Abstract
Curcumin's benefits on tumorigenesis are thought to be mediated by its antiinflammatory activity; however, these effects have not been well characterized in a mouse model of colon cancer. We examined the effects of curcumin on intestinal inflammation in the ApcMin/+ mouse. ApcMin/+ mice were given a placebo or curcumin (2%) diet from 4 to 18 weeks of age (n = 10/group). C57BL/6 mice were used as a wild-type control (n = 10/group). Intestines were analyzed for polyp burden (sections 1, 4, and 5) and for mRNA expression, and concentration of interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and chemokine ligand 2 (CCL2) (sections 2 and 3). Plasma was collected for concentration of CCL2. Curcumin decreased total intestinal polyps by 75% (P < 0.05) in all size categories [>2 mm (65%), 1–2 mm (72%), <1 mm (82%); P < 0.05]. mRNA expression of IL-1β, IL-6, tumor necrosis factor-α, and CCL2 was elevated (P < 0.05) and curcumin blunted this increase (P < 0.05). Protein concentration of IL-1β, IL-6 (section 3), and CCL2 was increased (P < 0.05) and curcumin reduced this response for IL-1β (section 2) and CCL2 (P < 0.05). Curcumin also offset the increase in plasma CCL2 (P < 0.05). The benefits of curcumin in colon cancer may be at least in part mediated by its antiinflammatory activity.
Introduction
Many plants contain biologically active chemicals that possess health-promoting capabilities (Aggarwal and others 2008; Kim and others 2009); such components of the diet are generally known to have a much wider safety margin with chronic use than most drugs. Many of these phytochemicals, such as curcumin (the major yellow pigment extracted from turmeric, and commonly used spice derived from the rhizome of plant Curcuma longa), have been examined for their chemopreventive potential (Aggarwal and others 2008; Kim and others 2009). While data from human trials are still limited, there is good preclinical data to support a benefit of curcumin on carcinogenesis. It has been shown to inhibit carcinogenesis in many different models, including colon cancer models, and at a wide range of physiological attainable doses (Corpet and Pierre 2003; Kim and others 2009). For example, evidence suggests that it can provide growth inhibitory effects on colon cancer cells in vitro (Cho and others 2007). Further, several studies in rodent models argue for curcumin's chemopreventive potential in colon cancer (Mahmoud and others 2000; Collett and others 2001; Perkins and others 2002; Volate and others 2005; Tunstall and others 2006).
Curcumin has a diverse range of molecular targets, including transcription factors, growth factors and their receptors, cytokines, enzymes, and genes regulating cell proliferation and apoptosis, which are all likely to contribute to its anticancer potential (Goel and others 2008). However, given the strong association between inflammation and cancer, it has been suggested that curcumin's beneficial effects on tumorigenesis are likely to largely involve the inhibition of cell signaling pathways involving nuclear factor kappa B (NFκB) (Siwak and others 2005; Singh and Khar 2006). NFκB has been linked to the initiation and progression of cancer through its ability to activate the inflammatory cascade (Karin 2006). It has also been shown that curcumin downregulates expression of cyclooxygenase-2 protein in colon cancer (Tunstall and others 2006), most likely through downregulation of NFκB. Curcumin also appears to exert antiinflammatory effects on colon cancer cells by inhibiting the NFκB-regulated proinflammatory cytokines interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) (Cho and others 2007), all of which have been associated with a poor outcome in colon cancer. Although not specifically in colon cancer, several investigators have documented a decrease in chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein 1, after treatment with curcumin (Woo and others 2007). CCL2 has been identified as a major chemokine for macrophage recruitment and has been associated with increased grade of the tumor in certain cancers (Amann and others 1998; Tanaka and others 2009).
The ApcMin/+ mouse model has been the most widely used genetically engineered mouse model for the study of dietary chemopreventive agents on intestinal tumorigenesis (Corpet and Pierre 2003). It has been shown to have a mutated Apc gene similar to that in patients with familial adenomatous polyposis and in many sporadic cancers. This mouse model of intestinal tumorigenesis has been shown to be responsive to treatment with antiinflammatory dietary components (Corpet and Pierre 2003), including curcumin (Mahmoud and others 2000; Collett and others 2001; Perkins and others 2002; Tunstall and others 2006). However, except for one study that examined curcumin's effect on cyclooxygenase-2 expression in intestinal adenomas in the ApcMin/+ mouse (Tunstall and others 2006), there have been no studies that have specifically linked the antiinflammatory effects of curcumin to intestinal tumorigenesis in this model.
