Abstract
HIV-1 Nef has been demonstrated to be integral for viral persistence, infectivity, and the acceleration of disease pathogenesis (AIDS) in humans. Nef has also been detected in the plasma of HIV-infected individuals and is released from infected cells. The form in which Nef is released from infected cells is unknown. However, Nef is a myristoylated protein and has been shown to interact with the intracellular vesicular trafficking network. Here we show that Nef is released in CD45-containing microvesicles. This microvesicular Nef (mvNef) is detected in the plasma of HIV-infected individuals at relatively high concentrations (10 ng/ml). It is also present in tissue culture supernatants of Jurkat cells infected with HIVMN. Interestingly, plasma mvNef levels in HIV+ patients did not significantly correlate with viral load or CD4 count. Microvesicular Nef levels persisted in the plasma of HIV-infected individuals despite the use of antiretroviral therapy, even in individuals with undetectable viral loads. Using cell lines, we found Nef microvesicles induce apoptosis in Jurkat T-lymphocytes but had no observed effect on the U937 monocytic cell line. Given the large amount of mvNef present in the plasma of HIV-infected individuals, the apoptotic effect of mvNef on T cells, and the observed functions of extracellular soluble Nef in vitro, it seems likely that in vivo mvNef may play a significant role in the pathogenesis of AIDS.
Introduction
The negative factor (Nef) of human immunodeficiency virus type 1 (HIV-1) is an accessory protein of approximately 27–34 kDa that is unique to primate lentiviruses. Nef is associated with the T cell depletion characteristic of HIV-induced acquired immunodeficiency syndrome (AIDS). Many studies have demonstrated the importance of Nef in the pathogenesis of AIDS. In humans, studies of individuals infected with human immunodeficiency virus type 1 (HIV-1) containing defective nef alleles demonstrate the role of Nef in the progression of disease. Such individuals are more likely to become long-term nonprogressors (LTNPs) and have delayed development of the symptoms of AIDS.1–5 LTNPs exhibit reduced viral loads and stable CD4 counts for over 10 years postinfection.1,3 This suggests that the absence of a functional Nef attenuates viral pathogenesis. This cohort was shown to actually consist of two distinct groups, LTNPs and long-term survivors (LTSs).6 Eventually, LTSs exhibit declining CD4 T cell counts with low viral loads and develop immunodeficiency after long asymptomatic periods suggesting that although Nef plays a key role in viral pathogenesis other factors play a role in progression to AIDS.6–8 Rhesus macaques infected with nef-defective molecular clones of simian immunodeficiency virus (SIV) also exhibit significant reductions in pathogenicity.9 These animals exhibit extended periods of asymptomatic disease, but eventually progress to simian AIDS much like their human counterparts.9 Interestingly, nef-transgenic mice suffer organ system dysfunction and severe CD4+ T cell depletion similar to AIDS patients.10–13 Taken together these studies provide evidence that HIV-1 Nef plays a central role in the pathogenesis leading to AIDS.
A hallmark of AIDS progression is the progressive loss of CD4+ T cells. The simplest model accounting for this depletion is that HIV infection directly leads to cell death. However, mathematical models of HIV infection suggest that many more T cells die than are actually infected.14,15 This implies that there are other “indirect” methods leading to T cell depletion and that have led to the “bystander” hypothesis of depletion. Soluble molecules released from infected cells are prime candidates for this “bystander” effect. In chronic infections, the HIV proteins Nef, Tat, gp120, and Vpr have all been implicated in the induction of apoptosis via the bystander effect.16–18 More specifically, early reports demonstrate that soluble extracellular Nef induces apoptosis in T cells via a CXCR4-dependent interaction.19,20 Given this result, we have considered whether soluble Nef is released from infected cells and could play a significant role in mediating bystander apoptosis in CD4+ T cells.
Nef has no known intrinsic enzymatic activities and yet it promotes pleiotropic effects such as enhancement of infectivity, immune evasion, and cellular activation. It promotes these functions by acting as a molecular adaptor linking various proteins to components within the intracellular trafficking machinery. Nuclear magnetic resonance (NMR) studies reveal that Nef consists of four regions: (1) an N-terminal region containing a flexible myristoylated anchor domain, (2) a proline-rich loop, (3) a globular core, and (4) a flexible C-terminal loop.21 Cellular localization of Nef is dependent on its conformation. Open conformation of Nef results in membrane association whereas closed confirmation yields a cytosolic localization.22,23 Differential localization coupled with “flexible” regions allows Nef to interact with many different cellular proteins that ultimately contribute to Nef's pleiotropic effects. Some of these effects are well documented, such as reducing the expression of CD4, MHC class I and II, and chemokine receptors (CCR5 and CXCR4) as well as disrupting key signaling pathways within target cells.24–28 To reduce expression of these proteins Nef binds to multiple proteins involved in intracellular trafficking: adapter protein complexes (AP1–AP4), subunit H of the vacuolar membrane ATPase (V1H), PACS-1a, and COP-I coatomers.21,29–31 As a result of these interactions, Nef has the capacity to exert multiple effects on intracellular trafficking. For example, interactions between Nef and β-Cop target CD4 to late acidic endosomes whereas interactions with AP-2 target CD4 to clathrin-coated pits (early endosomes).32–34
Studies have shown that Nef expression leads to changes within the endosomal compartment and increases endosome, lysosome, and late endosome/multivesicular body (MVB) production in hematopoietic cells.35–37 MVBs contain smaller membrane-bound vesicles that fuse with plasma membranes and are released via exocytosis into the extracellular space as exosomes ranging in size from 30 to 200 nm in diameter.38–41 Exosomes mediate several biological functions such as T cell stimulation, antigen presentation, immunological tolerance, and apoptosis.42–45 Microvesicles containing ecto-enzymes or other proteins are of similar size to exosomes and bud from the plasma membrane.46–48 Vesicles produced by hematopoietic cells will typically consist of both exosomes and microvesicles. Cells infected with HIV commonly release both virions and microvesicles that copurify with the virions due to similarity in size and density.49,50 Some studies have separated microvesicles from HIV-1 virions using (Optiprep) iodixonal gradients clearly showing the capacity to discriminate between microvesicles, virions, and cell debris.51,52 CD45 (human leukocyte antigen) is highly expressed on the surface of vesicles derived from hematopoietic cells but is not incorporated into HIV-1 virions.53,54 This differential incorporation of CD45 has been used to remove contaminating vesicles from virion preparations and we have used this method conversely to capture microvesicles.55
Nef, Tat, and gp120 have been shown to be released by HIV-infected cells and can be found in the extracellular compartment.56–58 The nature of released Nef and the pathways mediating this release are largely unknown. Given the association of intracellular Nef with vesicular compartments, it would not be farfetched to consider that Nef may be released in association with vesicles. Recent reports demonstrate that Nef interacts with Rab11, a GTPase protein integral to MVB formation.59 This interaction suggests a potential mechanism/pathway in which extracellular Nef can be released from infected cells. Indeed, we have previously shown that Nef-transfected cells release vesicles containing Nef that can be taken up by other cells.35 A recent report actually describes the secretion of Nef into exosomes and the subsequent induction of apoptosis in bystander CD4+ T cells by vesicular Nef.60
Here, we show that Nef is released in microvesicular form from both nef-transfected and HIV-1-infected cells. Nef microvesicles contain CD45 (leukocyte common antigen), an integral membrane protein that is excluded from virions. Using paramagnetic separation, we were able to separate Nef microvesicles from virions. Using this technique we show that Nef microvesicles are secreted from infected cells and are present in the plasma53,55,61 of HIV-1-infected individuals independent of viral load or treatment status. This suggests that Nef may be released from infected cells throughout the course of HIV infection, facilitating immune cell activation, viral spread, apoptosis, and/or other aspects of HIV-1 pathogenesis. Nef microvesicles, produced and released in high quantity, may be a significant factor driving T cell depletion in chronic HIV infection. In fact, a recent study indicates that HIV-1-infected T cells undergo a Nef-dependent massive increase in exocytosis of microvesicles containing Nef and/or FasL.62 This report supports our study and suggests that Nef microvesicles may play a factor in HIV-induced T cell apoptosis. Ultimately, Nef microvesicles make an attractive therapeutic target that could inhibit the loss of CD4+ T-lymphocytes, thereby extending the asymptomatic period of HIV-1 infection and attenuating HIV-1 disease.
