Abstract
Elite controllers or suppressors (ES) are HIV-1-infected patients who maintain viral loads of <50 copies/ml without antiretroviral therapy. While HLA-B*57 and B*5801 alleles are overrepresented in ES, many HLA-B*57/B*5801 patients become chronic progressors (CP). We show here that HIV-1 infection results in similar levels of downregulation of HLA-B*57 and HLA-B*5801 molecules on primary CD4+ T cells from ES and CP. Thus, differences in HIV-1-mediated downregulation of HLA-B*57/B*5801 molecules do not distinguish ES from CP.
Asmall subset of HIV-1 infected patients is able to control viral replication without antiretroviral therapy. These patients are known as elite controllers, HIV controllers, or elite suppressors (ES).1 Replication-competent virus has been isolated from some ES suggesting that host factors rather than defective virus are responsible for the elite suppression of HIV-1 replication.2,3 Thus, ES may provide insight into the mechanisms involved in the immune control of viral replication. The HLA-B*57 and the closely related HLA-B*58-01 alleles are overrepresented in ES.1,4 These class I molecules are thought to be involved in the presentation of immunodominant epitopes to HIV-1-specific CD8+ T cells. Many studies have shown that HIV-specific CD8+ T cell responses from ES are functionally superior to those from CP.5–8 Studies that have specifically compared HLA-B*57 ES to HLA-B*57 CP have shown that CD8+ T cells from ES are more likely to focus on HLA-B*57-restricted Gag epitopes4 and are more likely to proliferate in response to HIV-1-infected autologous CD4+ T cells.8 The mechanisms responsible for these differences in CD8+ T cell function between HLA-B*57 ES and HLA-B*57 CP remain unknown.
Downregulation of surface HLA A and B molecules is a strategy used by HIV-1 to evade the CD8+ T cell response.9,10 We previously have shown that replication-competent viruses isolated from ES and CP were equally efficient at downregulating surface HLA-A*2 and HLA-B*57 proteins on primary CD4+ T cells.11 In contrast, it has recently been shown that CD4+ T cells from macaques with rapidly progressive disease are more susceptible to SIV-mediated HLA class I downregulation than CD4+ T cells from macaques with normal disease progression.12 We thus tested the hypothesis that CD4+ T cells from HLA-B*57+ ES would be significantly less susceptible to HIV-1-mediated downregulation of surface HLA-B*57 protein than CD4+ T cells from HLA-B*57+ CP. This would lead to more effective killing of HIV-1-infected cells and could potentially explain the superior HIV-specific responses seen in ES.
