pulse were measured at the same membrane potentials in Ca2+-containing Tyrode's. Cells were held at −70 mV before stimulating them with the voltage protocol. Each value of Cm represents the average value obtained from 10 runs (n = 4 cells). C, Changes in Cm recorded after a single voltage pulse series in a different set of taste cells (n = 10 cells). Ba2+ currents are combined in A from both sets of cells. Error bars represent SEM. The asterisks adjacent to data points show the level of significance (***p < 0.001, **p < 0.01, *p < 0.05, in comparison with the response to −60 mV; one-way ANOVA with Tukey's multiple-comparison test).
Secretory responses were measured by determining the change in amplitude of the Im signal, proportional to the membrane capacitance (Cm), recorded before and after each pulse. We measured the average amplitude value of a 50 ms signal epoch starting 60 ms before the voltage pulse and a 50 ms signal epoch starting 10 ms after the end of the voltage pulse (Fig. 1B, middle trace, bars). The change in average capacitance was measured for each cell and these measurements were averaged. These epochs were additionally filtered by a digital Gaussian filter (Clampfit facility, low pass, 100 Hz). Close inspection of the middle trace of Figure 1B shows that, after the application of the voltage pulse, the amplitude of the averaged Im trace, representing changes in Cm, increased by ∼3 fF, without a correlated change in the Re part of the admittance signal, which reflects changes in membrane and access conductance. To insure that the voltage protocol application did not alter the phase setting, we repeated the phase adjustment after the voltage series (Fig. 1C). We also readjusted the phase after any solution changes to the recording chamber. All recordings were obtained at room temperature (∼20°C).
All statistics are in the form of mean ± SEM, unless otherwise stated. Statistical significance between averages was tested with a one-way ANOVA with Tukey's multiple-comparison test (GraphPad Prism, version 5).
Results
Acutely isolated taste cells have an ovoid to elongated spindle or fusiform shape with a maximal diameter of 3–10 μm and a length of 15–30 μm (Fig. 2A) (Romanov et al., 2007), which appears slightly smaller compared with electron micrographs published previously (Kinnamon et al., 1988; Royer and Kinnamon, 1988). Membrane capacitance (Cm) is a parameter proportional to membrane surface area (Neher and Marty, 1982) and considering a specific membrane capacitance of 5 fF/μm2 (=0.5 μF/cm2) (Solsona et al., 1998) and assuming a cylindrical shape, an estimate of resting Cm from 1 to 8 pF is expected in taste cells. Measurements on 75 cells revealed a resting Cm of 3.42 ± 0.18 pF (mean ± SEM), consistent with values reported previously (Bigiani, 2001). Resting Cm of type I cells, identified electrophysiologically (Fig. 2B–D) (Medler et al., 2003; Romanov and Kolesnikov, 2006), was 4.25 ± 0.34 pF (n = 19). Type III cells, identified by green fluorescence of GAD67-GFP mice (Fig. 2A) (DeFazio et al., 2006; Tomchik et al., 2007), had a resting capacitance of 2.60 ± 0.12 pF (n = 37), whereas type II cells, identified by the green fluorescence of TRPM5-GFP mice and/or by the profile of voltage-activated inward currents (Fig. 2B–D) (Medler et al., 2003; Clapp et al., 2006), exhibited a resting capacitance of 4.21 ± 0.46 pF (n = 19). Larger resting Cm of type I and type II taste cells (p < 0.001) compared with type III cells is consistent with their morphology (Royer and Kinnamon, 1994). Type I cells possess membranous extensions that envelop other taste cell types (Pumplin et al., 1997), contributing to their relatively high Cm for their apparent size.
Figure 2.