Materials and Methods
Plasmid constructs and transfections
A polymerase chain reaction (PCR)-generated fragment spanning the coding region of nef was amplified from the viral clone pNL4-3 and inserted into the pCDNA-3 expression vector by topo cloning (Invitrogen, Carlsbad, CA) as previously described.63,64 HEK293 or 293FT cells were transfected with Nef or a Nef-GFP expression plasmid using the Effectene transfection kit (Qiagen) according to the manufacturer's instructions. Briefly, cells were plated in 100-mm dishes and transfected at 90% confluency. DNA (∼ 5 μg) was diluted in DNA condensation buffer (EC) and 40 μl of Enhancer was added (per 1 μg of DNA). After a 2-min incubation at room temperature, 125 μl of Effectene (liposomal agent) was added to the DNA mixture. DNA–liposome complexes were subsequently formed during incubation (10 min) at room temperature and then added dropwise to the 100-mm dishes. Culture supernatants and cells were collected 72 h posttransfection and analyzed for Nef protein expression.
Cell culture
The T-lymphocytic cell line Jurkat (clone E6) and U937 promonocytic cell line were acquired from ATCC and maintained at 37°C in RPMI-1640 media supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
Human plasma samples
Plasma from HIV-1+/HCV−/HBV− individuals (n = 10) and HIV−/ HCV−/HBV− controls (n = 10) was obtained from Bioreclamation (Long Island, NY). Samples were separated into aliquots and stored at −80°C until immediately prior to use. Studies performed were approved by the Morehouse School of Medicine Institutional Review Board.
Virus and microvesicle preparation
Microvesicles were collected from both nef-transfected and HIV-1-infected cells. Microvesicles from transfected cells were prepared using a protocol adapted from Trubey et al.55 Culture supernatants from nef-transfected HEK293 cells were collected 3 days posttransfection and subjected to sequential differential centrifugation. Supernatants were initially centrifuged for 10 min at 300 × g, followed by 10,000 × g for 30 min and finally 400,000 × g for 1 h. Microvesicles were assumed to be the pellet of the 400,000 × g ultracentrifugation spin. Jurkat cells were infected in vitro with HIVMN (ABI) and supernatant was collected 14 days postinfection. Virus and microvesicles were purified from HIV-infected cell culture supernatants by sucrose density ultracentrifugation as previously described.55 Briefly, supernatants were layered over 20% sucrose, ultracentrifuged at 100,000 × g for 1.5 h, and the resultant pellet containing virions and microvesicles was resuspended in 200 μl of cold 1 × TNE buffer (10 mM Tris–HCl, 100 mM NaCl, and 1 mM EDTA). Microvesicles from culture supernatants of uninfected Jurkat cells were similarly prepared.
Isolation of CD45+ microvesicles
Microvesicles were separated from virions using a protocol adapted for CD45 affinity depletion of virion preparations.55 Briefly, tissue culture supernatants from nef-transfected HEK293 cells or HIV-1-infected Jurkat or macrophage cell lines were incubated with anti-CD45 magnetic microbeads for 1 h at 4°C. The mixture was then applied to a magnetized 10 M column (Miltenyi Biotec). Fluid not retained on the column was collected and termed the flow-through fraction. The column was washed three times with cold 1 × TNE buffer. Each wash was collected and pooled to produce the wash fraction. Protein and microvesicles retained on the column were recovered by removing the column from the magnet and forcing 250 μl of 1 × TNE buffer through the column using a 5-ml syringe plunge (Becton-Dickson) to generate the eluate/CD45-captured fraction. Each fraction: Flow-through (FT), wash (W), eluate (E), and starting material (SM)—was centrifuged at 400,000 × g at 4°C for 1 h in a TLA 120.2 rotor using a TLA ultracentrifuge (Beckman Instruments).
Subtilisin digest
Microvesicles isolated from tissue culture supernatant of HIV-infected Jurkat cells were treated with subtilisin (1 mg/ml) (Sigma-Aldrich, St. Louis, MO) at 37°C. After a 4-h incubation, PMSF (1 μg/ml) was added to inhibit digestion. Subtilisin-treated microvesicles were pelleted by ultracentrifugation at 100,000 × g for 1 h. Pellets were resuspended in 1 × PBS and NEF concentration were determined by ELISA.
Infectivity assay
A single-round infectivity assay developed as previously described was used to determine the relative infectivity of each fraction produced during CD45 affinity separation of microvesicles.65 Briefly, the multinuclear activation of a galactosidase indicator assay (MAGI) was performed utilizing 3596 MAGI/HeLa-CD4+-LTR β-gal cell (NIH AIDS Reagent program, catalogue #1470) cultures maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 0.1 mg/1 ml G418, 0.05 mg/1 ml of Hygromyocin B per 1 ml, glutamine, and 1% penicillin and streptomycin. Cells were seeded in six-well plates at a density of 2 × 105 cells per well 24 h prior to infection with HIV-1 virions (1 ng of p24) or fractions—SM (∼1 ng of p24), FT (∼1 ng of p24), W (100 μl), or E (100 μl)—produced during CD45 affinity separation of microvesicles. Following infection cells were incubated at 37°C for 48 h and then fixed for 5 min at room temperature with 0.2% glutaraldehyde and 1% formaldehyde. After fixation cells were washed with phosphate-buffered saline (PBS), stained with X-gal solution (5-bromo-4-chloro-3-indolyl-β-d-glactosidase dissolved in DMSO–4 mM potassium ferrocyanide, 4 mM ferricyanide, and 2 mM MgCl2 in PBS) for 50 min at 37°C, washed twice with PBS, and then scored. Results were reported as total number of blue cells per well.
