Abstract
Sphingolipids are intrinsic components of membrane lipid rafts. The abnormal accumulation of these molecules may introduce architectural and functional changes in these domains, leading to cellular dysfunction. Galactosylsphingosine (psychosine) is a pathogenic lipid-raft associated molecule whose accumulation leads to brain deterioration and irreversible neurological handicap in the incurable leukodystrophy Krabbe disease (KD). The relevance of clearing excessive levels of pathogenic psychosine from lipid rafts in therapy for KD has not been investigated. The work presented here demonstrates that psychosine inhibits raft-mediated endocytosis in neural cells. In addition, while in vitro enzyme reconstitution is sufficient for the reversal of related endocytic defects in affected neural cells, traditional in vivo enzyme therapies in the mouse model of KD appear insufficient for complete removal of pathogenic levels of raft-associated psychosine. This work describes a mechanism that may contribute to limit the in vivo efficacy of traditional therapies for KD.
Keywords: Twitcher, Krabbe disease, leukodystrophies, myelin, bone marrow transplants, lentiviral gene transfer, dying-back pathology, lipid raft, psychosine, endosomes
INTRODUCTION
Globoid cell leukodystrophy or Krabbe disease (KD) is caused by the genetic deficiency of the enzyme β-galactosylceramidase (GALC). The result of this deficiency is the progressive accumulation of sphingolipid metabolites such as galactosylsphingosine or psychosine. Psychosine is thought to be highly toxic and the consequences of its accumulation include severe demyelination and neurodegeneration, eventually leading to early death (Wenger 2001; Suzuki 2003; Galbiati et al. 2009). In recent years it has been shown that psychosine may affect different signaling pathways, including psychosine-mediated activation of phospholipase A2 (Giri et al. 2006) and upregulation of AP1 in oligodendrocytes (Haq et al. 2003). One recent mechanistic breakthrough regarding psychosine mediated toxicity is the finding that psychosine accumulates to high levels in lipid rafts (LRs) and that this affects the activity of protein kinase C (PKC) (White et al. 2009). This is important as it is the first specific demonstration of a mechanism by which psychosine may be able to act negatively within membrane domains in a cellular system.
In humans, the most common form of KD is the severe and progressive early infantile variant. Within six months of birth these patients show symptoms of irritability, spasticity and developmental regression which is generally followed by fatality before 2 years of age (Aicardi 1993). Since this disease is the result of the loss of GALC activity, the treatment of KD has mostly consisted of strategies for the replacement of the deficient enzyme (i.e. umbilical cord blood transplants and bone marrow transplants). In addition, some attention has also been given to the supplementation of these therapies through both viral-mediated GALC gene transfer and pharmacological inhibition of psychosine production using substrate reduction therapy (Biswas and LeVine 2002; Dolcetta et al. 2006; Galbiati et al. 2009). While these treatments have indeed been shown to delay the onset of some symptoms in KD patients, as of yet no cure for this disease has been described (Escolar et al. 2005; Krivit et al. 1998; Lim et al. 2008).
Since it is thought that toxicity in KD is mediated by the loss of GALC and the resultant accumulation of psychosine, the efficient treatment of this disease relies upon two phenomena: First, therapies must be able to deliver the missing enzyme to lysosomes in areas of the body, such as the central nervous system, that are heavily impacted by the disease. In addition, it is necessary that subcellular compartments in which psychosine is accumulated are somehow exposed to GALC-containing lysosomes in order for breakdown and clearance of this lipid to occur. Recent research has clearly demonstrated that psychosine is preferentially accumulated in LRs. This suggests that recovery of KD may rely on the exposure of these domains to lysosomal GALC activity. The following work shows that psychosine can inhibit the endocytosis of LRs and further demonstrates that traditional GALC replacement therapies in Twitcher (TWI) mice, a natural genetic model of KD (Duchen et al. 1980), are insufficient to completely remove psychosine from these domains. These results provide additional mechanistic interpretations to understand the limitations in the treatment of KD.
MATERIALS AND METHODS
Animal Care and Treatment
For the twitcher colony, heterozygous breeders were maintained under standard housing conditions with the approval of the Animal Care and Use Committee. Mice were left to survive as long as was possible with animals reaching a moribund condition being sacrificed. Genotyping was performed as previously described by extracting DNA from clipped tails of postnatal day 1 (P1) to P2 mice (Dolcetta et al. 2006; Sakai et al. 1996). GALC replacement was done using methods of lentiviral injection and bone marrow transplant that have previously been described (Dolcetta et al. 2006; Galbiati et al. 2009).
Cell Culture
Neural stem cell cultures were harvested from early postnatal mice as previously described (Givogri et al. 2006; Gritti et al. 2002). Briefly, primary cells were isolated and left to grow in control medium containing basic fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF) (Peprotech). Under these conditions neurospheres are formed from a fraction of proliferative cells. Primary neurospheres are then collected, mechanically dissociated and plated in complete medium for expansion. NSC34 cells were obtained from ATCC and grown under standard conditions in DMEM containing 5% fetal bovine serum (FBS). HeLa cells were also obtained from ATCC and grown under standard conditions in DMEM containing 10% FBS. Transfected HeLa cells expressing GALC or GALC-HA were obtained from frozen stocks which were developed as described previously (Dolcetta et al. 2006). For uptake assays, cells were singly dissociated and plated at a density of 3,000 cells per well in 16-well matrigel coated chamber slides. In the GALC replacement experiments, 5×107 cells were used per condition and were grown in 175mm flasks. Conditioned medium was obtained by growing HeLa cells to 75–80% confluence in 150mm dishes and incubating them in DMEM/F12 for 72 hours. Before use with singly dissociated NSCs, conditioned medium was diluted by 50% using complete medium with 1x DMEM/F12 and 2x concentrations of all other components.
Uptake Assays
Uptake assays were performed essentially as described (Shajahan et al. 2004a; Shajahan et al. 2004b) with minor alterations for the culture systems and reagents used in this study. Reagents used were Cholera Toxin B- AF488 (CTXB) (Molecular Probes), NBD-Lactosyl Ceramide (NBD-LacCer) (Matreya), Transferrin-AF546 (Molecular Probes) and GALC-HA which was obtained by conditioning medium and labeled using a FITC-HA antibody (Sigma). Following exposure to each marker, cells were washed three times in ice cold PBS and then immediately fixed for 15 minutes with 4% paraformaldehyde. Cells were co-labeled with DAPI in order to allow for their localization under the microscope without the photobleaching of the probes. Exposure times were dependent upon which probe was used and were as follows: 30 minutes for CTXB, 15 minutes for NBD-LacCer, 1hr for Transferrin and 1hr for GALC-HA. Immunocytochemical staining with cellular markers was done as described previously using antibodies recognizing O4 (Millipore), NeuN (Millipore) and Glial fibrillary acidic protein (Abcam) (Givogri et al. 2006; Givogri et al. 2008). Imaging of cells was done using a Zeiss LSM 510 Meta microscope. Microscope settings were optimized using control cells at the beginning of each experiment and were unchanged while acquiring images. Fluorescence intensity (20–40 cells per condition) was quantified using NIH ImageJ software.