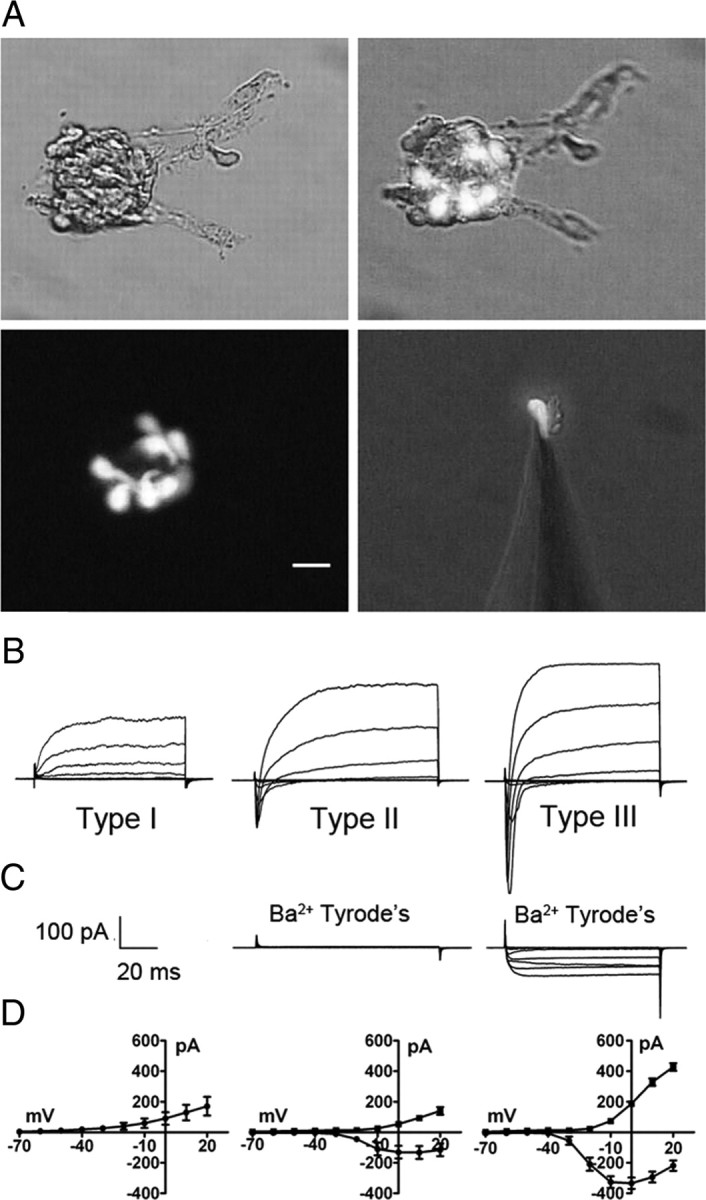
Optical and electrophysiological identification of taste cell type. A illustrates photomicrographs of circumvallate taste buds and a single isolated taste cell, isolated from a GAD67-GFP mouse, attached to a patch pipette. GFP-labeled cells are type III taste cells. Similarly, type II taste cells could be identified by GFP fluorescence from TrpM5-GFP mice (data not shown). Scale bar, 20 μm. B illustrates typical current profiles of type I, type II, and type III taste cells in Tyrode's. C, Although both type II and type III taste cells have voltage-gated Na+ currents in response to membrane depolarization, only type III taste cells have voltage-gated currents in Ba2+ Tyrode's (with Na+ and K+ currents blocked). D illustrates the averaged I/V curves for the voltage-gated Na+ (circles) and K+ (squares) currents in type I (n = 10), type II (n = 12), and type III (n = 18) cells in Tyrode's. Voltage was stepped in 10 mV increments from −60 to +20 mV from a holding potential of −70 mV. Error bars represent SEM.