The aim of this study was to examine the effects of the antiinflammatory dietary component curcumin on intestinal inflammation in the ApcMin/+ mouse as a potential mechanism for its benefits on tumorigenesis. On the basis of previous literature on curcumin's anticarcinogenic and antiinflammatory activity in vitro, we hypothesized that curcumin would decrease intestinal inflammation and that this would be associated with a decrease in tumorigenesis.
Materials and Methods
Animals
ApcMin/+ male mice on a C57BL/6 background (Jackson Laboratories) were purchased and bred with female C57BL/6 mice in the University of South Carolina's Center for Colon Cancer Research. Offspring were genotyped as heterozygotes by real-time polymerase chain reaction (RT-PCR) for the Apc gene by taking tail snips at weaning. The primer sequences were sense 5′ TGAGAAAGACAGAAGTTA 3′ and antisense 5′ TTCCACTTTGGCATAAGGC 3′. C57BL/6 mice were used as a wild-type control. Mice were maintained on a 12:12 h light–dark cycle in a low-stress environment (22°C, 50% humidity and low noise) and provided food and water ad libitum. All animal experimentation was approved by the University of South Carolina's Institutional Animal Care and Use Committee.
Curcumin treatment
Male and female ApcMin/+ mice were randomly assigned to either curcumin or placebo treatment (n = 10/group; 5 males and 5 females). Curcumin (Naturex) was incorporated into the basal diet at a concentration of 2% (BioServ). A wide range of doses (0.1%–2%) have been used to examine the effects of curcumin on tumorigenesis in the ApcMin/+ mouse model as well as other cancer models (Perkins and others 2002; Moghaddam and others 2009). We used a dose (2%) that has been successful at reducing tumorigenesis in our hands to examine the effects of curcumin on intestinal inflammation in the ApcMin/+ mouse. Placebo mice received an identical diet without curcumin. Curcumin feedings began at 4 weeks of age and continued for 14 weeks, until animals reached 18 weeks of age, at which time they were sacrificed. Age-matched C57BL/6 mice (n = 10/group; 5 males and 5 females) used as a wild-type control were fed an identical diet to the placebo mice. We did not include a wild-type curcumin group in our investigation; wild-type mice do not develop polyps and it is unlikely that they would display significant inflammation. Food intake and body weight were measured weekly throughout the treatment period.
Tissue collection
Mice were sacrificed at 18 weeks of age for tissue collection. The small intestine was carefully dissected distally to the stomach and proximal to the cecum. The large intestine (section 5) was removed from the distal end of the cecum to the anus. Mesentary tissue was removed with tweezers, and the small intestine was cut into 4 equal sections (sections 1–4). All intestinal sections were flushed with phosphate-buffered saline, opened longitudinally, and flattened with a cotton swab. Sections 1 and 4 of the small intestine and the large intestine (section 5) were fixed in 10% buffered formalin (Fisher Scientific) for 24 h. Sections 2 and 3 were divided into 2 equal parts, and mucosal scrapings were performed in iscoves medium (Invitrogen) [containing 5% fetal bovine serum and a cocktail enzyme inhibitor (10 mM EDTA, 5 mM benzamidine HCl, and 0.2 mM phenylmethyl sulfonyl fluoride)] and TRIzol reagent (Invitrogen) for protein and gene expression analysis, respectively. Samples were stored at −80°C until analysis of inflammatory mediators. Blood was collected from the inferior vena cava using a heparinized syringe and spun in a microcentrifuge at 4,000 rpm for 15 min. Plasma was then stored at −80°C until assayed for CCL2.
Polyp counts
Formalin-fixed intestinal sections from all animals were rinsed in deionized water, briefly stained in 0.1% methylene blue, and counted by the same investigator who was blinded to the treatments. Polyps were counted under a dissecting microscope, using tweezers to pick through the intestinal villi and identify polyps. Polyps were categorized by size [>2 mm (large), 1–2 mm (medium), and <1 mm (small)].