GALC Activity Assay
Analysis of GALC activity was done essentially as described previously (Wiederschain et al. 1992). Briefly, a 96 well plate was prepared in which each sample was left to incubate with 20uL of the GALC substrate for 17 hours at 37°C. After incubation, the reaction was stopped and the plate was measured using a Beckmann Coulter DTX 880 multimode detector and excitation/emission wavelengths of 385nm and 450nm, respectively.
Lipid raft preparation
Lipid raft preparation is performed as described previously (Vetrivel et al. 2005; Vetrivel et al. 2004; White et al. 2009). Briefly, brains are homogenized on ice with a dounce homogenizer followed by 5 passages through a 25 gauge needle. Homogenates are then centrifuged at 960g for 10 minutes at 4C. The supernatant is collected and the pellet is resuspended and once again passaged through a 25 gauge needle and centrifuged. The second supernatant is pooled with the first and supplemented with 5% lubrol wx (Serva) for a final concentration of 1%. The resultant mixture is nutated for 30 min at 4C before being loaded onto a discontinuous sucrose gradient and centrifuged in an SW41 rotor (Beckman) at 39000 RPM for 18–22 hours at 4C. After centrifugation fractions are collected and analyzed. For culture models, cells are collected and lysed on ice by 5 passages through a 25 gauge needle in a buffer containing lubrol. These lysates are then loaded directly on to a discontinuous sucrose gradient and centrifuged as above.
Mass Spectrometry
Lipid samples from tissue and cells were extracted in chloroform/methanol/water and analyzed by liquid chromatography tandem mass spectrometry, as previously described (Galbiati et al. 2007). Positive ion electrospray precursor ion scanning was done using an API 4000 triple quadrupole mass spectrometer from Applied Biosystems that was equipped with a Shimadzu HPLC system and Leap autosampler (Whitfield et al. 2001).
Western Blot
Western blots were performed using lipid raft and total samples as previously described (White et al. 2009). Briefly, of the 12 fractions collected from each lipid raft preparation fractions 4–12 were used for western blot analysis. Equal volumes of all fractions from a sample were loaded and normalization between samples was done by comparing protein concentrations in a pool of fractions 8–12. The following antibodies were used in this study: anti-flotillin-2, P115 (Beckton Dickson), caveolin-1 (Cell Signaling), PKC (Santa Cruz), phospho-PKC (Cell Signaling), and actin (Millipore Bioscience Research Reagents). Antibody-reactive products were detected using peroxidase-labeled secondary antibodies and ECL chemiluminescent substrate (Pierce).
Statistical Analysis
For experiments in which statistics are presented data were given as the mean +/− SE. The statistical significance of the differences between these values was determined using a Student’s t-test or analysis of variance (ANOVA) test, where appropriate.
RESULTS
The caveolar pathway of endocytosis is inhibited in neural cells from a mouse model of Krabbe disease
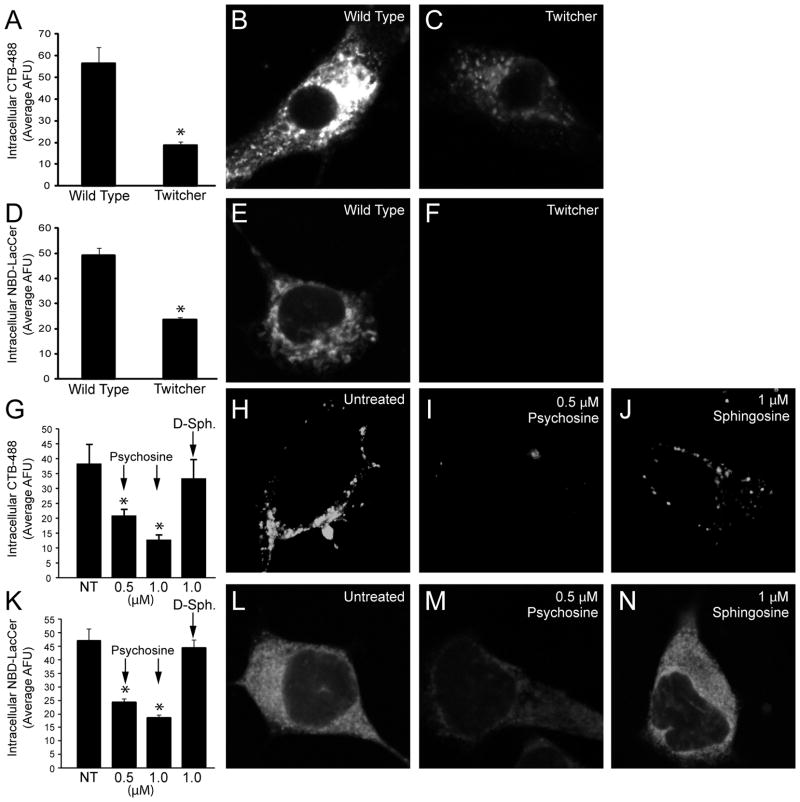
As a lysosomal enzyme, GALC requires an acidic environment to actively degrade psychosine. This means that, in order for GALC replacement to be effective in removing psychosine from LRs, both the enzyme and LRs must be endocytosed by a cell and localized within a lysosome. In order to examine whether one or both of these processes may be altered in KD, the endocytosis of both GALC, which is primarily mediated by the mannose-6-phosphate receptor (M6PR)/clathrin pathway (Rafi et al. 1996; Zhang et al. 2008), and of LRs was measured in differentiating cultures of wild type (WT) and twitcher (TWI) neural stem cells (NSC). The TWI mouse is a natural genetic model of the infantile variant of KD. It shows a rapidly progressing demyelinating and neurodegenerative phenotype that is concomitant with progressive accumulation of psychosine (Duchen et al. 1980; Igisu and Suzuki 1984). For the following experiments NSCs were isolated from the subventricular zone of early postnatal WT and TWI mice and expanded in EGF/FGF2 medium. Cells were differentiated by growth factor removal before experiments. Figure 1 shows the results of the analysis of raft-mediated endocytosis as measured by the uptake of cholera toxin B-Alexa 488 (CTB) and NBD-Lactosyl Ceramide (LacCer), two markers used to study caveolar and raft mediated endocytosis (Shajahan et al. 2004a; Shajahan et al. 2004b; Singh et al. 2007). The uptake of both CTB and LacCer was decreased by greater than 50% in TWI cells with respect to the WT cells (Figure 1A–1F). This shows that cells deficient of GALC activity have decreased endocytosis of LRs and related cargo.