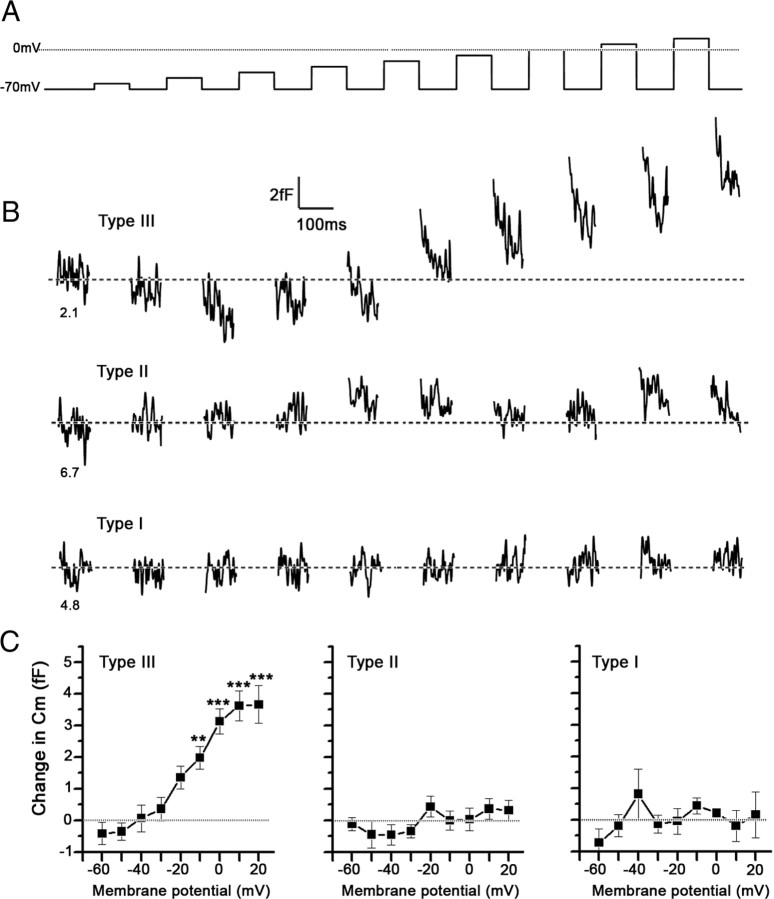
To detect voltage-induced surface area changes monitored as Cm in the femtofarad range and to monitor the voltage-activated currents, we applied a series of nine depolarizing pulses (Fig. 3A) averaged over 10 cycles (to reduce noise) to determine whether the cell exhibited voltage-activated currents and showed concomitant changes in Cm. Figure 3B shows representative responses to the averaged series of voltage pulses in the three types of taste cells. Note that, at the highest voltage pulse amplitudes, significant increases in Cm are recorded in type III cells (Fig. 3B, top trace). In contrast, in type II (Fig. 3B, middle trace) and in type I (Fig. 3B, bottom trace) cells, such changes were not observed. Average changes in Cm (Fig. 3C), measured before and after each voltage pulse, show that only in type III cells voltage-induced increases in Cm are present. In the other two cell types, voltage-induced changes in Cm are not significantly different from zero. Moreover, when capacitance changes were normalized to the resting Cm of each cell and represented as a percentage of resting Cm, similar results were obtained (Table 1).
Figure 3.
Type III, but not type II and type I, taste cells exhibit voltage-induced increases in membrane capacitance (Cm). A, The sequence of voltage pulses, in 10 mV increments, applied to the taste cells clamped at −70 mV. B, Representative depolarization-induced changes in Cm (100 Hz, low pass, Gaussian filter) in type III, type II, and type I taste cells. The numbers beneath each trace represent the resting Cm of each taste cell. C, Average depolarization-induced changes in Cm in type III, type II, and type I cells, recorded in 18, 12, and 10 cells, respectively. Error bars represent SEM. The asterisks represent the significance level compared with the Cm at the first voltage step (***p < 0.001; **p < 0.01; one-way ANOVA with Tukey's multiple-comparison test).
Table 1.
Maximum values of ΔCm normalized to individual cell resting capacitance, expressed as percentage max ΔCm
| Type III (n = 18) | Type II (n = 12) | Type I (n = 10) | |
|---|---|---|---|
| Resting Cm (pF) | 2.47 ± 0.17 | 3.79 ± 0.50 | 4.06 ± 0.57 |
| Max ΔCm at +20 mV (fF) | 3.67 ± 0.59 | 0.32 ± 0.32 | 0.16 ± 0.73 |
| % Max ΔCm at +20 mV | 0.16 ± 0.03 | 0.003 ± 0.012 | 0.012 ± 0.03 |
The cells included in this table represent only those cells whose change in Cm were measured. The percentage max ΔCm of type III taste cells is significantly different (p < 0.05; one-way ANOVA with Tukey's multiple-comparison test) from both type I and type II taste cells. The percentage max ΔCm values for type I and type II cells are not significantly different from each other.