Electron microscopy
High-speed pellets from tissue culture supernatants of nef-transfected HEK293 cells were embedded in 1% agar. Agar was removed from tubes, cut into blocks that were immediately fixed in 2.5% glutaraldehyde, and embedded in Epon plastic. Ultrathin sections were stained with 2% uranyl acetate and placed on grids. Analysis of grids was performed using a Hitachi 700 transmission electron microscope.
Nef ELISA
The concentration of HIV-1 Nef in 1 ml of plasma pelleted at 400,000 × g or culture supernatants of HIV-1-infected cells was determined using a commercially available anti-Nef enzyme-linked immunosorbent assay (ELISA) (Immunodiagnostics, Bedford, MA) according to the manufacturer's instructions. Prior to ELISA measurement, immune complexes in human plasma were disrupted using acid dissociation (ICD). For ICD 100 μl of plasma was added to 100 μl of 0.33 N HCl and incubated at 37°C for 1 h. Following incubation acid-treated plasma was neutralized by adding 100 μl of 0.33 N NaOH. Microvesicles and virions from the neutralized plasma were lysed by the addition of 1% Triton and Nef ELISA was performed according to protocol. Briefly, 100 μl of acid-treated plasma or culture supernatants of HIV-infected cells was added to ELISA plates coated with anti-Nef (Component A) and incubated for 1 h at room temperature. Plates were washed three times with wash buffer (Component B); 100 μl of anti-Nef-HRP-labeled antibody solution (component E) was then added to each well and incubated for 1 h at room temperature. Plates were washed three times with component B; 100 μl of alkaline phosphatase substrate (component F) was added to each well and then incubated for 10 min at room temperature. After approximately 10 min, 100 μl of stop solution (Component G) was added to each well and the absorbance at 450 nm was determined using a Spectramax spectrophotometer.
SDS–PAGE and immunoblot
Proteins were separated by SDS–PAGE using 8–16% Tris–HCl gradient gels (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Proteins were transferred to 0.2 μM PVDF membranes (Bio-Rad), blocked with blotto (5% milk in TBS) for 60 min, and probed with one of the following primary antibodies: anti-Nef (Immuno Diagnostics Inc., Woburn, MA), anti-p24 (Immunodiagnostic), anti-Alix1 (Santa Cruz), anti-CD63 (AbCam), or anti-CD45 (AbCam) antibodies. Following incubation with primary antibody membranes undergo three 10-min washes in T-TBS [20 mM Tris-HCl (pH 6.7), 137 mM NaCl with 1% Tween-20 (Sigma-Aldrich)] before being probed with horseradish peroxidase-conjugated antimouse (Jackson Laboratories) or protein A (Rockland Laboratories) secondary antibodies as previously described.35 Immune complexes were detected using advanced chemiluminescence (Amersham Biosciences). Immunoblots were reprobed by initially incubating the membrane in stripping buffer [100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl (pH 6.7)] at 60°C with agitation for 15 min followed by three 10-min washes in T-TBS. Blots were incubated with blocking for 30 min and immunoblotting was performed as described above.
Apoptosis assay
Caspase-3 activation along with poly(ADP-ribose) polymerase (PARP) substrate were measured in cell lysates using a human capase-3 kit (Millipore, catalog #48-670) according to the manufacturer's instructions. Briefly, 1 × bead solution (25 μl/well) was added to a 96-well filter plate that was prewet with cell signaling assay Buffer #1 (25 μl/well). Cell lysates diluted 1:1 in Beadlyte cell signaling assay Buffer #1 was then added (25 μl/well) to the plate, placed on a shaker (∼500 rpm) at 4°C, and incubated overnight. Following incubation, the lysates were removed via vacuum filtration and the plate washed with cell assay Buffer #1 (100 μl/well). Buffer #1 was removed via vacuum filtration and the wash step was repeated. Biotin reporter-antibody was added to each well (25 μl) and the plate was incubated for 1 h at room temperature. The reporter antibody was removed via vacuum and streptavidin-PE (25 μl) was added to each well. The plate was then incubated for 30 min at room temperature while shaking (∼500 rpm) and protected from light. Streptavidin-PE was removed from the wells via vacuum and the remaining beads were resuspended in Beadlyte Cell Signaling Assay Buffer #1 (100 μl/well). Experimental samples were analyzed using the BioPlex 200 system (Bio-Rad).
Statistical analysis
Statistical analyses were performed using the statistical component of Graphpad Prism. A nonparametric Mann-Whitney test was used to compare Nef plasma levels in uninfected and HIV-infected groups and significance was achieved with a p-value < 0.05.
Results
Nef expression induces cellular release of Nef-containing microvesicles
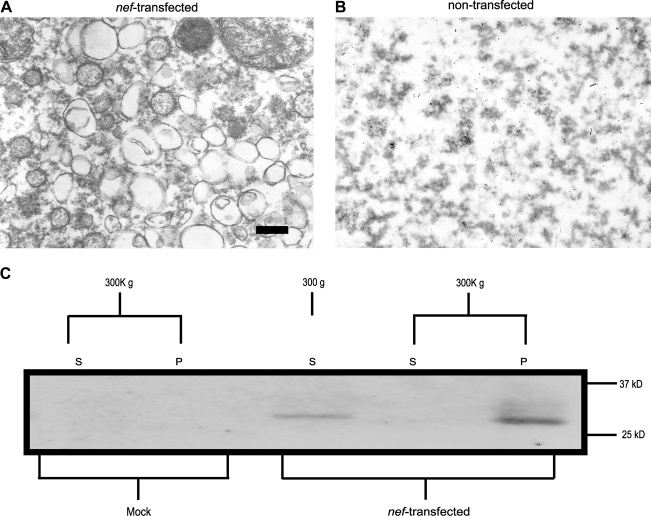
We have previously shown that Nef can be found in the extracellular medium taken from nef-transfected HEK293 cells.35 To determine the nature of Nef expressed into the culture medium, we examined the culture medium by differential centrifugation. Conditioned medium was first centrifuged at 300 × g to remove cells. Our results show that Nef was present in the supernatant of clarified medium (Fig. 1A). When cell-free medium was subjected to further centrifugation at 300,000 × g all of the detectable Nef was present in the pellet and none remained in the supernatant (Fig. 1A). This suggests that the Nef released into medium was present in a higher molecular weight form. To determine the nature of the pelleted material we examined the high-speed pellet by electron microscopy (Fig. 1B). The high-speed pellet contained numerous vesicles that varied in appearance, but typically had diameters of approximately 100 nm. HEK293 cells mock transfected did not contain Nef in the conditioned medium and high-speed pellets contained no visible vesicles (Fig. 1B and C).
FIG. 1.