Expression of inflammatory markers
Procedures for mRNA expression of inflammatory mediators were performed as previously described (Nieman and others 2007). Briefly, muscosal tissue was homogenized under liquid nitrogen with a polytron, and total RNA was extracted using TRIzol reagent (Invitrogen). The extracted RNA (2.5 μL of sample) was dissolved in diethyl pyrocarbonate (DEPC)-treated water and quantified spectrophotometrically at 260 nm wavelength. Quality of RNA was determined using the 260:280 ratio; RNA with a ratio >1.6 was included in the analysis. RNA was reverse transcribed into cDNA in a 50 μL reaction volume containing 19.25 μL RNA (1.5 μg) in RNase-free water, 5 μL 10 × RT buffer, 11 μL 25 mM MgCl2, 10 μL deoxyNTPs mixture, 2.5 μL random hexamers, 1 μL RNase inhibitor, and 1.25 μL multiscribe reverse transcriptase (50 U/μL). Reverse transcription was performed at 25°C for 10 min, 37°C for 60 min, and 95°C for 5 min, followed by quick chilling on ice and storage at −20°C until subsequent amplification. Quantitative RT-PCR analysis was done per manufacturer's instructions (Applied Biosystems) using TaqMan® Gene Expression Assays (IL-1β, IL-6, TNF-α, and CCL2). DNA amplification was carried out in 12.5 μL TaqMan Universal PCR Master Mix (AmpliTaq Gold DNA Polymerase, Passive Reference 1, Buffer, dNTPs, and AmpErase UNG), 1 μL cDNA, 9 μL RNase-free water, and 1.25 μL 18S primer (VIC) and 1.25 μL primer (FAM) (for endogenous reference and target gene) in a final volume of 25 μL/well. Samples were loaded in a MicroAmp 96-well reaction plate. Plates were run using Applied Biosystems Sequence Detection System. After 2 min at 50°C and 10 min at 95°C, samples were coamplified by 50 repeated cycles of which 1 cycle consisted of 15 s denaturing step at 95°C and 1 min annealing/extending step at 60°C. All samples were run in duplicate. Quantification of cytokine gene expression for IL-1β, IL-6, TNF-α, and CCL2 was calculated using the ΔCT method. This method uses a single sample, the calibrator sample, for comparison of every unknown sample's gene expression. This method of analysis and quantification has been shown to give similar results as the standard curve method. Briefly, ΔCT [CT(FAM) − CT(VIC)] is calculated for each sample and calibrator. ΔΔCT [ΔCT(calibrator) − ΔCT(sample)] is then calculated for each sample, and relative quantification is calculated as 2ΔΔCT. Initial exclusion criteria consist of FAM CT ≥40 and VIC CT ≥23.
Concentration of inflammatory markers
Mucosal tissue was homogenized using a polytron, and samples were centrifuged at 10,000 rpm and 4°C for 10 min, and the supernatants removed and stored at 4°C before the assay of IL-1β, IL-6, TNF-α, and CCL2 via ELISA (R&D Systems). The assay was performed according to the manufacturer's instructions. Total soluble protein was also determined using supernatant of homogenized samples via bicinchoninic acid protein assay (Pierce). Cytokine levels are expressed as a ρg per 100 μg total protein. Plasma levels of CCL2 were also measured using ELISA techniques (R&D Systems).
Statistical analysis
Data were analyzed using a one-way analysis of variance with Student Newman–Keuls post hoc analysis. All data were analyzed using commercial software (SigmaStat; SPSS). Statistical significance was set with an α value of P < 0.05. Data are presented as mean (±standard error of the mean).
Results
Body weight and food intake
Food intake and body weight were measured weekly throughout the treatment period. Consistent with previous reports of curcumin feedings, there was no difference in body weight or food intake between the placebo and curcumin group, which suggests that the 2% curcumin diet was well tolerated.