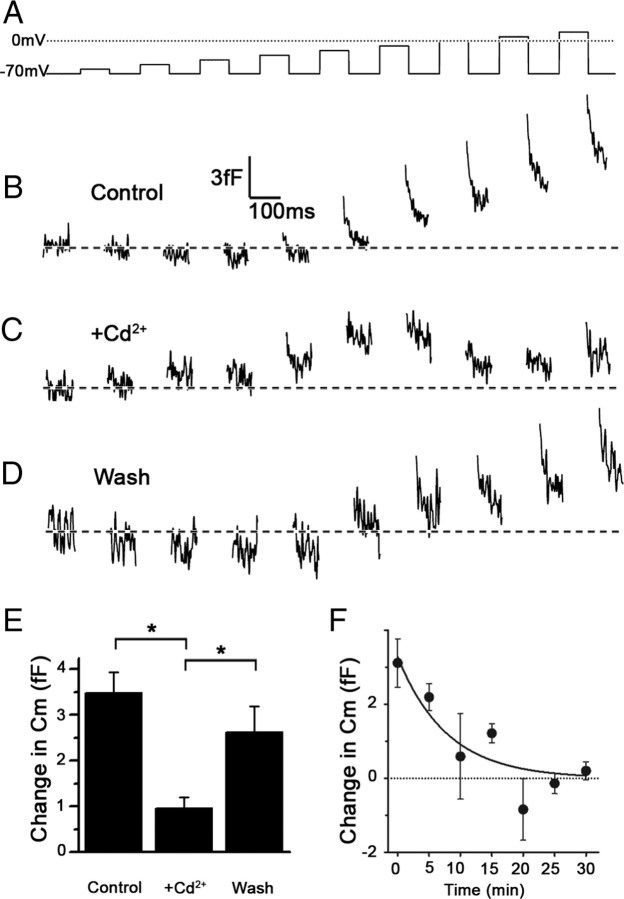
As shown in Figure 2B, high-voltage-activated Ca2+ currents, determined in the presence of extracellular Ba2+ (see Materials and Methods), are expressed in type III cells, consistent with previous reports (Medler et al., 2003; Romanov and Kolesnikov, 2006). A recent study demonstrated that the currents in the GAD67-GFP subset of type III cells are carried primarily by P/Q-type Ca2+ channels, since they are blocked by Ω-agatoxin IVA (Roberts et al., 2009). We confirmed that the peak Ba2+ currents in our preparation are blocked by Ω-agatoxin IVA (data not shown). Since the general Ca2+ channel blocker Cd2+ is more readily reversible in our preparation, we elected to use Cd2+ to determine the Ca2+ dependence of the changes in Cm in type III taste cells. Compared with the control (Fig. 4B), the application of 0.5 mm Cd2+ reduced the depolarization-induced Cm changes (Fig. 4C) and the effect was reversible (Fig. 4D). The effect was significant (p < 0.05; n = 5), based on the average of voltage-induced responses in Cm, determined from the last five voltage pulses in the series (Fig. 4E). Partial, rather than full, recovery is likely attributable to a time-dependent amplitude reduction of voltage-induced responses in Cm under control conditions, as shown on Figure 4F, in which we observed a decline with a time constant of ∼8 min.
Figure 4.
Cd2+-mediated attenuation of voltage-induced changes in Cm in a type III taste cell. A, The sequence of voltage pulses applied to the taste cell clamped at −70 mV. B, Control depolarization-induced changes in Cm, recorded before the application of Cd2+ Tyrode's solution. C, Depolarization-induced changes in Cm recorded 3 min after the application of a Tyrode's solution containing 0.5 mm Cd2+. D, Recovery of depolarization-induced changes in Cm after 5 min wash of the cell with Tyrode's. The traces in B–D were low-pass filtered (100 Hz, Gaussian filter). E, Average amplitude of depolarization-induced increases in Cm, determined from the last five voltage pulses (−20 to +20 mV) on five cells was significantly reduced (p < 0.05) after the application of Cd2+. There was a significant (*p < 0.05; one-way ANOVA with Tukey's multiple-comparison test) recovery after washout of the Cd2+ Tyrode's. F, Time-dependent amplitude reduction of depolarization-induced Cm in type III taste cells (n = 6 cells). The voltage pulse protocol was applied to a single cell in 5 min intervals. The response in Cm was averaged for the voltage pulses from −20 to +20 mV. The line represents the best fit to the equation of the form: Cm (fF) = (3.28 ± 0.63)e(0.127 ± 0.047)*time(min), yielding a time constant of the decay of ∼8 min. Error bars represent SEM.