Nef expression and HIV-1 infection induce vesicle formation. TEM image of conditioned media from HEK293 cells transfected with (A) Nef expression plasmid or (B) mock-transfected. Conditioned media collected 3 days posttransfection and subjected to ultracentrifugation (400,000 × g). Pellets were fixed, embedded, and stained with 2% uranyl acetate. Samples were visualized using a JEOL 1200 EX transmission microscope. (C) Culture supernatant from HEK293 transfected with a Nef expression plasmid was subjected high-speed differential centrifugation. Supernatants were centrifuged for 10 min at 300 × g, followed by 30 min at 10,000 × g, and then centrifuged for 1 h at 300,000 × g. Nef was detected in vesicles pelleted at 300,000 by immunoblot using anti-Nef monoclonal. S, supernatants; P, pellet.
Nef microvesicles released from HIV-infected cells are isolated by anti-CD45 immunoaffinity
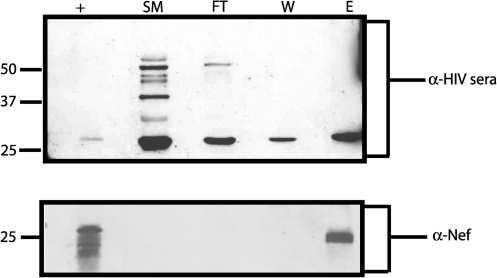
Extracellular vesicles are derived primarily from two sources: (1) budding from the plasma membrane47,48 or (2) exocytosis of late endosomal bodies/multivesicular bodies.38,39 Microvesicles are protein rich and are often found in virion preparations due to their similar size and density. During assembly and budding, HIV-1 selectively incorporates some cellular proteins while excluding others, such as CD45 (human leukocyte antigen).53,66 Microvesicles, on the other hand, contain CD45 and this has been exploited to form the basis of a method to separate vesicles from virion particles using paramagnetic anti-CD45 beads.55 Cell-free medium was collected from Jurkat cells infected with HIV-1NL4-3. The cell-free medium was analyzed by immunoblot using anti-HIV serum and revealed bands consistent in size and amount with virions (Fig. 2). The supernatant was subjected to fractionation using an anti-CD45 magnetic column as described. Fractions produced during separation of microvesicles and virions via CD45 magnetic bead separation were analyzed by Western blot. The flow through fraction contained many of the same viral proteins found in the starting material, including a prominent p24 band. Likewise, washes of the column contained viral proteins (Fig. 2). The eluate consistently contained only one viral protein approximately 27 kDa in size (note the band is slightly higher than the p24 in the other fractions). When the same fractions were reprobed with anti-Nef antibody only the 27-kDa band in the eluate was present. This suggests that the majority of the Nef present in cell-free medium from infected cells is contained within the vesicle fraction and undetectable levels were present in virions.
FIG. 2.
Nef vesicles captured using anti-CD45 magnetic bead separation. Supernatants from HIV-1-infected cells were harvested 15 days postinfection and vesicles/virions were concentrated by centrifugation at 200,000 × g for 1 h on a 20% sucrose cushion. The pellet was resuspended in 1 × TNE buffer and subjected to CD45 magnetic bead separation. Each fraction was collected, ultracentrifuged at 400,000 × g for 1 h, and then separated by SDS-PAGE. Fractions (SM = starting material, FT = flow through, W = wash, and E = eluate) were analyzed by Western blot for HIV proteins using anti-HIV sera (upper panel). The singular band present in the eluate was confirmed to be Nef via an immunoblot using an anti-Nef monoclonal antibody (lower panel). Nef+, recombinant Nef protein.
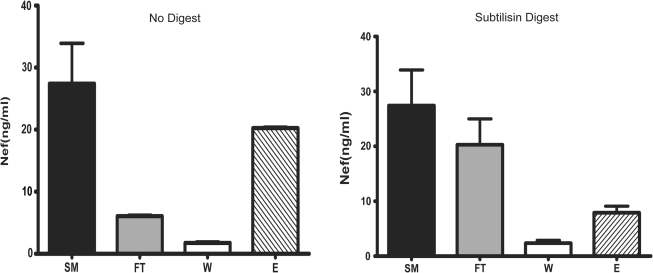
To confirm that CD45 paramagnetic separation isolates microvesicles from virions, each fraction was tested for infectivity using a MAGI cell assay. As expected, infectious virions were found only in the starting virus suspension and flow thorough and the CD45 capture material was noninfectious (data not shown). To confirm that the majority of the Nef present was colocalized with CD45+, we pretreated microvesicles with subtilisin prior to CD45 affinity purification. The subtilisin treatment dramatically reduced the amount of Nef that was captured on the anti-CD45 column (Fig. 3, compare A and B). The small amount remaining bound to the column was likely due to incomplete removal of CD45 from treated vesicles. This experiment confirms that a majority of Nef was being captured in a CD45+-dependent fashion.
FIG. 3.
Subtilisin treatment reduces CD45 capture of Nef microvesicles capture. Nef microvesicles from supernatants of HIV-infected Jurkat cells (15 days postinfection) were isolated as described in Fig. 2, treated for 4 h with subtilisin (2 mg/ml), and then CD45 affinity purified. Nef contents in subsequent SM, FT, W, and E fractions were measured by ELISA. SM = starting material, FT = flow through, W = wash, and E = eluate.
THP-1 monocytic cells produce more Nef microvesicles than Jurkat T cells
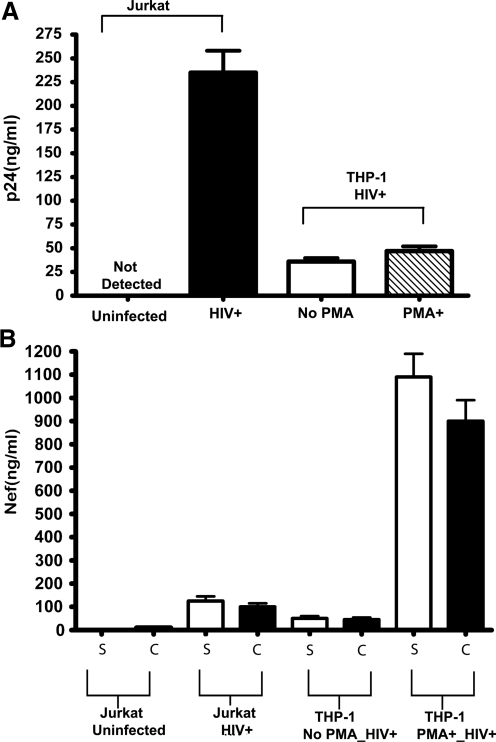
We wished to determine if production of Nef vesicles was influenced by cell type. Culture supernatants from HIV-1-infected Jurkat T cells and THP-1 macrophage cells were harvested; HIV-1 p24 was measured and the amount of Nef was measured in the culture supernatant and CD45 captured material as previously described. As expected, the total amount of p24 isolated from Jurkat cells was about 5-fold higher than either stimulated or nonstimulated PMA cells (see Fig. 4A). Even though the amount of p24 produced by Jurkat cells was much higher than THP-1 cells, the amount of Nef secreted into conditioned medium was about 20-fold less than stimulated THP-1 cells (Fig. 4B). This suggests that even though PMA-treated THP-1 may produce fewer virions than Jurkat T cells these macrophage-like cells produce many more Nef vesicles.