Polyp incidence
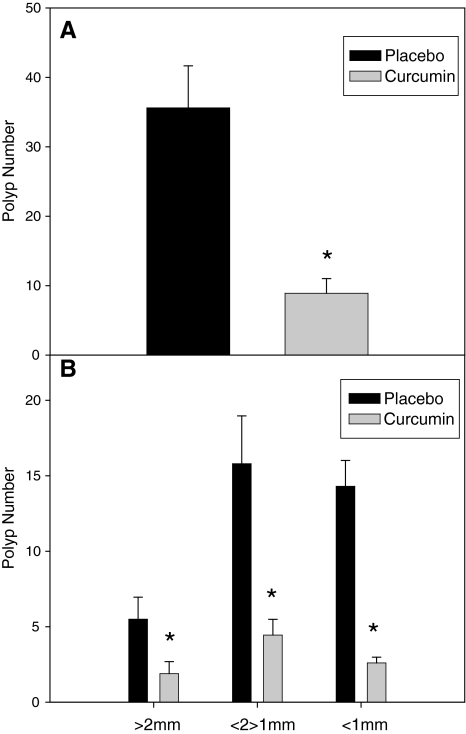
Polyps were counted on formalin-fixed, methylene-blue-stained intestinal sections. Curcumin feedings for 14 weeks (age 4–18 weeks) resulted in a 75% decrease in total polyp (sections 1, 4, and 5) number compared with mice fed a placebo diet (36 ± 6 versus 9 ± 2; P < 0.05) (Fig. 1A). Colon polyps (section 5) were reduced by 86% (5.7 ± 2.5 versus 0.8 ± 0.5) (data not shown), but this did not reach statistical significance (P = 0.06). Curcumin feedings also reduced polyp size in all size categories (Fig. 1B). Large (>2 mm), medium (1–2 mm), and small (<1 mm) polyps were reduced by 65% (5.5 ± 1.5 versus 1.9 ± 0.9; P < 0.05), 72% (15.8 ± 3.3 versus 4.4 ± 1.2; P < 0.05), and 82% (14.3 ± 1.8 versus 2.6 ± 0.4; P < 0.05), respectively. Statistical analyses were not performed across gender due to the small sample sizes (n = 10/group; 5 males and 5 females).
FIG. 1.
Curcumin reduces (A) total intestinal polyp number and (B) polyp number in all size categories [>2 mm (large), <2 mm, >1 mm (medium), and <1 mm (small)] in the ApcMin/+ mouse model of intestinal tumorigenesis (n = 10/group). Mice were fed a placebo or curcumin diet from 4 to 18 weeks of age. After the treatment period mice were sacrificed and polyps were counted in sections 1 and 4 of the small intestine and the colon (section 5) and categorized as >2 mm, 1–2 mm, and <1 mm. Values are means ± standard error of the mean (SEM). *Significantly different from placebo, P < 0.05.
Tissue mRNA expression of inflammatory markers
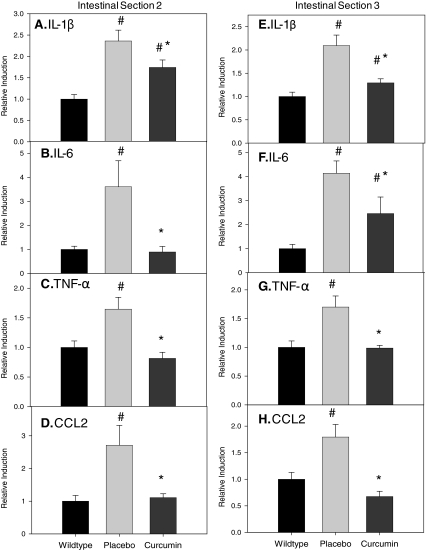
mRNA expression of inflammatory markers (IL-1β, IL-6, TNF-α, and CCL2) was examined in regions 2 and 3 of the intestines of ApcMin/+ after curcumin treatment. A wild-type control group was included to illustrate inflammation in the ApcMin/+ mouse and is included only for comparison purposes. Regions 2 and 3 were chosen so as to represent a section of the intestine with low and high polyp incidence, respectively. In section 2, mRNA expression of IL-1β, IL-6, TNF-α, and CCL2 (Fig. 2A–D) was increased by ∼2.5-, 4-, 1.5-, and 2.5-fold, respectively (P < 0.05). Curcumin blunted this effect for IL-1β (P < 0.05) and completely blocked the increased expression of IL-6, TNF-α, and CCL2 in this region (P < 0.05). Similar effects were noted for region 3; expression of IL-1β, IL-6, TNF-α, and CCL2 (Fig. 2E–H) was increased by 2-, 4-, 1.5-, and 2-fold, respectively (P < 0.05), and curcumin feedings blunted this effect for IL-1β and IL-6 (P < 0.05) and completely blocked the increase in TNF-α and CCL2 (P < 0.05).