To further test the role of Ca2+, we studied the relationship between voltage-induced increases in Cm and the amplitude of voltage-activated Ba2+ currents. After the recording of voltage-induced changes in Cm in type III cells (measured with Ca2+-containing Tyrode's), we then recorded the voltage-activated Ba2+ currents (Fig. 2B, right panel) in the same cells. Figure 5A shows the relationship between the average amplitude of voltage-activated Ba2+ currents as a function of membrane potential. The threshold of activation of these currents is related to the increase in voltage-induced changes in Cm, recorded in the same cells (Fig. 5B); however, the Cm change did not decrease at voltages at which the Ba2+ current begins to decrease. To determine whether the lack of decrease in the Cm at higher voltage steps was attributable to averaging 10 runs, which might lead to Ca2+ buildup, we recorded Cm changes in taste cells with a single voltage series. Although the Cm changes in these cells were more variable because of the lack of averaging, they showed a clear decrease in Cm at higher voltage steps, paralleling the decrease in Ba2+ current at the same voltage steps. These results corroborate and extend the results obtained with Cd2+ and are consistent with the hypothesis that type III taste cells exhibit Ca2+-associated voltage-dependent changes in Cm, likely reflecting regulated exocytosis of transmitter.
Discussion
The aim of this study was to test whether taste cells exhibit depolarization-induced regulated exocytosis, consistent with the fusion of vesicle membrane with the plasma membrane, as monitored by patch-clamp membrane capacitance measurements (Neher and Marty, 1982). We show that a sequence of depolarizing stimuli in type III taste cells reliably evokes increases in Cm that are not observed in either type II or type I taste cells. Although the depolarization-evoked Cm responses in the taste cells are small compared with those of mammalian sensory receptors with ribbon synapses [i.e., 4–5 fF in taste cells compared with 30–40 fF for mouse vestibular hair cells (Dulon et al., 2009) and >100 fF for photoreceptors (Innocenti and Heidelberger, 2008)], the responses are robust and repeatable. This finding is consistent with the comparatively small synaptic area in taste cells compared with hair cells and photoreceptors (Royer and Kinnamon, 1988, 1994). Furthermore, capacitance changes in taste cells are likely to be Ca2+-dependent, involving the entry of Ca2+ through the voltage-activated Ca2+ channels. Cm responses were inhibited by the Ca2+ channel blocker Cd2+ and the threshold voltage for Cm increases mirrored the threshold for voltage-dependent Ba2+ current activation. Furthermore, when a single voltage series was used to stimulate the taste cells, there was a clear decline in Cm at higher voltages at which the Ba2+ current is declining. It is interesting that there was no obvious linear relationship between Ca2+ current density and Cm change when 10 runs were averaged. Although membrane Cm peaked at the peak of the Ba2+ current, it did not decay with the decreasing Ba2+ current at high voltages. The lack of decrease of Cm at higher voltages may be attributable to a residual buildup of intracellular Ca2+, since the voltage pulses were separated by only 100 ms and the voltage series was averaged over 10 cycles. However, this rate of stimulation is physiological, since type III cells are highly electrically excitable and generate trains of action potentials to taste stimuli (Yoshida et al., 2009). Repetitive voltage steps, such as paired-pulse protocols, are commonly used to test the presence of facilitation of current amplitudes. The augmentation of responses is thought to be attributable to a buildup of Ca2+.
Type III taste cells contain two classes of vesicles: small clear synaptic vesicles with a diameter of 40–70 nm and vesicles with a dense core with a diameter of 90–120 nm (Royer and Kinnamon, 1994). Considering that type III cells in mouse form on average two synapses with the afferent nerve, and that each presynaptic site consists of ∼250 small clear vesicles, the fusion of all these vesicles with the plasma membrane on stimulation will result in a surface area increase of the taste cell of ∼4 μm2, or an equivalent of 20 fF. Furthermore, it was shown that there are approximately five dense-core vesicles per slice of 250 nm thickness in type III taste cells [Royer and Kinnamon (1994), their Fig. 5]. Considering that a synapse consists of five sections (Royer and Kinnamon, 1994), each synapse will contain ∼25 dense-core vesicles. Assuming that all dense-core vesicles fuse with the plasma membrane on stimulation, a membrane surface area of ∼1 μm2, or an equivalent of 5 fF is expected. These data, combined with our Cm measurements, suggest that ∼20% of the vesicles are released in response to each depolarizing stimulus. Since this number is much lower than that observed with sensory cells with ribbon synapses that rely on fast communication with sensory afferents (Innocenti and Heidelberger, 2008; Dulon et al., 2009), it suggests that the readily releasable pool of vesicles in type III taste cells is much lower. Alternatively, the relatively small increase in Cm may also be attributable to the ongoing endocytosis. A very small decline in Cm was observed during the first few voltage steps, which may reflect background endocytosis, especially since the data were averaged over a 10 pulse series (Figs. 3B, top trace; 4B, top and bottom traces). Moreover, at higher amplitude voltage steps, a more rapid decline in Cm was observed, which may reflect a more rapid component of endocytosis, observed in other sensory cells (Matthews, 1996). However, the observed slow decline of Cm was much slower that the voltage pulse duration, which should minimally affect the measured increases in Cm. However, it cannot be ruled out completely that the voltage-induced steps in Cm are somehow underestimated by the slow decline in Cm because of slow endocytosis.