Peripheral blood mononuclear cells (PBMCs) were obtained from eight HLA-B*57/5801 ES and from eight HLA-B*57/5801 CP. The CP had a median CD4 nadir of 155 cells/μl (range: 18–503 cells/μl) and a median peak viral load of 130,325 copies/ml (range: 13,308 to >750,000 copies/ml). Six of the eight CP were currently on HAART regimens. The ES all maintained viral loads of <50 copies/ml and had a median CD4 count of 1150 cells/μl (range 636–2033 cells/μl). The patients' cells were activated with PHA and CD4+ T cells were purified by negative selection with magnetic beads (Miltenyi) on day 3. Pseudotyped virus expressing GFP and capable of single-round infection was generated as described previously.13 The isolated CD4+ T cells were then infected with these pseudotyped viruses by spinoculation. On day 3 of infection, cells were collected and stained with Tricolor conjugated anti-CD4 (Caltag Laboratories) and biotinylated anti-HLA-B*57 (One lambda) monoclonal antibodies. PE-conjugated Streptavidin (Ebioscience) was then added, and the cells were then fixed with 2% formaldehyde. FACS analysis was performed and 50,000–100,000 cells in the lymphocyte gate were analyzed for each. The median infection rate of CD4+ T cells was lower in CP (9%) than in ES (23%) due to the residual effect of antiretrovirals in CP CD4+ T cells. The HLA-B*57 monoclonal antibody has been previously described in a Caucasian population14 and it shows perfect concordance with molecular HLA typing performed on a previously described cohort of African-American ES.15
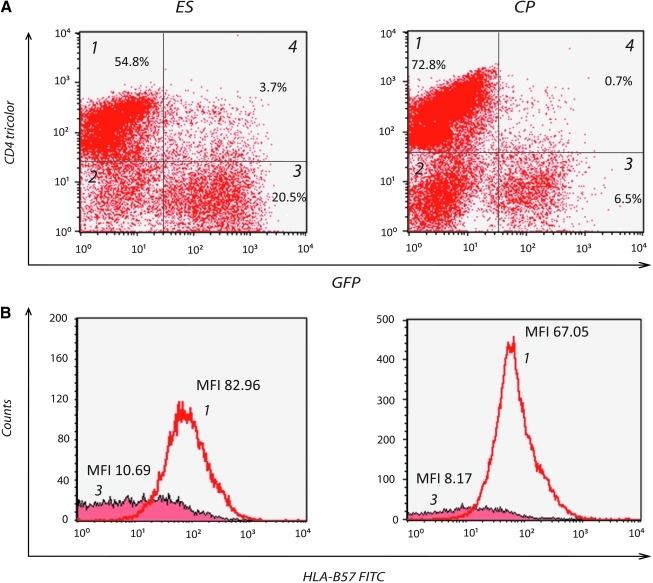
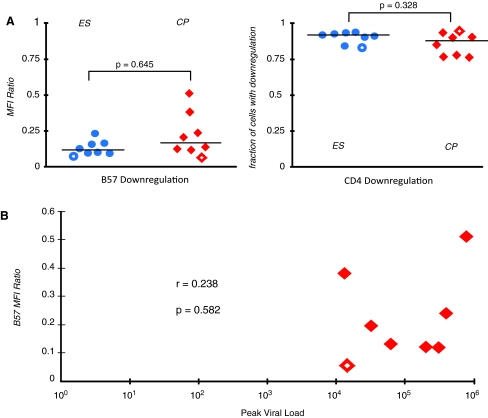
As previously reported, HIV infection resulted in significant downregulation of surface CD4 molecules16 (Fig. 1A, quadrant 3). The infected cells that had downregulated CD4 also had markedly less surface HLA-B*57 molecules than uninfected CD4+ T cells (Fig. 1B). There was no significant difference in the percent of infected cells that had downregulated CD4 in ES and CP as determined by the Mann–Whitney test (p = 0.328, Fig. 2A). There was also no significant difference in HLA-B*57 expression on stimulated uninfected CD4+ T cells in CP and ES (data not shown). The mean fluorescence intensity (MFI) of HLA-B*57/5801 expression on CD4 low, GFP-positive infected cells (quadrant 3 in Fig. 1A) was divided by that of the MFI on CD4-positive GFP-negative uninfected cells in each sample (quadrant 1 in Fig. 1A). The resulting MFI ratios are compared in Fig. 2A. There was no significant difference in the ratio of HLA-B*57 expression on infected versus uninfected CD4+ T cells in CP and ES (p = 0.645). We next determined whether the peak viral load in HLA-B*57 CP was related to the degree of HIV-1-mediated HLA-B*57 downregulation on CD4 T cells. As shown in Fig. 2B, Spearman's rank correlation analysis showed no significant correlation between the two parameters (r = 0.238, p = 0.582).
FIG. 1.
Representative dot plots (A) and histograms (B) showing downregulation of CD4 and HLA-B*57 proteins, respectively, on HIV-1-infected cells (GFP positive) for an elite suppressor (ES) and a chronic progressor (CP). The expressions of HLA-B*57 on uninfected CD4+ T cells (quadrant 1) and infected CD4low cells (quadrant 3) are shown in the histogram. Color images available online at www.liebertonline.com/aid.
FIG. 2.