FIG. 4.
Production of CD45+ Nef microvesicles is cell-type dependent. Concentrations of p24 (A) microvesicles and Nef (B) were measured by ELISA in culture supernatants and CD45 captured microvesicles of HIV-1-infected Jurkat T-lymphocytes, THP-1 monocytes, and PMA-treated THP-1 (macrophage-like). T-lymphocytes release the most HIV-1 virus as measured by p24 ELISA whereas macrophage-like THP-1 releases the most Nef microvesicles.
Nef microvesicles are detected in plasma of HIV-infected individuals
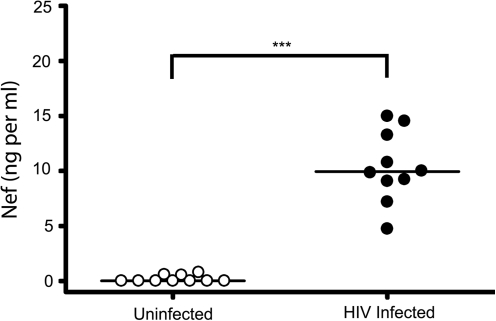
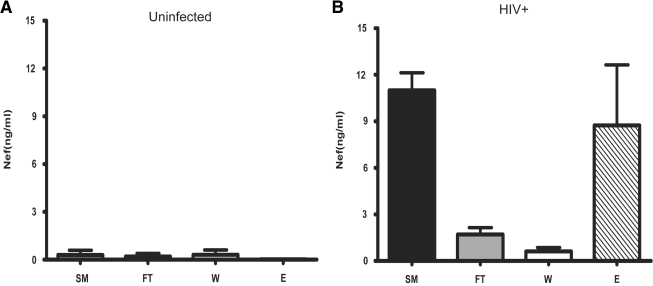
Because Nef microvesicles are released from infected cells we wished to determine whether they might also be present in the plasma of HIV-infected individuals. To determine this, we tested plasma from HIV-infected and uninfected donors. Plasma was subjected to high-speed centrifugations followed by paramagnetic CD45 immunoaffinity capture as previously described. We determined plasma levels of Nef microvesicles in a small cohort of HIV-infected subjects using a Nef ELISA. To ensure that antibodies in the plasma were not masking the detection of Nef, we used acid dissociation techniques similar to those that have been used in measuring p24 protein levels in the plasma. In agreement with earlier reports (Fujii et al.56), the median Nef level in plasma was approximately 10.4 ng/ml (Fig. 5). Plasma from uninfected controls showed no significant Nef in any fraction (Fig. 6A). Plasma from HIV-infected individuals had significant Nef in the plasma (Fig. 6B). The majority of this Nef was captured on anti-CD45 columns and was present in the eluate, whereas minimal amounts were present in the flow through and wash. This finding clearly indicated that the majority of the Nef present in the plasma of infected individuals is in the form of CD45+ microvesicles whereas only small amounts are associated with the HIV virion.
FIG. 5.
Nef microvesicles are detected in plasma of HIV-infected subjects. Plasma was collected from uninfected (n = 15) and HIV+-infected individuals (n = 10). Microvesicles were ultracentrifuged (400,000 × g); pellets were resuspended in PBS and assayed for Nef using a Nef-capture ELISA. The median value of Nef in plasma is 10.3 (ng/1 ml plasma). Statistical significance was determined using a two-tailed Mann–Whitney analysis. ***p-value < 0.001.
FIG. 6.
Nef microvesicles in plasma of HIV-infected subjects are isolated by CD45 affinity. CD45+ microvesicles in plasma of (A) uninfected and (B) HIV+ donors were isolated as described previously in Fig. 2. Fractions generated during CD45 affinity (SM, FT, W, and E) were ultracentrifuged (400,000 × g); the pellets were resuspended in PBS and assayed for Nef via Nef capture ELISA. Nef was detected in the SM and CD45 captured material (E). SM = starting material, FT = flow through, W = wash, and E = CD45 captured eluate.
Microvesicular Nef levels did not appear to correlate with viral load or CD4 count (data not shown). Overall, these preliminary studies show that of Nef is released from infected cells largely in vesicular form and is present in the plasma of HIV-infected individuals.
Nef microvesicles induce apoptosis in Jurkat T-lymphocytes but not in U937 monocytic cells
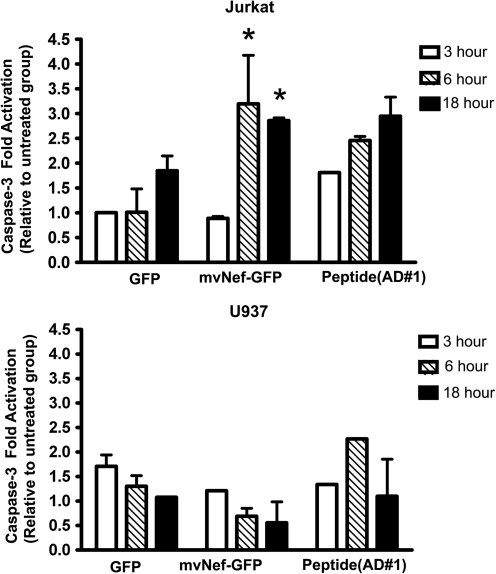
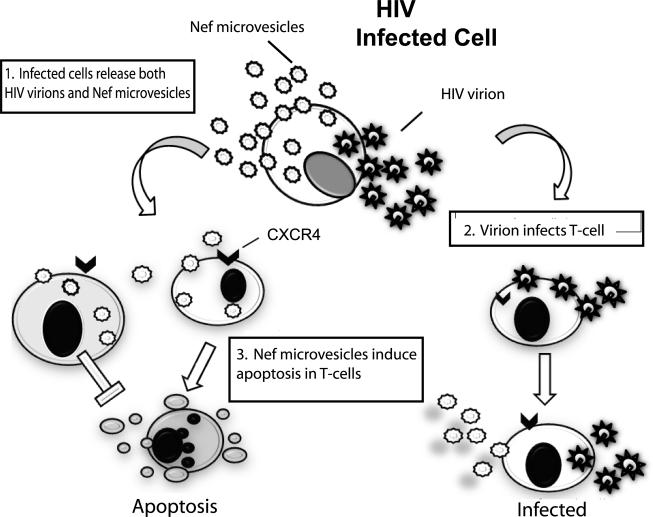
We have previously shown that recombinant, soluble Nef can induce apoptosis in T-lymphocytes via CXCR4-dependent mechanisms.19,20 We have also shown that cell-free tissue culture supernatant contains Nef, which can also induce apoptosis in T cells. To confirm the apoptotic activity of the microvesicular Nef isolated in this study we exposed Jurkat and U937 cells to Nef-GFP microvesicles for 3, 6, or 18 h (Fig. 7). For comparison, a peptide containing one of the apoptotic motifs previously described was also included. Following exposure to the Nef-GFP microvesicles, the degree of apoptosis was measured by caspase-3 activation using a Luminex assay. Interestingly, the interaction of Jurkat T-lymphocytes with Nef-GFP resulted in caspase-3 activation after only 6 h of treatment whereas the U937 monocytic cells did not show evidence of apoptosis until after 18 h of exposure. Taken together, these data provide evidence that the microvesicular Nef isolated in this study was proapoptotic. Based on this work and previous reports from our laboratory and others we propose a general model for the roles of released Nef (Fig. 8).