FIG. 2.
Curcumin blunts increased mRNA expression of interleukin (IL)-1β (A, E), IL-6 (B, F), tumor necrosis factor (TNF)-α (C, G), and chemokine ligand 2 (CCL2) (D, H) in sections 2 and 3 of the small intestine of the ApcMin/+ mouse (n = 10/group). Mice were fed a placebo or curcumin diet from 4 to 18 weeks of age. After the treatment period mice were sacrificed and intestinal sections 2 and 3 were removed for analysis of mRNA expression of IL-1β, IL-6, TNF-α, and CCL2 using real-time polymerase chain reaction. Wild-type mice were used as a control. Values are means ± SEM. #Significantly different from wild type, P < 0.05; *significantly different from placebo, P < 0.05.
Tissue concentration of inflammatory markers
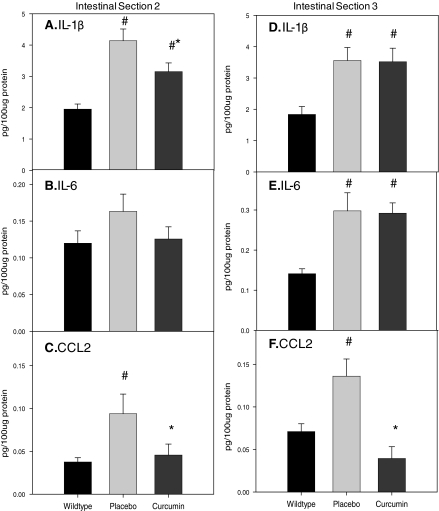
The protein concentration of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and CCL2 was compared in regions 2 and 3 of the intestines of ApcMin/+ with and without curcumin treatment. For section 2, IL-1β (Fig. 3A) and CCL2 (Fig. 3C) were significantly increased in the ApcMin/+ mouse (P < 0.05) and curcumin blunted this increase (P < 0.05). There was no significant increase in IL-6 (Fig. 3B). For region 3, concentration of IL-1β, IL-6, and CCL2 was increased in the ApcMin/+ mouse (P < 0.05) (Fig. 3D–F) and curcumin offset this effect for CCL2 only (P < 0.05). TNF-α levels were below the detection limit of the assay for both regions 2 and 3.
FIG. 3.
Effects of curcumin on concentration of IL-1β (A, D), IL-6 (B, E), and CCL2 (C, F) in sections 2 and 3 of the small intestine of the ApcMin/+ mouse (n = 10/group). Mice were fed a placebo or curcumin diet from 4 to 18 weeks of age. After the treatment period mice were sacrificed and intestinal sections 2 and 3 were removed for analysis of protein concentration of IL-1β, IL-6, and CCL2 using ELISA. Wild-type mice were used as a control. Values are means ± SEM. #Significantly different from wild type, P < 0.05; *significantly different from placebo, P < 0.05.
Plasma CCL2
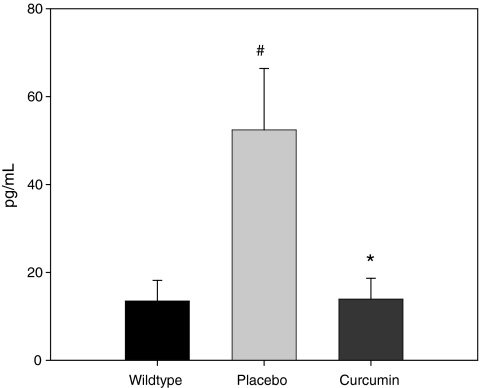
Plasma concentration of CCL2 (Fig. 4), a marker of systemic inflammation and the most important chemokine for macrophage recruitment, was also measured in the ApcMin/+ mouse after 14 weeks of curcumin feedings. CCL2 was significantly increased in the placebo ApcMin/+ mice versus wild type (P < 0.05) and curcumin blocked this effect (P < 0.05).