Figure 1.
Lipid raft-mediated/caveolar endocytosis is inhibited in GALC deficient cells and in cells treated with psychosine. Endocytosis was examined by measuring the uptake of fluorescent cholera toxin B (CTB) and lactosyl ceramide (LacCer) in differentiated twitcher neural stem cells (NSC) and in NSC34 cells treated with psychosine. A–C. Uptake of CTB measured in wild type and twitcher NSCs after 30 minute exposures. D–F. LacCer uptake in Wild type and twitcher NSCs after 15 minutes of exposure to the fluorescent probe. G–J. CTB uptake is measured in NSC34 cells exposed to 0.5 and 1μM psychosine for 1 hour. K–N. Uptake of LacCer in NSC34 cells exposed 0.5 and 1 μM psychosine for 1 hour. In I and M, sphingosine is used as a non-toxic sphingolipid control for the specificity of the activity of psychosine in these cells. Representative cell images are shown for relevant experimental conditions. All data is expressed as the mean fluorescence intensity per cell +/− SE with an N of at least 25 for all conditions. *P=<0.0001.
Psychosine is sufficient for inhibition of caveolar endocytosis
To determine whether the decreased endocytosis of LRs was due to psychosine, the same experiments were carried out in cultures of the motoneuronal cell line NSC34 in the presence and absence of sub-lethal concentrations of psychosine. It was found that one hour of incubation in the presence of 0.5 μM or 1μM psychosine reduced the uptake of CTB in NSC34 cells by ~50% and ~70%, respectively (Figure 1G–1J) and, as expected, the uptake of LacCer was similarly reduced (Figure 1K–1N). Non-galactosylated D-sphingosine (D-Sph.) was unable to affect the endocytosis of either marker (Figure 1G, 1J, 1K, 1N). These results confirm that psychosine alone is sufficient to produce sizable decreases in raft-mediated endocytosis and strongly suggests that this lipid is the mediator of similar reductions observed in TWI cells.
Clathrin-mediated endocytosis is not affected in KD and is not a target of psychosine
To determine whether similar effects are seen with mechanisms of endocytosis that are not raft-mediated, such as the mannose-6-phosphate receptor (M6PR)/clathrin-mediated uptake of the GALC enzyme, endocytosis of GALC and of transferrin (Ehrlich et al. 2004; Pearse 1982) was measured. To measure uptake of the enzyme, GALC was tagged with the human influenza hemagglutinin (HA) epitope (Dolcetta et al. 2006). These results showed that the uptake of both GALC-HA (Figure 2A–2C) and transferrin (Figure 2D–2F) were not significantly affected in TWI neural cells. Furthermore, ectopic addition of psychosine to motoneuronal NSC34 cells was unable to affect the endocytosis of either GALC-HA (Figure 2G–2J) or transferrin (Figure 2K–2N). Taken together, these findings suggest that the presence of psychosine significantly inhibits raft-mediated endocytic processes while leaving M6PR-mediated and clathrin-mediated endocytosis unaffected.
Figure 2.
Non raft-mediated endocytosis is not altered by GALC deficiency or psychosine exposure. Mannose-6-phosphate receptor/clathrin mediated endocytosis was measured by quantification of the uptake of either HA-tagged GALC or fluorescent transferrin in differentiated twitcher NSCs and in NSC34 cells exposed to psychosine. A–C. Analysis of the uptake of GALC-HA by twitcher and wild type NSCs after 1 hour of exposure. D–F. Analysis of transferrin uptake in twitcher and wild type NSCs after 1 hour exposure. G–J. Uptake of GALC-HA is measured in NSC34 cells treated with 0.5 or 1 μM psychosine for 1 hour. K–N. Fluorescent transferrin uptake in NSC34 cells treated with 0.5 or 1 μM psychosine for 1 hour. In I and M, sphingosine is used as a non-toxic sphingolipid control for the specificity of the activity of psychosine in these cells. Representative cell images are shown for relevant experimental conditions. All data is expressed as the mean fluorescence intensity per cell +/− SE with an N of at least 25 for all conditions.
In vitro enzyme reconstitution is capable of removing psychosine from LR, improving caveolar endocytosis and recovering raft architecture
Importantly, the above results set up a paradigm in which the preferential accumulation of psychosine in LRs not only disrupts raft architecture and related activities, such as signaling (White et al., 2009), but also inhibits their ability to be endocytosed. Because of the fundamental relevance of this effect on the metabolism of LR components (i.e. the degradation of psychosine at the lysosomal compartment, after endocytosis of LRs at the plasmalema), the ability of enzyme reconstitution to remove psychosine from LRs was tested.
First, the efficiency of enzyme correction in TWI neural cells, which lack GALC activity, was determined by supplementing their culture medium containing recombinant GALC. Conditioned medium with high levels of the enzyme was produced using Hela cells that overexpressed GALC (Dolcetta et al. 2006). Once exposed to the conditioned medium, TWI neural cells were collected at 8, 30 and 72 hours. Figure 3A shows that GALC activity was recovered to ~8% with respect to WT NSC levels after 8 hours and leveled off to around 5–6% thereafter. While enzymatic recovery seemed modest, figure 3B demonstrates that this was sufficient to induce a significant decrease in total levels of psychosine in the TWI cells. Specifically, psychosine concentrations that, after 8 hours, were unchanged from those of untreated TWI cells had decreased by over 90% after 72 hours (Figure 3B). Figure 3C demonstrates that levels of psychosine after 72 hours of exposure to GALC were not significantly different from concentrations detected in WT NSCs. Since it was shown in figure 1 that psychosine can significantly inhibit LR endocytosis, it was important to determine whether this phenomenon had an impact on the clearance of psychosine from LRs. To answer this question, LRs were isolated from TWI neural cells that had been exposed to GALC for 30 and 72 hours. Figure 3D shows that correction of GALC deficiency in TWI cells reduced the levels of psychosine in LR fractions by ~30% after 30 hours and by ~70% after 72 hours. This demonstrates that modest levels of enzymatic recovery were capable of removing psychosine from LRs to a great extent in an in vitro system.
Figure 3.