An important question that remains is the identity of the transmitter(s) that are released by each type of vesicle. Both serotonin (Y. J. Huang et al., 2005; Y. A. Huang et al., 2009) and norepinephrine (Y. A. Huang et al., 2008) are released from type III taste cells, and both likely involve dense-core vesicles. The transmitter(s) contained in the small clear vesicles are not known, but could be GABA, since type III cells express GAD67 (DeFazio et al., 2006). Additional studies will be required to determine whether type III cells release GABA in response to depolarization.
The lack of depolarization-induced Cm changes in the other types of taste cells is noteworthy. Type I cells are similar to astrocytes (Lawton et al., 2000; Bartel et al., 2006) in expressing proteins for uptake and degradation of transmitters. Unlike astrocytes, type I taste cells contain abundant, membrane-associated dense granules in the apical region supposedly released to contribute to the dense substance in the taste pore (Takeda and Hoshino, 1975). Recent data also suggest that type I cells may play a role in transduction. They express the amiloride-sensitive Na+ channel ENaC, suggesting that type I cells may be the primary transducer of amiloride-sensitive salt taste (Vandenbeuch et al., 2008). How salt taste information is transmitted from type I taste cells to the nervous system or neighboring cells is unclear. These cells do not form morphologically identifiable synapses with nerve fibers. Our data indicate that depolarization does not result in release of either vesicles or dense granules.
Type II cells release ATP via hemichannels composed of pannexin-1 and/or connexin subunits (Y. J. Huang et al., 2007; Romanov et al., 2007; Dando and Roper, 2009) to activate P2X receptors on the afferent nerve fibers (Finger et al., 2005). However, type II cells also contain some components of synaptic machinery, including synaptobrevin-2 (Yang et al., 2004) and synaptophysin (Asano-Miyoshi et al., 2009). In addition, type II cells express several peptide transmitters, including GLP-1 (glucagon-like peptide I) (Shin et al., 2008); NPY (neuropeptide Y), CCK (cholecystokinin), and VIP (vasoactive intestinal peptide) (Shen et al., 2005; Zhao et al., 2005; Herness and Zhao, 2009); and galanin (Seta et al., 2006). Furthermore, many type II cells express choline acetyltransferase and vesicular acetylcholine transporter (Ogura and Lin, 2005; Ogura et al., 2007), suggesting ACh may also be released. If and how these transmitter candidates are released remains in question. Romanov et al. (2007) demonstrated that ATP is released from type II cells in response to depolarizing voltage steps; however, our data appear to rule out voltage-regulated exocytosis. Type II cells use IP3-mediated release of Ca2+ from intracellular stores in the transduction of bitter, sweet, and umami taste stimuli (Zhang et al., 2003), and this may provide sufficient intracellular Ca2+ to evoke vesicular exocytosis. Additional experiments will be required to test this possibility.
Footnotes
This work was supported by Bilateral Grant BI-US/08-10-034 (R.Z., S.C.K.); R.Z. is supported by Grants P3-310-0381, J3-0133, J3-0031, and J3-9417 from the Research Agency of the Republic of Slovenia. S.C.K. is supported by National Institutes of Health Grants DC000766 and DC007495. We thank Dr. William Betz for the use of the SWAM IIC amplifier, Dr. Robert Margolskee for the TrpM5-GFP mice, and Catherine Anderson and Daniel Sanculi for technical assistance. We also thank Dr. Jack Kinnamon for helpful discussions and Drs. Thomas Finger and Katie Rennie for comments on this manuscript.