FIG. 4.
Curcumin blocks the increase in plasma CCL2 in the ApcMin/+ mouse (n = 10/group). Mice were fed a placebo or curcumin diet from 4 to 18 weeks of age. After the treatment period mice were sacrificed, and blood was collected for plasma analysis of CCL2 using ELISA. Wild-type mice were used as a control. Values are means ± SEM. #Significantly different from wild type, P < 0.05; *significantly different from placebo, P < 0.05.
Discussion
The dietary polyphenol curcumin has received a lot of attention for its diverse biological properties in preclinical models of tumorigenesis at a wide range of physiological attainable doses (Goel and others 2008). One such property of curcumin is its ability to block NFκB activation and subsequent inflammation (Aggarwal and others 2006). However, the effects of curcumin on intestinal inflammation in colon cancer have not been well characterized. This study used a well-established mouse model of intestinal tumorigenesis to examine the effects of the antiinflammatory dietary component curcumin on intestinal (IL-1β, IL-6, TNF-α, and CCL2) and systemic (CCL2) inflammation and associated tumorigenesis (polyp number and size). Our findings confirm the previously reported benefits of curcumin on intestinal tumorigenesis in the ApcMin/+ mouse (Mahmoud and others 2000; Collett and others 2001; Perkins and others 2002; Tunstall and others 2006) and further indicate that curcumin can inhibit mRNA gene expression and protein concentration of various inflammatory mediators in the small intestine and plasma. To our knowledge these are the first data to show a curcumin-induced benefit on mRNA expression and protein concentration of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and CCL2 in a mouse model of intestinal tumorigenesis. Further, this is the first report of an effect of curcumin on plasma CCL2 in any mouse model of tumorigenesis.
To date there are several studies that show clinical evidence for a chemopreventive effect of curcumin against colon cancer (Sharma and others 2004; Cruz-Correa and others 2006). Cruz-Correa and others (2006), for example, have reported a beneficial effect of curcumin in patients with familial adenomatous polyposis. Curcumin has also been shown to inhibit intestinal tumorigenesis in several rodent models and at a wide range of physiological attainable doses. Because rodents almost never develop spontaneous colon cancer, investigators have used either a carcinogen or genetically engineered mouse model for cancer studies that involve the gastrointestinal tract. The ApcMin/+ mouse model and the azoxymethane (AOM) rat model are the most widely used models in this regard (Corpet and Pierre 2003). Curcumin has been reported to reduce the number of aberrant crypts by ∼2-fold in the AOM rat model (Volate and others 2005). In the ApcMin/+ mouse model, several studies have reported a beneficial effect of curcumin on intestinal tumorigenesis (Mahmoud and others 2000; Collett and others 2001; Perkins and others 2002; Tunstall and others 2006). For example, Perkins and others (2002) reported that curcumin decreased polyp number by 39% and 40% after a 0.2% and 0.5% curcumin diet. Our findings using a 2% curcumin diet indicate a 75% decrease in intestinal tumorigenesis, confirming the previously reported beneficial effects of curcumin in this model. Further, our data indicate that this dose of curcumin reduced polyp number across all size categories [>2 mm (large), <2, >1 mm (medium), and <1 mm (small)]. One drawback of this model is that the tumors occur predominately in the small intestine and not the colon; nonetheless, the development of polyps in this model is thought to closely mimic the human disease.