Psychosine is removed from lipid rafts and endocytic defects are reversed in NSCs incubated with GALC conditioned medium. Enzymatic correction was tested in vitro by incubating twitcher NSCs with conditioned medium from HeLa cells overexpressing GALC. A. Analysis of GALC activity in NSCs after 8, 30 and 72 hours of exposure to GALC conditioned medium. Recovery of enzymatic activity is represented as a percent recovery of GALC activity with respect to WT levels. B. Measurement of psychosine levels in twitcher NSCs after GALC exposure. Data is expressed as the percentage of psychosine retained in treated cells with respect to untreated twitcher NSCs at time zero. C. Total levels of psychosine are shown for twitcher cells in non-conditioned medium (NCM), GALC-conditioned medium (GCM) and wild type cells in NCM. Data is shown as nmol/mg protein and was collected after 72 hours of exposure to conditioned medium. D. Levels of psychosine in lipid rafts are shown after exposure to either non-conditioned medium (NCM) or GALC conditioned medium (GCM) for 30 and 72 hours. Data is expressed as a fold change with respect to normal wild type levels. Data for A–D is represented as the mean +/− SE.
Next, it was determined whether the removal of psychosine from LRs after enzyme therapy also normalized the caveolar endocytic pathway and recovered raft architecture. TWI neural cells were exposed to culture medium supplemented with GALC for 48 hours and then caveolar endocytosis of CTB and LacCer was measured as described for Figure 1. These results showed that the defects in endocytosis of CTB (Figure 4A–4C) and LacCer (Figure 4D–4F) were significantly recovered. In addition, LR’s prepared from treated cells were subjected to western blot analysis of the raft marker flotillin 2. This data demonstrated that levels of flotillin in raft fractions are decreased in untreated TWI cells and that 48 hour exposure to GALC was sufficient to induce a recovery of the raft marker to near wild type levels (Figure 4G). Combined, these results suggest that the blockage of raft endocytosis and disruption of domain architecture observed in TWI cells are phenomena that are not irreversible. This indicates that raft disruption can be ameliorated by the removal of psychosine from LRs after enzyme replacement in vitro.
Figure 4.
Lipid raft-mediated/caveolar endocytosis and lipid raft architecture are recovered in vitro with GALC enzyme replacement. The uptake of markers of raft-mediated endocytosis was assayed after incubation of twitcher neural cells with GALC conditioned medium (GCM). A–C Uptake of cholera toxin B (CTB) is measured in wild type NSCs and in twitcher NSCs incubated in GCM for 48 hours. D–F NBD-Lactosyl Ceramide (NBD-LacCer) uptake assays in wild type NSCs and in twitcher NSCs after 48 hour incubation in GCM. Data in A and D are expressed as the mean fluorescence intensity per cell +/− SE with an N of at least 25 for all conditions. B–C and E–F are representative cell images for each experimental condition. The recovery of raft architecture was assayed by western blot analysis of the levels and distribution of the lipid raft marker flotillin 2. G. Blots were performed using antibodies against Flotillin 2 (raft marker) and P115 (non-raft control). Samples were collected by lipid raft preparation of wild type, twitcher, and treated twitcher NSCs.
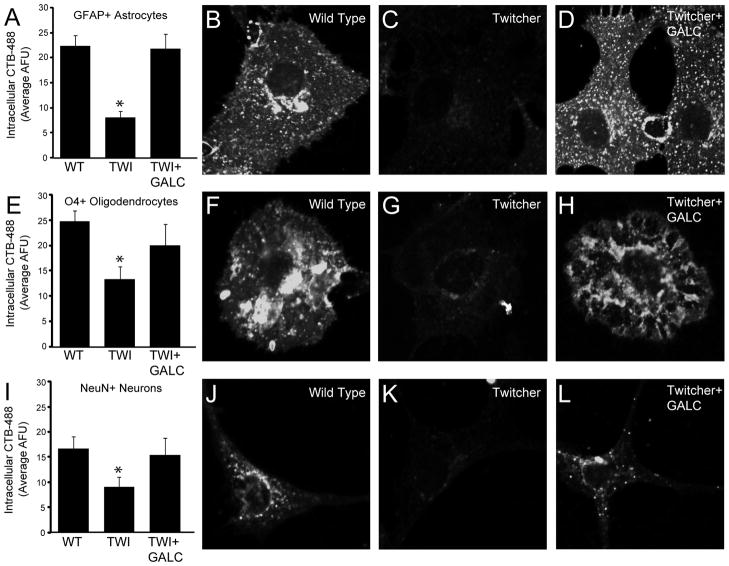
An interesting question that remained following the conduction of the above experiments was whether endocytotic defects could be attributed to a particular neural cell type. To investigate this possibility, uptake assays using CTB were performed on NSCs that were differentiated for 7–9 days. Importantly, the potential recovery of any defects was also addressed by exposing the TWI cells to GALC as described for figure 4. After CTB uptake, cells were processed immunocytochemically using markers for the three major neuronal cells types; glial fibrillary acidic protein for astrocytes, O4 for oligodendrocytes and NeuN for neurons (see supplementary figure 1). Figure 5 shows that, in all cell types, GALC deficiency was sufficient to reduce the uptake of CTB by ~50–70%. Interestingly, these experiments also demonstrated that enzyme replacement was able to induce recovery of these defects (Figure 5). This indicates that caveolar endocytosis is altered in TWI cells, regardless of the specific cell type. Importantly, this highlights the idea that psychosine-based induction of LR defects is a general phenomenon that may affect the health of any cell that is within the TWI nervous system.
Figure 5.
Inhibition of Lipid raft-mediated/caveolar endocytosis and its recovery by enzyme replacement are independent of neural lineage specificity in GALC deficient cells. Wild type and twitcher cells were cultured in differentiating conditions for 7–9 days. A subset of twitcher cells was exposed to GALC conditioned medium for the final 48 hours. Endocytosis was measured by uptake of cholera toxin B-488 (CTB-488) after 30 minutes of exposure. Cell types were identified by immunocytochemical staining using specific markers for each lineage. A–D. Measurement of CTB-488 uptake in glial fibrillary acidic protein (GFAP) positive astrocytes. EH. CTB-488 uptake assayed in oligodendrocytes that were positive for the lineage marker O4. I–L. Analysis of CTB-488 uptake in neurons that were identified by labeling with NeuN. Representative cell images are shown for all experimental conditions. All data is expressed as the mean fluorescence intensity per cell +/− SE. *P=<0.001.