FIG. 7.
Exposure to vesicular Nef induces apoptosis. Caspase-3 activation in Jurkat and U937 monocytic cells exposed to Nef for 3, 6, and 18 h was measured using a 3-plex Luminex kit. Statistical significance was determined using ANOVA, *p-value <0.05.
FIG. 8.
Model of Nef microvesicle in HIV pathogenesis. HIV-infected cells release both virions and Nef microvesicles. Depending on the cell type of the microvesicle, the virion ratio varies. In step 1 the infected cells release both virions and Nef microvesicles. Cells (both T cells and monocytes) that come in contact with virions become infected (step 2). T cells encountering only Nef microvesicles undergo apoptosis whereas monocytes/macrophages do not (step 3). In this model Nef microvesicles are integral to HIV pathogenesis.
Discussion
The role of Nef in HIV-1 pathogenesis has been difficult to clearly define. One of the major reasons for confusion has been the multitude of effects that have been ascribed to Nef. Most of the known functions attributed to Nef are based on the intracellular expression of the protein. Downregulation of CD4, MHC class I and II, and dysregulation of signal transduction are a few. The role of Nef in the enhancement of infectivity remains controversial, but it appears that membrane association is also important for this effect as well.67,68
Using both nef-transfected and HIV-1-infected cells, we show that Nef is released from cells in the form of microvesicles. The presence of contaminating microvesicles in virus preparations is a common observance. Procedures have been developed to purify virions from vesicles using density gradients. However, because of the similarity of contaminating vesicles and virions in size and density it has been difficult to separate the two by physical means alone. More recently, Trubey et al.55 developed a procedure to purify virions based on the observation that virions exclude CD45 (common leukocyte antigen) whereas contaminating vesicles contain CD45. We have used this procedure to investigate the possibility that the microvesicles that commonly contaminate virus preparations are actually microvesicles containing Nef.
The observation that Nef is released in the form of vesicles is not surprising. Nef is myristoylated at its N-terminus and most of its known functions are dependent on its ability to interact with membranes. Nef has been shown to upregulate the endosomal pathway and actually increase the numbers of endosomes within the cell.36,37,54,69–72 Given Nef interactions with the endosomal pathway it is not unlikely that Nef could be released into exosome-like microvesicles.
Isolation of CD45+ microvesicles from viral supernatants has allowed us to compare the relative quantity of HIV virions and Nef microvesicles released from HIV-infected Jurkat T cells, THP-1 monocytic cells, and PMA-treated macrophage-like THP-1. Interestingly, Jurkat T cells that produce high levels of virus as measured by p24 released 5-fold less CD45+ Nef microvesicles than PMA-stimulated macrophage-like THP-1, which produced much less virus than Jurkat T cells. This suggests that high levels of viral replication are not needed to produce nef-containing microvesicles and that the degree of vesicle production appears to be dependent on cell type. Results from this study clearly demonstrate that Nef vesicles are released from PMA-treated THP-1 at an almost 20-fold greater amount than HIV-1 virions, suggesting that Nef microvesicles are actually the primary product of acute HIV-1 infection in monocytes/macrophages. This finding fits well with current paradigms of acute HIV infection and disease progression. In the Trojan Horse model of trans-infection, DC-SIGN+ macrophages and dendritic cells internalize HIV and home to lymph nodes where they expose CD4 T cells to HIV.73–75 If these macrophages release high levels of Nef microvesicles within the lymph node then this could account for the depletion of CD T cells. Elevated Nef microvesicle release from macrophages also fits the paradigm of acute HIV infection involving the gut-associated lymphatic tissue (GALT). In this model of disease progression the GALT is the principal site of virus production and depletion of CD4+ T cells. Using the SIV model, Li et al.76 have shown that intestinal memory CD4 T cells in the lamina propria of the GALT are preferentially infected and depleted within days of infection.76 Results from this study suggest that only ∼7% of CD4 T cells in GALT are productively infected by SIV and a large portion of these cells undergoes bystander apoptosis on exposure to viral proteins. It is very plausible that this viral protein is the microvesicular Nef released from monocytes/macrophages exposed/infected with HIV in the GALT. Because macrophages appear to be refractive to Nef-induced apoptosis, these cells can continue to release microvesicles without undergoing significant depletion. We know from experiments in our laboratory that Nef microvesicles can be taken up by other cells,35 and that Nef can stimulate endosomal trafficking. Therefore, we are investigating whether the early production of Nef microvesicles by infected macrophages could actually prime other cells for subsequent infection. An observation that lends credence to this idea is that Nef is present in the plasma of HIV-infected individuals. Our results are consistent with previously published findings (Fuji et al.56) that Nef is present at the level of 5–15 ng/ml in plasma. By comparison, it is typical to find p24 present in plasma at levels of picograms. This observation supports our cell culture findings in which Nef could be produced in greater quantities than p24 in infected cells.
Our results suggest no significant direct correlation between Nef levels in the HIV+ plasma and viral load or CD4+ counts (although our sample size is small). Larger studies are underway. A major goal of these studies will be to correlate Nef levels with various markers of immune function. The main hypothesis is that released microvesicular Nef can interact with various cells of the immune system and cause effects that result in T cell depletion and immune dysregulation.
Surprisingly, Nef was still present in HIV-infected patients with undetectable viral loads. This suggests that active viral replication is not required for Nef secretion. Thus, Nef vesicles may be released throughout all phases HIV-1 infection including asymptomatic clinical latency and contributes to the observed continuing decline in CD4+ T cells. With a larger HIV+ patient cohort, we hope to continue to redefine clinical correlates with Nef levels and elucidate the nature of the interaction of Nef vesicles with monocytes/macrophage and other cells of the immune system.