What mediates the chemopreventive benefits of curcumin in colon cancer has yet to be completely elucidated. On the basis of numerous reported in vitro studies, it is hypothesized that its antiinflammatory properties play a significant role (Jobin and others 1999; Pan and others 2000; Surh and others 2001; Aggarwal and others 2006); however, these effects have not yet been well characterized in animal models of colon cancer. To our knowledge these data are the first to show a curcumin-induced reduction in mRNA expression and protein concentration of the inflammatory mediators CCL2, IL-1β, IL-6, and TNF-α in intestinal tissue and CCL2 in plasma in any mouse model of intestinal tumorigenesis. Further, these are the first data to show a reduction in plasma CCL2 in any mouse model of cancer after curcumin feedings. CCL2 has been identified as a major chemokine for macrophage recruitment in several human tumors, including the colon (Grimm and others 1996; Ueno and others 2000), and has been associated with increased grade of the tumor in certain cancers (Amann and others 1998; Tanaka and others 2009). Similarly, expression of CCL2 within tumors has also been shown to correlate with poor prognosis (Ueno and others 2000; Tanaka and others 2009). Recruitment of macrophages by CCL2 has been shown to promote growth and/or facilitate survival of neoplastic cells, which is in part due to the increased release of proinflammatory cytokines from macrophages; proinflammatory cytokines have well-established associations with the progression of colon cancer. For example, mice exposed to AOM develop IL-6-dependent tumors in the gastrointestinal tract (Becker and others 2004) and a role for IL-6 has been implicated in human colorectal carcinomas (Becker and others 2005). Further, we have previously shown that IL-6 overexpression using electron gene transfer techniques can increase polyp burden in the ApcMin/+ mouse (Baltgalvis and others 2008).
While curcumin blunted increased expression of CCL2, IL-1β, IL-6, and TNF-α in both sections 2 and 3 of the intestine, concentration of IL-1β in section 2 and CCL2 in sections 2 and 3 of the intestine, as well as CCL2 in the plasma, it did not blunt the increased concentration of IL-1β and IL-6 in section 3. A potential explanation for this may be that because section 3 of the intestine is generally considered a region of high polyp burden, the inflammation in this area may be too severe to detect a beneficial antiinflammatory effect of this dose of curcumin on these cytokines; however, our data do not support this hypothesis. It is also possible that the absorption of curcumin may have been greater in section 2 than in section 3, resulting in a greater potential for its antiinflammatory effects in this region. Perkins and others (2002) reported large variability in curcumin levels between the small intestinal mucosa and colonic mucosa in C57BL/6 mice after dietary curcumin; this certainly raises the possibility that curcumin levels may differ throughout the small intestine. It is also important to note that the analysis of inflammatory mediators was performed in the small intestine where most of the polyps develop in this model, as opposed to the colon, which is the site of polyp development in the human disease.
Unfortunately, oral administration of curcumin to mice and humans has been reported to have low bioavailability; however, it does result in detectable levels in the plasma and tissue (Anand and others 2007). The low bioavailability of curcumin appears to be due to low absorption, rapid metabolism, and rapid systemic elimination, all of which continue to be highlighted as major concerns with the efficacy of this compound. Despite the low bioavailability of curcumin, its therapeutic efficacy against cancer in preclinical models is widely recognized (Strimpakos and Sharma 2008).
In summary, these findings confirm the previously reported beneficial effects of dietary curcumin on intestinal tumorigenesis in rodent models of colon cancer. However, these are the first data to indicate a curcumin-induced reduction in mRNA expression and protein concentration of proinflammatory cytokines and CCL2 in the intestines in any model of intestinal tumorigenesis. Further, these are the first data to show a reduction in plasma CCL2 in any mouse model of cancer after curcumin feedings. If these data can be clinically translated, curcumin may lead to an important target of intestinal and systemic inflammation in colon cancer.
Acknowledgments
This work was supported by a grant from the National Cancer Institute (R21 CA135377). The authors would like to thank Dr. Franklin Berger, Director of the Center for Colon Cancer Research, University of South Carolina, for kindly providing the ApcMin/+ mice used in this study.
Author Disclosure Statement
E. Angela Murphy: No conflict of interest.
J. Mark Davis: No conflict of interest.
Jamie L. McClellan: No conflict of interest.
Benjamin T. Gordon: No conflict of interest.
Martin D. Carmichael: No conflict of interest.