Removal of psychosine from brain LRs is incomplete in Twitcher mice after in vivo enzyme replacement
The above experiments have provided an important first indication that LR related defects can be reversed in vitro. However, the question remained whether similar corrections could be achieved in vivo. For the following experiments, TWI mice were treated with enzyme replacement therapies within the first two postnatal days. The therapies used were divided into three groups: intravenous bone marrow transplant (BMT), intravenous lentiviral gene transfer with a GALC Lentivirus (LV), and a combination of both (BMT+LV). Ample details of these therapies have been described previously (Galbiati et al. 2009). BMT and BMT+LV groups but not the LV group experienced a significant albeit modest increase in lifespan (Supp. Figure 2A), with modest improvement of electrophysiological parameters such as peripheral motor conduction velocities (Supp. Figure 2B) and some recovery of myelination measured as expression of MBP (Supp. Figure 2C). Representative levels of engraftment (measured as chimerism in blood, Supp. Figure 3A) and infiltration of donor cells in the brain (Supp. Figure 3B–3F), confirmed that marrow replacement was stable and that donor cells were able to infiltrate, to a certain extent, the mutant brain. Supplementary figure 4A shows the efficiency of enzyme replacement as measured by total GALC activity in brain homogenates. This demonstrated that there was little to no recovery of GALC activity in the brains of TWI mice that received LV-treatment only; BMT and BMT+LV treatments recovered total GALC activity in the brain to ~10% and 20%, respectively. Supplementary figure 4B, shows the results of the LC-MS-MS measurement of total psychosine concentrations in TWI brains after treatment. These data demonstrated that, for each condition, psychosine was still detected at significantly high levels. In the LV-GALC animals, concentrations were found to be around 630pmol/mg protein which is at or above those of untreated end-stage TWI animals. For the BMT group, levels of psychosine were near those previously found in post-natal day 20 (P20) animals, around 350pmol/mg protein. Long-term survived mutants treated with the combination of both LV and BMT contained ~370 pmol/mg protein of psychosine. These data showed that psychosine accumulation was significantly lower in both BMT and LV+BMT groups with respect to untreated TWI control mice, consistent with some enzymatic recovery.
Since the above experiment demonstrated that high levels of psychosine were still present in treated animals and since recent work has demonstrated that psychosine preferentially accumulates in LRs, it was of interest to investigate whether this lipid was retained in LRs from treated animals. To do this, LR fractions were isolated from the brains of WT, untreated TWI at 20 and 45 days of age and from mutant mice subjected to either LV, BMT and BMT+LV treatments. TWI mice treated with the combination of LV and BMT were analyzed at P45 and maximal survival (range P75-P125). Those from LV and BMT groups were analyzed at their maximal survival (~P50, LV and ~P70, BMT). Average psychosine concentrations (pmol/mg protein) in raft fractions #4–6 and non-raft fractions #8–12 were measured by LC-MS-MS. Figure 6A demonstrates that a significant portion of residual psychosine remained associated to raft fractions in these brains. Specifically, concentrations of LR psychosine in untreated animals were found to be near zero in the WT, ~30 pmol/mg protein in the P20 TWI and ~60 pmol/mg protein in the P45 TWI. For the single treatment conditions levels were ~90 pmol/mg protein with LV treatment and ~30 pmol/mg protein with BMT (Figure 6A). For the combined treatment, levels were ~25 pmol/mg protein at P45 and ~45 pmol/mg protein at maximal survival (Figure 6A). Figure 6B shows representative chromatograms of the peak of psychosine detected with a retention time of 1.82 minutes in fraction #4 for each experimental group.
Figure 6.
Lipid raft disruption persists after enzyme replacement therapy in the twitcher mouse. A. Analysis of psychosine concentrations in lipid rafts prepared from twitcher and wild type mice. Enzyme replacement in the twitcher mice was achieved by bone marrow transplant (BMT), injection of lentiviral GALC (LV) or a combination of the two (BMT+LV). Results indicate that significant amounts of psychosine can be detected in twitcher lipid rafts in all conditions with respect to normal wild type levels. Data is shown for raft and non-raft membranes and represents the average concentration of fractions 4–5 and 8–12, respectively. Psychosine concentrations in rafts of P20 twitchers are not significantly different from P45 twitcher+BMT+LV or twitcher+BMT. However, P45 twitcher, twitcher+BMT+LV at max survival and twitcher+LV all show psychosine concentrations that are significantly above these P20 levels. B. Signal intensity is shown for representative mass spectrometric peaks resulting from the detection of psychosine in each treatment group. C. Western blots of total levels of raft markers are shown with respect to actin for each of the twitcher treatment groups as well as for the wild type animals. D–E. Western blots are shown for each treatment group and represent loading of fractions 4–12 from lipid raft preparations. Fractions 4–5 are labeled as rafts and 6–12 are notated as non-raft fractions. The levels of both markers are quantified in fraction 4 and 5 for each of the treatment groups and are labeled as follows: twitcher (TWI), twitcher+BMT+LV (TWI+BMT+LV), twitcher+LV (TWI+LV), twitcher+BMT (TWI+BMT), wild type (WT).
LR-associated changes in Twitcher mice are incompletely corrected after in vivo enzyme replacement
Interestingly, relatively low accumulations of psychosine have been shown to be sufficient to disrupt lipid raft architecture (White et al. 2009). In all treatment conditions tested in our study, psychosine concentrations were several times these levels and, therefore, raft disruptions were expected. To confirm this, western blots were performed using brain raft fractions collected from these animals to analyze the levels and distribution of the LR marker proteins Caveolin-1 and Flotillin 2. This analysis indicated that changes in the LR distribution (Figure 6D–E), but not in the total content (Figure 6C), of both Caveolin-1 and Flotillin-2 in LR fractions (fractions 4–5) are detectable in most treatment conditions (Figure 6D–E).
In addition to physical measures of membrane architecture it was of interest to determine whether functional changes associated with raft disruption could also be detected. Since previous work has demonstrated that psychosine can interfere with the activity of PKC (Hannun and Bell 1987; White et al. 2009), western blots for total (tPKC) and phosphorylated (active) PKC (pPKC) were used to measure its raft-associated activity in treated TWI brains. Compared with WT animals, a reduction in the level of pPKC and tPKC that is in LR fractions can be seen in each of the treatment conditions. Figure 7A shows examples of these immunoblot analyses for an untreated TWI mouse, BMT+LV TWI mouse and wild type control. Analysis of the ratio of pPKC/tPKC was then used to determine quantitatively whether alterations in PKC activity could be observed in LR fractions 4–5. These results show a reduction in activity of ~70% for both LV and BMT treated animals and a reduction of ~45% in the long-survived BMT+LV condition (Figure 7B). A similar quantitative analysis for pPKC/tPKC in brain homogenates (Figure 7C) shows no significant differences among all experimental groups, underlining the specific defect of this signaling pathway at the raft level.
Figure 7.