The questions of how and why Nef is released from cells still remain unknown. A recent report demonstrating that Nef interacts with Rab1159 suggests that Nef microvesicle release is via the MVB pathway. This would mean that Nef vesicles could be thought of as exosomes. This is consistent with the idea that HIV-1 has actually taken advantage of existing cell mechanisms (such as exosome release) to facilitate an aspect of viral pathogenesis (T cell depletion). The release of Nef microvesicles has many potential implications for viral pathogenesis. We have attempted to account for these various effects in the form of a model for Nef vesicles (see Fig. 7). In this model, Nef microvesicles along with HIV virions are released from infected cells. The microvesicles via apoptotic domains identified on extracellular soluble Nef can interact with CXCR4 on the surface of T cells and induce apoptosis as previously described by Huang et al.19 Microvesicles can also interact with monocytes and macrophages. Although vesicles do not typically induce apoptosis in these cells, they can be internalized. We do not yet know the effects of internalized Nef on monocytes or macrophages; however, it has been shown that the uptake of soluble Nef by macrophages can reproduce many of the same effects (i.e., CD4 downregulation) that endogenously expressed Nef can produce. Exactly what occurs when CD8+ T cells or other cells of the immune system are exposed to vesicular Nef is yet to be determined. Studies investigating the responses of other cells (such as dendritic cells) to microvesicular Nef are ongoing in the laboratory. Importantly, many of the characteristics of HIV-1 Nef microvesicles are conserved in SIV.
Overall, this study documents a novel finding in HIV-1 pathogenesis. We demonstrate that Nef is released from infected cells in CD45+ exosome-like microvesicles. Most importantly, these Nef microvesicles could be detected in plasma of HIV+ patients in large quantities. Given the capacity for these microvesicles to induce apoptosis in T cells and interact with other cells in the body, it seems likely that mvNef could play an important role in HIV-1 pathogenesis.
Acknowledgments
We would like to acknowledge the support of RCMI core facilities at Morehouse School of Medicine, the Emory Center for AIDS Research, and the NIH AIDS Reagent program. We are grateful for funding support from Grants S06-GM08428, R21 A1060370, and RR003034.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Deacon NJ. Tsykin A. Solomon A, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270(5238):988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhoff F. Greenough TC. Brettler DB. Sullivan JL. Desrosiers RC. Brief report: Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332(4):228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 3.Learmont JC. Geczy AF. Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340(22):1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 4.Lewin SR. Lambert P. Deacon NJ. Mills J. Crowe SM. Constitutive expression of p50 homodimer in freshly isolated human monocytes decreases with in vitro and in vivo differentiation: A possible mechanism influencing human immunodeficiency virus replication in monocytes and mature macrophages. J Virol. 1997;71(3):2114–2119. doi: 10.1128/jvi.71.3.2114-2119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvi R. Garbuglia AR. Di CA, et al. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72(5):3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birch MR. Learmont JC. Dyer WB, et al. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC) J Clin Virol. 2001;22(3):263–270. doi: 10.1016/s1386-6532(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 7.Gorry PR. Churchill M. Learmont J. Cherry C. Dyer WB. Wesselingh SL, et al. Replication-dependent pathogenecity of attenuated nef-deleted HIV-1 in vivo. J Acquir Immune Defic Syndr. 2007;46:390–394. doi: 10.1097/QAI.0b013e31815aba08. [DOI] [PubMed] [Google Scholar]
- 8.Greenough TC. Sullivan JL. Desrosiers RC. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N Engl J Med. 1999;340(3):236–237. doi: 10.1056/NEJM199901213400314. [DOI] [PubMed] [Google Scholar]
- 9.Baba TW. Liska V. Khimani AH, et al. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5(2):194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 10.Dickie P. Ramsdell F. Notkins AL. Venkatesan S. Spontaneous and inducible epidermal hyperplasia in transgenic mice expressing HIV-1 Nef. Virology. 1993;197(1):431–438. doi: 10.1006/viro.1993.1607. [DOI] [PubMed] [Google Scholar]
- 11.Dickie P. HIV type 1 Nef perturbs eye lens development in transgenic mice. AIDS Res Hum Retroviruses. 1996;12(3):177–189. doi: 10.1089/aid.1996.12.177. [DOI] [PubMed] [Google Scholar]
- 12.Dickie P. Nef modulation of HIV type 1 gene expression and cytopathicity in tissues of HIV transgenic mice. AIDS Res Hum Retroviruses. 2000;16(8):777–790. doi: 10.1089/088922200308774. [DOI] [PubMed] [Google Scholar]
- 13.Dickie P. Roberts A. Lee R. A defect in HIV-1 transgenic murine macrophages results in deficient nitric oxide production. J Leukoc Biol. 2001;70(4):592–600. [PubMed] [Google Scholar]
- 14.Ho DD. Neumann AU. Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 15.Perelson AS. Essunger P. Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387(6629):188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 16.Badley AD. Pilon AA. Landay A. Lynch DH. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000;96(9):2951–2964. [PubMed] [Google Scholar]
- 17.Boya P. Pauleau AL. Poncet D, et al. Viral proteins targeting mitochondria: Controlling cell death. Biochim Biophys Acta. 2004;1659(2–3):178–189. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Chase A. Zhou Y. Siliciano RF. HIV-1-induced depletion of CD4+ T cells in the gut: Mechanism and therapeutic implications. Trends Pharmacol Sci. 2006;27(1):4–7. doi: 10.1016/j.tips.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Huang MB. Jin LL. James CO, et al. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J Virol. 2004;78(20):11084–11096. doi: 10.1128/JVI.78.20.11084-11096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James CO. Huang MB. Khan M, et al. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol. 2004;78(6):3099–3109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer M. Fackler OT. Peterlin BM. Structure–function relationships in HIV-1 Nef. EMBO Rep. 2001;2(7):580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arold ST. Baur AS. Dynamic Nef and Nef dynamics: How structure could explain the complex activities of this small HIV protein. Trends Biochem Sci. 2001;26(6):356–363. doi: 10.1016/s0968-0004(01)01846-1. [DOI] [PubMed] [Google Scholar]
- 23.Harris M. From negative factor to a critical role in virus pathogenesis: The changing fortunes of Nef. J Gen Virol. 1996;77(Pt 10):2379–2392. doi: 10.1099/0022-1317-77-10-2379. [DOI] [PubMed] [Google Scholar]
- 24.Garcia JV. Miller AD. Downregulation of cell surface CD4 by nef. Res Virol. 1992;143(1):52–55. doi: 10.1016/s0923-2516(06)80080-4. [DOI] [PubMed] [Google Scholar]
- 25.Hrecka K. Swigut T. Schindler M. Kirchhoff F. Skowronski J. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J Virol. 2005;79(16):10650–10659. doi: 10.1128/JVI.79.16.10650-10659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz O. Marechal V. Le GS. Lemonnier F. Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2(3):338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 27.Stumptner-Cuvelette P. Morchoisne S. Dugast M, et al. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc Natl Acad Sci USA. 2001;98(21):12144–12149. doi: 10.1073/pnas.221256498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildum S. Schindler M. Munch J. Kirchhoff F. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol. 2006;80(16):8047–8059. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdtmann L. Janvier K. Raposo G, et al. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the mu 1 chain of AP1 complex. Traffic. 2000;1(11):871–883. doi: 10.1034/j.1600-0854.2000.011106.x. [DOI] [PubMed] [Google Scholar]
- 30.Geyer M. Yu H. Mandic R, et al. Subunit H of the V-ATPase binds to the medium chain of adaptor protein complex 2 and connects Nef to the endocytic machinery. J Biol Chem. 2002;277(32):28521–28529. doi: 10.1074/jbc.M200522200. [DOI] [PubMed] [Google Scholar]