References
- Asano-Miyoshi M, Hamamichi R, Emori Y. Synaptophysin as a probable component of neurotransmission occurring in taste receptor cells. J Mol Histol. 2009;40:59–70. doi: 10.1007/s10735-009-9214-5. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béhé P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigiani A. Mouse taste cells with glialike membrane properties. J Neurophysiol. 2001;85:1552–1560. doi: 10.1152/jn.2001.85.4.1552. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus K, Lindau M. Resolution of patch capacitance recordings and of fusion pore conductances in small vesicles. Biophys J. 2000;78:2983–2997. doi: 10.1016/S0006-3495(00)76837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci. 2009;29:10474–10487. doi: 10.1523/JNEUROSCI.1009-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Meiri H, Horstmann H, Lindau M, Almers W. Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. EMBO J. 2000;19:84–93. doi: 10.1093/emboj/19.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Heidelberger R. Mechanisms contributing to tonic release at the cone photoreceptor ribbon synapse. J Neurophysiol. 2008;99:25–36. doi: 10.1152/jn.00737.2007. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds: III. Patterns of synaptic connectivity. J Comp Neurol. 1988;270:1–10. 56–57. doi: 10.1002/cne.902700102. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;12:3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Marty A, Neher E. Tight-seal whole-cell recording. In: Sakmann B, Neher E, editors. Single-channel recording. New York: Plenum; 1983. pp. 107–113. [Google Scholar]
- Matthews G. Synaptic exocytosis and endocytosis: capacitance measurements. Curr Opin Neurobiol. 1996;6:358–364. doi: 10.1016/s0959-4388(96)80120-6. [DOI] [PubMed] [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Lin W. Acetylcholine and acetylcholine receptors in taste receptor cells. Chem Senses. 2005;30(Suppl 1):i41. doi: 10.1093/chemse/bjh103. [DOI] [PubMed] [Google Scholar]
- Ogura T, Margolskee RF, Tallini YN, Shui B, Kotlikoff MI, Lin W. Immuno-localization of vesicular acetylcholine transporter in mouse taste cells and adjacent nerve fibers: indication of acetylcholine release. Cell Tissue Res. 2007;330:17–28. doi: 10.1007/s00441-007-0470-y. [DOI] [PubMed] [Google Scholar]
- Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Roberts CD, Dvoryanchikov G, Roper SD, Chaudhari N. Interaction between the second messengers cAMP and Ca2+ in mouse presynaptic taste cells. J Physiol. 2009;587:1657–1668. doi: 10.1113/jphysiol.2009.170555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Kolesnikov SS. Electrophysiologically identified subpopulations of taste bud cells. Neurosci Lett. 2006;395:249–254. doi: 10.1016/j.neulet.2005.10.085. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer SM, Kinnamon JC. Ultrastructure of mouse foliate taste buds: synaptic and nonsynaptic interactions between taste cells and nerve fibers. J Comp Neurol. 1988;270:11–24. 58–59. doi: 10.1002/cne.902700103. [DOI] [PubMed] [Google Scholar]
- Royer SM, Kinnamon JC. Application of serial sectioning and three-dimensional reconstruction to the study of taste bud ultrastructure and organization. Microsc Res Tech. 1994;29:381–407. doi: 10.1002/jemt.1070290508. [DOI] [PubMed] [Google Scholar]
- Seta Y, Kataoka S, Toyono T, Toyoshima K. Expression of galanin and the galanin receptor in rat taste buds. Arch Histol Cytol. 2006;69:273–280. doi: 10.1679/aohc.69.273. [DOI] [PubMed] [Google Scholar]
- Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130:229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solsona C, Innocenti B, Fernández JM. Regulation of exocytotic fusion by cell inflation. Biophys J. 1998;74:1061–1073. doi: 10.1016/S0006-3495(98)74030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Hoshino T. Fine structure of taste buds in the rat. Arch Histol Jpn. 1975;37:395–413. doi: 10.1679/aohc1950.37.395. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol. 2004;471:59–71. doi: 10.1002/cne.20021. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol. 2006;96:3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Henigman F, Mason WT, Kordas M. Electrophysiological study of hormone secretion by single adenohypophyseal cells. Methods Neurosci. 1991;4:194–210. [Google Scholar]