Psychosine in lipid rafts is concomitant with a loss of raft-associated protein kinase C activity in treated twitcher animals. Lipid raft fractions from wild type, twitcher and treated twitcher animals were analyzed for total protein kinase C (tPKC) and phosphorylated protein kinase C (pPKC). A. Western blots show the distribution of pPKC and tPKC in lipid raft fractions from wild type and twitcher animals including twitchers treated with a combination of lentiviral GALC and bone marrow transplant (+LV+BMT). B. Quantification of the ratio of pPKC/tPKC in fractions 4–5 from wild type mice or twitcher mice that are either untreated or treated with lentiviral GALC (LV), bone marrow transplant (BMT) or a combination of the two (BMT+LV). C. Quantification of PKC activity in whole brain extracts as indicated by the ratio of pPKC/tPKC. This was done for each treatment group and shows no detectable changes in PKC activity indicating that loss of localization of active PKC in lipid rafts may be the mechanism of its inhibition by psychosine.
Taken together, the data presented here show that, while achievable in cell culture, in vivo enzyme replacement techniques, such as those examined here, provide modest levels of therapeutic correction of biochemical and functional alterations of raft microdomains in the Twitcher brain.
DISCUSSION
The work presented in this study was originally designed to determine functional consequences of psychosine accumulation in LRs. Indeed, for the first time, it is demonstrated that raft-mediated endocytosis in KD is negatively and reversibly affected by the accumulation of psychosine in these domains.
Over the past several years a significant effort has been put forth with the goal of treating and ultimately curing KD (Hoogerbrugge et al. 1988; Scaravilli and Suzuki 1983; Yeager et al. 1984). Since it is known that the catalyst for disease is the lack of GALC activity, much of this work has focused on the reconstitution of the enzyme activity. To some extent, treatments developed out of this effort, such as bone marrow and umbilical cord blood transplants, have been successful at altering the natural progression of KD (Escolar et al. 2005; Krivit et al. 1998; Lim et al. 2008) (see also Supplementary figure 1A–B). However, it is unfortunate to note that so far infants with KD remain very affected and a definite cure is not available yet. In light of this, some recent research has been conducted with the idea that psychosine may be inducing detrimental changes to KD patients much earlier and in different ways than previously thought (Galbiati et al. 2007; Galbiati et al. 2009) (Cantuti & Bongarzone, submitted). In essence, minute levels of psychosine may be enough to induce alterations in cellular structure that may set the course of disease long before classically cytotoxic accumulations occur. The ideas presented here that most psychosine-based raft alterations persist throughout the course of enzyme correction as performed in our experiments is a previously undescribed feature of KD and may be an example of this phenomenon. In addition, it is important to mention that the fundamental implications of the apparent sensitivity of these cellular domains may apply in alternate paradigms such as other leukodystrophies (i.e. metachromatic leukodystrophy) or even aging.
One important question that is raised by this study is how minimal levels of GALC replacement are able to efficiently reverse psychosine-based LR defects in vitro but yet are relatively unsuccessful in vivo. One interpretation for this observation may be the differential complexity of the two systems. In the cell culture model, most if not all cells will be exposed evenly to the conditioned medium containing GALC and will thus have the opportunity to take up the enzyme and subsequently degrade psychosine. In vivo, however, a much more complex series of events is necessary, including the need for stable engraftment, generation of the proper hematopoietic lineages, infiltration of donor macrophages in the nervous system, production of GALC by transplanted cells (or by those lentivirally transduced), secretion of the enzyme and its diffusion and uptake by endogenous cellular populations throughout the brain of the host and importantly, its intracellular transport to the lysosomal compartment. Throughout the time needed for all of these steps to be successfully completed, the nervous system continues to synthesize and to accumulate psychosine. This means that lack of efficiency in vivo might be the result of insufficient diffusion and uptake of the enzyme in areas of the brain not immediately exposed to treatment (and/or corrected in a more timely fashion). The direct consequence of this paradigm is that, in spite of the fact that most cells may be exposed to GALC at certain point in time, its actual activity in a significant portion of them may not be high enough to overcome the accumulation of psychosine in LRs and the inhibition of raft-endocytosis and thus, raft defects in these cells are not corrected. In addition, these complications could be further exacerbated by differential levels of psychosine production in different cell types. For example, if all cell types are able to take up and use equal levels of GALC, it may be that populations producing lower levels of psychosine are corrected faster than those making more of this toxin. This may leave the CNS as a whole in a diseased state even while some cell populations may be rescued, as shown in the above in vitro experiments. Importantly, this is just a single example of the many ways in which cell specific functions or effects may complicate disease recovery.
The results presented in this study also raise the question of how psychosine is able to exert its effects on LR-mediated processes such as endocytosis. The most obvious explanation for this phenomenon lies within the biophysical characteristics of psychosine itself. In other words, since psychosine has a large head group and a single fatty acid chain it would seem that it might preferentially interact with cholesterol (Simons and Ikonen 1997; White et al. 2009). This may result in an increase in the energy requirements necessary for membrane alterations involved in the formation of endocytic vesicles and may thus result in decreases in the levels of this process (Bruno et al. 2007; Nowak and Chou 2008; Roux et al.; Sharma and Grant 1986; Singh et al. 2007). Another explanation may be that psychosine-based raft interactions may interfere with associated signals that are necessary for endocytosis to occur. For example, inhibition of PKC, which has previously been shown to be involved in endocytosis, may result in the decreases demonstrated in this study (Jayanthi et al. 2006; Padovano et al. 2009; Tossidou et al. 2009).
In conclusion, the data presented in this study show that psychosine accumulation in LRs has functional consequences that range from interference with cell signaling to deregulation of raft-mediated endocytosis. However, this study also provides evidence that clearance of psychosine from LRs and the recovery of endocytosis are achievable with GALC replacement. This idea, while still insufficient at the in vivo level, provides a promising line of research for the improvement of current therapies. This work underlines the need for improvement in the efficiency of GALC replacement and the recapitulation of the catabolism of psychosine at the raft level. These findings offer an additional mechanistic explanation for the reduced efficacy of enzyme corrective therapies in KD and, underline the need that future studies attempt to overcome these challenges by either increasing enzyme timely correction and/or by the innovation of pharmacological agents for the correction of raft function.
Supplementary Material
Identification of neural cell type lineages by cell specific markers. Neural stem cells were differentiated for 7–9 days and uptake assays were performed by 30 minute incubations with cholera toxin B-488 (CTB-488) (green) prior to fixation. Immunocytochemistry was carried using cell type specific markers in order to identify specific neural lineages for uptake quantification. A–C. Neurons were identified through the use of the neuronal nuclei marker NeuN (red). D–F. Identification of oligodendrocytes was carried out by labeling differentiated cultures with antibodies against O4 (red). G–I. Glial fibrillary acidic protein (GFAP) (red) was used to identify astrocytes in these cultures. Images shown are representative of those acquired for quantification of CTB-488 in figure 5.