- 31.Janvier K. Craig H. Le GS, et al. Nef-induced CD4 downregulation: A diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to beta-COP. J Virol. 2001;75(8):3971–3976. doi: 10.1128/JVI.75.8.3971-3976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig HM. Pandori MW. Guatelli JC. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95(19):11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig HM. Reddy TR. Riggs NL. Dao PP. Guatelli JC. Interactions of HIV-1 nef with the mu subunits of adaptor protein complexes 1, 2, and 3: Role of the dileucine-based sorting motif. Virology. 2000;271(1):9–17. doi: 10.1006/viro.2000.0277. [DOI] [PubMed] [Google Scholar]
- 34.Faure J. Stalder R. Borel C, et al. ARF1 regulates Nef-induced CD4 degradation. Curr Biol. 2004;14(12):1056–1064. doi: 10.1016/j.cub.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Campbell TD. Khan M. Huang MB. Bond VC. Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis. 2008;18(2 Suppl 2):S2–S9. [PMC free article] [PubMed] [Google Scholar]
- 36.Sandrin V. Cosset FL. Intracellular versus cell surface assembly of retroviral pseudotypes is determined by the cellular localization of the viral glycoprotein, its capacity to interact with Gag, and the expression of the Nef protein. J Biol Chem. 2006;281(1):528–542. doi: 10.1074/jbc.M506070200. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz O. Utry-Varsat A. Goud B, et al. Human immunodeficiency virus type 1 Nef induces accumulation of CD4 in early endosomes. J Virol. 1995;69(1):528–533. doi: 10.1128/jvi.69.1.528-533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan BT. Teng K. Wu C. Adam M. Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoorvogel W. Kleijmeer MJ. Geuze HJ. Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 40.Van NG. Raposo G. Candalh C, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121(2):337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 41.Van NG. Porto-Carreiro I. Simoes S. Raposo G. Exosomes: A common pathway for a specialized function. J Biochem. 2006;140(1):13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 42.Jonuleit H. Schmitt E. Steinbrink K. Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22(7):394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 43.Raposo G. Nijman HW. Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thery C. Duban L. Segura E, et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 45.Vincent-Schneider H. Stumptner-Cuvelette P. Lankar D, et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14(7):713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 46.Heijnen HF. Debili N. Vainchencker W, et al. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91(7):2313–2325. [PubMed] [Google Scholar]
- 47.Heijnen HF. Schiel AE. Fijnheer R. Geuze HJ. Sixma JJ. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 48.Trams EG. Lauter CJ. Salem N., Jr Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 49.Bess JW., Jr. Gorelick RJ. Bosche WJ. Henderson LE. Arthur LO. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230(1):134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 50.Gluschankof P. Mondor I. Gelderblom HR. Sattentau QJ. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230(1):125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 51.Ali SA. Huang MB. Campbell PE, et al. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses. 2010;26(2):173–192. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantin R. Diou J. Belanger D. Tremblay AM. Gilbert C. Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J Immunol Methods. 2008;338(1–2):21–30. doi: 10.1016/j.jim.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Esser MT. Graham DR. Coren LV, et al. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: Implications for viral pathogenesis and immune regulation. J Virol. 2001;75(13):6173–6182. doi: 10.1128/JVI.75.13.6173-6182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wubbolts R. Leckie RS. Veenhuizen PT, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 55.Trubey CM. Chertova E. Coren LV, et al. Quantitation of HLA class II protein incorporated into human immunodeficiency type 1 virions purified by anti-CD45 immunoaffinity depletion of microvesicles. J Virol. 2003;77(23):12699–12709. doi: 10.1128/JVI.77.23.12699-12709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujii Y. Otake K. Tashiro M. Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393(1):93–96. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- 57.Oh SK. Cruikshank WW. Raina J, et al. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5(3):251–256. [PubMed] [Google Scholar]
- 58.Xiao H. Neuveut C. Tiffany HL, et al. Selective CXCR4 antagonism by Tat: Implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA. 2000;97(21):11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaudhry A. Das SR. Jameel S, et al. HIV-1 Nef induces a Rab11-dependent routing of endocytosed immune costimulatory proteins CD80 and CD86 to the Golgi. Traffic. 2008;9(11):1925–1935. doi: 10.1111/j.1600-0854.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenassi M. Cagney G. Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chertova E. Chertov O. Coren LV, et al. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol. 2006;80(18):9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muratori C. Cavallin LE. Kratzel K, et al. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6(3):218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Khan M. Jin L. Huang MB, et al. Chimeric human immunodeficiency virus type 1 (HIV-1) virions containing HIV-2 or simian immunodeficiency virus Nef are resistant to cyclosporine treatment. J Virol. 2004;78(4):1843–1850. doi: 10.1128/JVI.78.4.1843-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan M. Jin L. Miles L. Bond VC. Powell MD. Chimeric human immunodeficiency virus type 1 virions that contain the simian immunodeficiency virus nef gene are cyclosporin A resistant. J Virol. 2005;79(5):3211–3216. doi: 10.1128/JVI.79.5.3211-3216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan M. Garcia-Barrio M. Powell MD. Restoration of wild-type infectivity to human immunodeficiency virus type 1 strains lacking nef by intravirion reverse transcription. J Virol. 2001;75(24):12081–12087. doi: 10.1128/JVI.75.24.12081-12087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen DH. Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74(7):3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kotov A. Zhou J. Flicker P. Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73(10):8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fackler OT. Moris A. Tibroni N, et al. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology. 2006;351(2):322–339. doi: 10.1016/j.virol.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 69.Kasper MR. Roeth JF. Williams M, et al. HIV-1 Nef disrupts antigen presentation early in the secretory pathway. J Biol Chem. 2005;280(13):12840–12848. doi: 10.1074/jbc.M413538200. [DOI] [PubMed] [Google Scholar]
- 70.Olivetta E. Percario Z. Fiorucci G, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: Involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol. 2003;170(4):1716–1727. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- 71.Piguet V. Gu F. Foti M, et al. Nef-induced CD4 degradation: A diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97(1):63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 72.Roeth JF. Williams M. Kasper MR. Filzen TM. Collins KL. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol. 2004;167(5):903–913. doi: 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geijtenbeek TB. Kwon DS. Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100(5):587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 74.McDonald D. Wu L. Bohks SM, et al. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300(5623):1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 75.Turville S. Wilkinson J. Cameron P. Dable J. Cunningham AL. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J Leukoc Biol. 2003;74(5):710–718. doi: 10.1189/jlb.0503208. [DOI] [PubMed] [Google Scholar]
- 76.Li Q. Duan L. Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434(7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]