References
- Aggarwal BB. Kunnumakkara AB. Harikumar KB. Tharakan ST. Sung B. Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74(13):1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- Aggarwal S. Ichikawa H. Takada Y. Sandur SK. Shishodia S. Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69(1):195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Amann B. Perabo FG. Wirger A. Hugenschmidt H. Schultze-Seemann W. Urinary levels of monocyte chemo-attractant protein-1 correlate with tumour stage and grade in patients with bladder cancer. Br J Urol. 1998;82(1):118–121. doi: 10.1046/j.1464-410x.1998.00675.x. [DOI] [PubMed] [Google Scholar]
- Anand P. Kunnumakkara AB. Newman RA. Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA. Berger FG. Pena MM. Davis JM. Muga SJ. Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- Becker C. Fantini MC. Schramm C. Lehr HA. Wirtz S. Nikolaev A. Burg J. Strand S. Kiesslich R. Huber S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. others. [DOI] [PubMed] [Google Scholar]
- Becker C. Fantini MC. Wirtz S. Nikolaev A. Lehr HA. Galle PR. Rose-John S. Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4(2):217–220. [PubMed] [Google Scholar]
- Cho JW. Lee KS. Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19(3):469–474. [PubMed] [Google Scholar]
- Collett GP. Robson CN. Mathers JC. Campbell FC. Curcumin modifies Apc(min) apoptosis resistance and inhibits 2-amino 1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) induced tumour formation in Apc(min) mice. Carcinogenesis. 2001;22(5):821–825. doi: 10.1093/carcin/22.5.821. [DOI] [PubMed] [Google Scholar]
- Corpet DE. Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12(5):391–400. [PMC free article] [PubMed] [Google Scholar]
- Cruz-Correa M. Shoskes DA. Sanchez P. Zhao R. Hylind LM. Wexner SD. Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Goel A. Jhurani S. Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52(9):1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- Grimm MC. Elsbury SK. Pavli P. Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol. 1996;59(6):804–812. doi: 10.1002/jlb.59.6.804. [DOI] [PubMed] [Google Scholar]
- Jobin C. Bradham CA. Russo MP. Juma B. Narula AS. Brenner DA. Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163(6):3474–3483. [PubMed] [Google Scholar]
- Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45(6):355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Kim YS. Young MR. Bobe G. Colburn NH. Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res (Phila Pa) 2009;2(3):200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud NN. Carothers AM. Grunberger D. Bilinski RT. Churchill MR. Martucci C. Newmark HL. Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21(5):921–927. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- Moghaddam SJ. Barta P. Mirabolfathinejad SG. Ammar-Aouchiche Z. Garza NT. Vo TT. Newman RA. Aggarwal BB. Evans CM. Tuvim MJ. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30(11):1949–1956. doi: 10.1093/carcin/bgp229. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DC. Henson DA. Davis JM. Angela Murphy E. Jenkins DP. Gross SJ. Carmichael MD. Quindry JC. Dumke CL. Utter AC. Quercetin's influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007;103(5):1728–1735. doi: 10.1152/japplphysiol.00707.2007. others. [DOI] [PubMed] [Google Scholar]
- Pan MH. Lin-Shiau SY. Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60(11):1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- Perkins S. Verschoyle RD. Hill K. Parveen I. Threadgill MD. Sharma RA. Williams ML. Steward WP. Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11(6):535–540. [PubMed] [Google Scholar]
- Sharma RA. Euden SA. Platton SL. Cooke DN. Shafayat A. Hewitt HR. Marczylo TH. Morgan B. Hemingway D. Plummer SM. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. others. [DOI] [PubMed] [Google Scholar]
- Singh S. Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6(3):259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- Siwak DR. Shishodia S. Aggarwal BB. Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104(4):879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- Strimpakos AS. Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10(3):511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Chun KS. Cha HH. Han SS. Keum YS. Park KK. Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Kurebayashi J. Sohda M. Nomura T. Prabhakar U. Yan L. Sonoo H. The expression of monocyte chemotactic protein-1 in papillary thyroid carcinoma is correlated with lymph node metastasis and tumor recurrence. Thyroid. 2009;19(1):21–25. doi: 10.1089/thy.2008.0237. [DOI] [PubMed] [Google Scholar]
- Tunstall RG. Sharma RA. Perkins S. Sale S. Singh R. Farmer PB. Steward WP. Gescher AJ. Cyclooxygenase-2 expression and oxidative DNA adducts in murine intestinal adenomas: modification by dietary curcumin and implications for clinical trials. Eur J Cancer. 2006;42(3):415–421. doi: 10.1016/j.ejca.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Ueno T. Toi M. Saji H. Muta M. Bando H. Kuroi K. Koike M. Inadera H. Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–3289. [PubMed] [Google Scholar]
- Volate SR. Davenport DM. Muga SJ. Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26(8):1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- Woo HM. Kang JH. Kawada T. Yoo H. Sung MK. Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80(10):926–931. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]