Enzyme replacement therapy results in modest improvements in the twitcher phenotype. Twitcher mice were treated with one of the following conditions: Lentiviral-GALC (+LV), bone marrow transplant (+BMT) or a combination of both (+BMT+LV). A. Lifespan was monitored for each of the above treatment conditions and is shown in days. B. Nerve conduction velocity was measured as a functional indication of nervous system recovery. This was done for each treatment condition and is expressed as meters/second (m/sec). C. Myelin basic protein (MBP) staining was carried out as a measure of myelin recovery in treated animals. Staining is shown in the cerebellum, cortex, corpus callosum and hippocampus for representative samples from post-natal day 90 (P90) wild type, P40 twitcher and P90 twitcher+BMT+LV. Graphical data is represented as the mean +/− SE.
Bone marrow transplant leads to successful engraftment and CNS infiltration of donor cells in the twitcher mouse. A. Analysis of the level of engraftment of donor bone marrow in host mice is measured as chimerism in the blood. Donor cells are identified by their expression of the CD45.1 marker. B. Infiltration of white blood cells into the mutant brain was done by identifying and quantifying CD45.1 (Donor) and CD45.2 (Host) positive cells. Results are presented for wild type, twitcher and twitcher +BMT+LV mice. Data for A and B are presented as the mean +/− SE. C–F. Immunohistochemical staining for CD45.1+ donor cells is shown for twitcher +BMT+LV in the cortex (C), cerebellum (D) and corpus callosum (F) in addition to the cortex of an untreated twitcher (F).
GALC replacement induces minor increases in enzymatic activity in twitcher mice as well as minor decreases in psychosine accumulations. Total brain homogenates were analyzed for GALC activity and psychosine concentration after enzyme replacement therapy. A. Analysis of GALC activity was carried out in twitcher mice treated with lentiviral-GALC (+LV), bone marrow transplant (+BMT) or a combination (+BMT+LV). These data are expressed as the mean +/− SE. N is greater than or equal to three for each treatment group. B. Total levels of psychosine were measured in all treatment groups using LC-MS-MS. Data is expressed as psychosine content per milligram of total protein. Data are represented as the mean +/− SE with an N of at least three.
Acknowledgments
The authors wish to thank the anonymous reviewers for their positive inputs, which contributed to improve our studies. We wish to dedicate this work to baby Grace and Gina and their families. This work was partially funded by grants from NIH (RNS065808A) (ERB), the Morton Cure paralysis foundation (ERB), the Board of Trustees at the University of Illinois (ERB) and a Ruth L. Kirschstein National Research Service Award (NRSA) Fellowship (F31NS065646) (ABW).
References
- Aicardi J. The inherited leukodystrophies: a clinical overview. J Inherit Metab Dis. 1993;16:733–743. doi: 10.1007/BF00711905. [DOI] [PubMed] [Google Scholar]
- Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Bruno MJ, Koeppe RE, 2nd, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci U S A. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetta D, Perani L, Givogri MI, Galbiati F, Amadio S, Del Carro U, Finocchiaro G, Fanzani A, Marchesini S, Naldini L, Roncarolo MG, Bongarzone E. Design and optimization of lentiviral vectors for transfer of GALC expression in Twitcher brain. J Gene Med. 2006;8:962–971. doi: 10.1002/jgm.924. [DOI] [PubMed] [Google Scholar]
- Duchen LW, Eicher EM, Jacobs JM, Scaravilli F, Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980;103:695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Basso V, Cantuti L, Givogri MI, Lopez-Rosas A, Perez N, Vasu C, Cao H, van Breemen R, Mondino A, Bongarzone ER. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J Neurosci. 2007;27:13730–13738. doi: 10.1523/JNEUROSCI.3379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Givogri MI, Cantuti L, Rosas AL, Cao H, van Breemen R, Bongarzone ER. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J Neurosci Res. 2009;87:1748–1759. doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- Giri S, Khan M, Rattan R, Singh I, Singh AK. Krabbe disease: psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. J Lipid Res. 2006;47:1478–1492. doi: 10.1194/jlr.M600084-JLR200. [DOI] [PubMed] [Google Scholar]
- Givogri MI, Bottai D, Zhu HL, Fasano S, Lamorte G, Brambilla R, Vescovi A, Wrabetz L, Bongarzone E. Multipotential neural precursors transplanted into the metachromatic leukodystrophy brain fail to generate oligodendrocytes but contribute to limit brain dysfunction. Dev Neurosci. 2008;30:340–357. doi: 10.1159/000150127. [DOI] [PubMed] [Google Scholar]
- Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006a;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- Givogri MI, Galbiati F, Fasano S, Amadio S, Perani L, Superchi D, Morana P, Del Carro U, Marchesini S, Brambilla R, Wrabetz L, Bongarzone E. Oligodendroglial progenitor cell therapy limits central neurological deficits in mice with metachromatic leukodystrophy. J Neurosci. 2006b;26:3109–3119. doi: 10.1523/JNEUROSCI.4366-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Bell RM. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987;235:670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Haq E, Giri S, Singh I, Singh AK. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J Neurochem. 2003;86:1428–1440. doi: 10.1046/j.1471-4159.2003.01941.x. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge PM, Suzuki K, Poorthuis BJ, Kobayashi T, Wagemaker G, van Bekkum DW. Donor-derived cells in the central nervous system of twitcher mice after bone marrow transplantation. Science. 1988;239:1035–1038. doi: 10.1126/science.3278379. [DOI] [PubMed] [Google Scholar]
- Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J, Wenger DA, Kolodny EH, Vanier MT, Loes DJ, Dusenbery K, Lockman LA. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med. 1998;338:1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- Lim ZY, Ho AY, Abrahams S, Fensom A, Aldouri M, Pagliuca A, Shaw C, Mufti GJ. Sustained neurological improvement following reduced-intensity conditioning allogeneic haematopoietic stem cell transplantation for late-onset Krabbe disease. Bone Marrow Transplant. 2008;41:831–832. doi: 10.1038/sj.bmt.1705984. [DOI] [PubMed] [Google Scholar]
- Nowak SA, Chou T. Membrane lipid segregation in endocytosis. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;78:021908. doi: 10.1103/PhysRevE.78.021908. [DOI] [PubMed] [Google Scholar]
- Padovano V, Massari S, Mazzucchelli S, Pietrini G. PKC induces internalization and retention of the EAAC1 glutamate transporter in recycling endosomes of MDCK cells. Am J Physiol Cell Physiol. 2009;297:C835–844. doi: 10.1152/ajpcell.00212.2009. [DOI] [PubMed] [Google Scholar]