MFI ratio of HLA-B*57/5801 expression and fraction of infected cells that had downregulated CD4 are shown for eight ES and eight CP (A). The open symbols represent HLA-B*5801-positive patients and the closed symbols represent HLA-B*57-positive patients. The horizontal bars represent the median value for each group. The relationship between peak viral load and the MFI ratio of HLA-B*57/5801 expression is shown (B). Color images available online at www.liebertonline.com/aid
We have previously reported an HLA-B*5703 transmission pair with a transmitter who developed full-blown AIDS and a recipient who became an ES.3 Functional studies showed that primary CD8+ T cells from the ES were much more effective at controlling replication of the transmitted virus in autologous CD4+ T cells than CD8+ T cells from the transmitter.3 To determine whether differences in HLA-B*57 downregulation induced by the transmitted virus were responsible for the difference in CTL responses, CD4+ T cells from both patients were infected with the transmitted replication-competent virus and after staining surface CD4 and HLA-B*57 molecules, the cells were then fixed and permeabilized and stained with the KC57 gag-specific monoclonal antibody (Beckman Coulter) as previously described.11 The MFI ratio of B57 expression on infected versus uninfected CD4+ T cells was comparable in the two patients (0.41 for ES, 0.25 for CP) and was similar to the range we have seen in cells from two other ES.11
Although the relatively small size of our study limits our ability to detect subtle differences between ES and CP, the data suggest that marked differences in HIV-1-mediated downregulation of HLA-B*57 proteins do not explain the superior CD8+ T cell responses seen in ES and do not explain elite suppression of HIV-1 replication. This supports a prior study that showed there was no difference in the sequence of the cytoplasmic tail of HLA-B*5701 in five ES and five CP.4 Furthermore, our data demonstrate there is no direct correlation between the extent of HLA-B*57 downregulation and viral load in CP, although we cannot rule out the possibility that treatment with HAART has affected the modulation of this HLA molecule in some patients. Although it is possible that different human HLA A and B proteins differ in their susceptibility to HIV-mediated downregulation, our data show that HLA-B*57 proteins are downregulated to a similar degree in HLA-B*57 ES and HLA-B*57 CP. Further study will be needed to determine why some HLA-B*57 individuals become ES whereas others develop progressive disease. The results will have major implications for the design of HIV-1 vaccines.
Acknowledgments
This study was supported by NIH grant R01 AI080328 (J.N.B.). M.S. Sampah and C.M. Ceccato contributed equally to this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Blankson JN. Effector mechanisms in HIV-1 infected elite controllers: Highly active immune responses? Antiviral Res. 2010;85(1):295–302. doi: 10.1016/j.antiviral.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blankson JN. Bailey JR. Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81(5):2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey JR. O'Connell K. Yang HC, et al. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J Virol. 2008;82(15):7395–7410. doi: 10.1128/JVI.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migueles SA. Sabbaghian MS. Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migueles SA. Osborne CM. Royce C, et al. Lytic granule loading of CD8 + T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts MR. Nason MC. West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8 + T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez-Cirion A. Lacabaratz C. Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migueles SA. Laborico AC. Shupert WL, et al. HIV-specific CD8 + T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunol. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz O. Marechal V. Le Gall S. Lemonnier F. Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 nef protein. Nature Med. 1996;2(3):338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 10.Cohen GB. Gandhi RT. Davis DM, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10(6):661–71. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 11.Nou E. Zhou Y. Nou DD. Blankson JN. Effective downregulation of HLA-A*2 and HLA-B*57 by primary human immunodeficiency virus type 1 isolates cultured from elite suppressors. J Virol. 2009;83(13):6941–6946. doi: 10.1128/JVI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich TC. Piaskowski SM. Leon EJ, et al. High viremia is associated with high levels of in vivo major histocompatibility complex class I downregulation in rhesus macaques infected with simian immunodeficiency virus SIVmac239. J Virol. 2010;84(10):5443–5447. doi: 10.1128/JVI.02452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H. Zhou Y. Alcock C, et al. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78(4):1718–1729. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin AM. Krueger R. Almeida CA. Nolan D. Phillips E. Mallal S. A sensitive and rapid alternative to HLA typing as a genetic screening test for abacavir hypersensitivity syndrome. Pharmacogenet Genomics. 2006;16(5):353–357. doi: 10.1097/01.fpc.0000197468.16126.cd. [DOI] [PubMed] [Google Scholar]
- 15.Han Y. Lai J. Barditch-Crovo P, et al. The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS (London, England) 2008;22(4):541–544. doi: 10.1097/QAD.0b013e3282f470e4. [DOI] [PubMed] [Google Scholar]
- 16.Garcia JV. Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350(6318):508–5011. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]