- Pearse BM. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982;79:451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi MA, Fugaro J, Amini S, Luzi P, de Gala G, Victoria T, Dubell C, Shahinfar M, Wenger DA. Retroviral vector-mediated transfer of the galactocerebrosidase (GALC) cDNA leads to overexpression and transfer of GALC activity to neighboring cells. Biochem Mol Med. 1996;58:142–150. doi: 10.1006/bmme.1996.0042. [DOI] [PubMed] [Google Scholar]
- Roux A, Koster G, Lenz M, Sorre B, Manneville JB, Nassoy P, Bassereau P. Membrane curvature controls dynamin polymerization. Proc Natl Acad Sci U S A. 2010;107:4141–4146. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Inui K, Tatsumi N, Fukushima H, Nishigaki T, Taniike M, Nishimoto J, Tsukamoto H, Yanagihara I, Ozono K, Okada S. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe’s disease. J Neurochem. 1996;66:1118–1124. doi: 10.1046/j.1471-4159.1996.66031118.x. [DOI] [PubMed] [Google Scholar]
- Scaravilli F, Suzuki K. Enzyme replacement in grafted nerve of twitcher mouse. Nature. 1983;305:713–715. doi: 10.1038/305713a0. [DOI] [PubMed] [Google Scholar]
- Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004a;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004b;279:48055–48062. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- Sharma RJ, Grant DA. A differential effect between the acute and chronic administration of ethanol on the endocytotic rate constant, ke, for the internalisation of asialoglycoproteins by hepatocytes. Biochim Biophys Acta. 1986;862:199–204. doi: 10.1016/0005-2736(86)90483-9. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Singh RD, Holicky EL, Cheng ZJ, Kim SY, Wheatley CL, Marks DL, Bittman R, Pagano RE. Inhibition of caveolar uptake, SV40 infection, and beta1-integrin signaling by a nonnatural glycosphingolipid stereoisomer. J Cell Biol. 2007;176:895–901. doi: 10.1083/jcb.200609149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. Globoid cell leukodystrophy (Krabbe’s disease): update. J Child Neurol. 2003;18:595–603. doi: 10.1177/08830738030180090201. [DOI] [PubMed] [Google Scholar]
- Tossidou I, Starker G, Kruger J, Meier M, Leitges M, Haller H, Schiffer M. PKC-alpha modulates TGF-beta signaling and impairs podocyte survival. Cell Physiol Biochem. 2009;24:627–634. doi: 10.1159/000257518. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger DA, Suzuki K, Suzuki Y, Suzuki K. In: The metabolic and molecular bases of inherited disease. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. New York: McGraw-Hill; 2001. p. 3669.p. 3670.p. 3687. [Google Scholar]
- White AB, Givogri MI, Lopez-Rosas A, Cao H, van Breemen R, Thinakaran G, Bongarzone ER. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield PD, Sharp PC, Taylor R, Meikle P. Quantification of galactosylsphingosine in the twitcher mouse using electrospray ionization-tandem mass spectrometry. J Lipid Res. 2001;42:2092–2095. [PubMed] [Google Scholar]
- Wiederschain G, Raghavan S, Kolodny E. Characterization of 6-hexadecanoylamino-4-methylumbelliferyl-beta-D- galactopyranoside as fluorogenic substrate of galactocerebrosidase for the diagnosis of Krabbe disease. Clin Chim Acta. 1992;205:87–96. doi: 10.1016/s0009-8981(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Yeager AM, Brennan S, Tiffany C, Moser HW, Santos GW. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science. 1984;225:1052–1054. doi: 10.1126/science.6382609. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Dinh A, Cronin J, Li SC, Reiser J. Cellular uptake and lysosomal delivery of galactocerebrosidase tagged with the HIV Tat protein transduction domain. J Neurochem. 2008;104:1055–1064. doi: 10.1111/j.1471-4159.2007.05030.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of neural cell type lineages by cell specific markers. Neural stem cells were differentiated for 7–9 days and uptake assays were performed by 30 minute incubations with cholera toxin B-488 (CTB-488) (green) prior to fixation. Immunocytochemistry was carried using cell type specific markers in order to identify specific neural lineages for uptake quantification. A–C. Neurons were identified through the use of the neuronal nuclei marker NeuN (red). D–F. Identification of oligodendrocytes was carried out by labeling differentiated cultures with antibodies against O4 (red). G–I. Glial fibrillary acidic protein (GFAP) (red) was used to identify astrocytes in these cultures. Images shown are representative of those acquired for quantification of CTB-488 in figure 5.
Enzyme replacement therapy results in modest improvements in the twitcher phenotype. Twitcher mice were treated with one of the following conditions: Lentiviral-GALC (+LV), bone marrow transplant (+BMT) or a combination of both (+BMT+LV). A. Lifespan was monitored for each of the above treatment conditions and is shown in days. B. Nerve conduction velocity was measured as a functional indication of nervous system recovery. This was done for each treatment condition and is expressed as meters/second (m/sec). C. Myelin basic protein (MBP) staining was carried out as a measure of myelin recovery in treated animals. Staining is shown in the cerebellum, cortex, corpus callosum and hippocampus for representative samples from post-natal day 90 (P90) wild type, P40 twitcher and P90 twitcher+BMT+LV. Graphical data is represented as the mean +/− SE.
Bone marrow transplant leads to successful engraftment and CNS infiltration of donor cells in the twitcher mouse. A. Analysis of the level of engraftment of donor bone marrow in host mice is measured as chimerism in the blood. Donor cells are identified by their expression of the CD45.1 marker. B. Infiltration of white blood cells into the mutant brain was done by identifying and quantifying CD45.1 (Donor) and CD45.2 (Host) positive cells. Results are presented for wild type, twitcher and twitcher +BMT+LV mice. Data for A and B are presented as the mean +/− SE. C–F. Immunohistochemical staining for CD45.1+ donor cells is shown for twitcher +BMT+LV in the cortex (C), cerebellum (D) and corpus callosum (F) in addition to the cortex of an untreated twitcher (F).
GALC replacement induces minor increases in enzymatic activity in twitcher mice as well as minor decreases in psychosine accumulations. Total brain homogenates were analyzed for GALC activity and psychosine concentration after enzyme replacement therapy. A. Analysis of GALC activity was carried out in twitcher mice treated with lentiviral-GALC (+LV), bone marrow transplant (+BMT) or a combination (+BMT+LV). These data are expressed as the mean +/− SE. N is greater than or equal to three for each treatment group. B. Total levels of psychosine were measured in all treatment groups using LC-MS-MS. Data is expressed as psychosine content per milligram of total protein. Data are represented as the mean +/− SE with an N of at least three.