Abstract
Background and Aims
Understanding the fate and dynamics of cells during callus formation is essential to understanding totipotency and the mechanisms of somatic embryogenesis. Here, the fate of leaf explant cells during the development of embryogenic callus was investigated in the model legume Medicago truncatula.
Methods
Callus development was examined from cultured leaf explants of the highly regenerable genotype Jemalong 2HA (2HA) and from mesophyll protoplasts of 2HA and wild-type Jemalong. Callus development was studied by histology, manipulation of the culture system, detection of early production of reactive oxygen species and visualization of SERK1 (SOMATIC EMBRYO RECEPTOR KINASE1) gene expression.
Key Results
Callus formation in leaf explants initiates at the cut surface and within veins of the explant. The ontogeny of callus development is dominated by the division and differentiation of cells derived from pluripotent procambial cells and from dedifferentiated mesophyll cells. Procambium-derived cells differentiated into vascular tissue and rarely formed somatic embryos, whereas dedifferentiated mesophyll cells were competent to form somatic embryos. Interestingly, explants incubated adaxial-side down had substantially less cell proliferation associated with veins yet produced similar numbers of somatic embryos to explants incubated abaxial-side down. Somatic embryos mostly formed on the explant surface originally in contact with the medium, while in protoplast microcalli, somatic embryos only fully developed once at the surface of the callus. Mesophyll protoplasts of 2HA formed embryogenic callus while Jemalong mesophyll protoplasts produced callus rich in vasculature.
Conclusions
The ontogeny of embryogenic callus in M. truncatula relates to explant orientation and is driven by the dynamics of pluripotent procambial cells, which proliferate and differentiate into vasculature. The ontogeny is also related to de-differentiated mesophyll cells that acquire totipotency and form the majority of embryos. This contrasts with other species where totipotent embryo-forming initials mostly originate from procambial cells.
Key words: Callus, dedifferentiation, leaf veins, Medicago truncatula, pluripotency, procambium, protoplasts, reactive oxygen species, SERK, somatic embryogenesis, stem cells, totipotency
INTRODUCTION
The in vitro culture of plant tissue and cells has a long history and has contributed much to contemporary biotechnology (Sussex, 2008; Vasil, 2008). Somatic embryos can be induced in vitro from cultured explants and protoplasts. While an understanding of somatic embryo induction is starting to emerge, details of the signalling mechanisms involved remain poorly understood (Rose and Nolan, 2006; Braybrook and Harada, 2008; Zheng et al., 2009; Rose et al., 2010).
Most morphological studies of somatic embryogenesis (SE) demonstrate that regeneration occurs via SE rather than organogenesis. However, an important and unresolved question in SE concerns the nature of the callus cells ultimately programmed to become totipotent and produce embryos (Vogel, 2005; Kwaaitaal et al., 2007; Verdeil et al., 2007). Historically, this question has been investigated primarily using Daucus carota (carrot) cell suspension cultures (Halperin, 1966). A study by Guzzo et al. (1994) on the origin of totipotent cells in carrot cell-suspension cell cultures, derived from hypocotyl explants, showed that procambial cells gave rise to the embryogenic cell lines. The cell-tracking studies of Schmidt et al. (1997) in cultures prepared from carrot hypocotyl explants support this finding. Procambial cells are pluripotent vascular stem cells that originate from the apical meristem and ultimately generate the xylem and phloem (Fukuda, 2004). Indeed, in the pioneering work on SE by Steward et al. (1958, 1964), embryogenic cell suspensions were derived from the phloem region of carrot storage root and likely contained procambial-like cells. A range of other morphological studies also supports the link between SE and procambium or vascular tissue (Lu and Vasil, 1985; Schmidt et al., 1997; Schwendiman et al., 1988; Somleva et al., 2000). A recent study by Kwaaitaal et al. (2007) suggests that in tissue cultures of arabidopsis, embryogenically competent cells mainly derive from procambial cells expressing SERK1 in planta.
While it is clear pluripotent procambial cells have a capacity for induced embryogenesis, providing an explanation for the association between somatic embryos and vascular tissue, a number of morphological studies also show embryos form at the periphery of callus distal to vascular tissue (Lu and Vasil, 1985; Sagare et al., 1995; Loiseau et al., 1998; Sharma and Millam, 2004).
The model legume Medicago truncatula is used to study the mechanism of SE (Rose and Nolan, 2006). In M. truncatula, SE requires an appropriate genotype, such as Jemalong 2HA (2HA), a cytokinin and an auxin (Nolan and Rose, 1998; Rose et al., 1999). Culturing M. truncatula leaf explants (either Jemalong wild-type or 2HA) with auxin alone, results in a small amount of callus and root organogenesis (Rose et al., 2006). In this situation, it is clear that root meristems form from procambial-like cells, because callusing does not obscure the relationship. However, culturing 2HA with both auxin and cytokinin triggers SE, producing a complex callus with substantially more vascular development.
The fate of cells associated with the ontogeny of embryogenic callus has been examined in M. truncatula. Despite its ultimate importance in understanding the molecular mechanisms of SE or organogenesis, the dynamics and fate of cells during callus morphogenesis has received little attention. Explants and mesophyll protoplasts were used as the source material together with the optimized hormonal protocol that has become the standard approach to investigate SE in M. truncatula (Nolan and Rose, 1998; Nolan et al., 2003). This optimized protocol has been used to characterize SE in 2HA via proteomic (Imin et al., 2004, 2005), transcriptomic (Imin et al., 2008; Mantiri et al., 2008) and functional genomic approaches (Mantiri et al., 2008; Chen et al., 2009). Here, different histological strategies, manipulation of the culture system, early ROS (reactive oxygen species) detection and analysis of SERK1 (SOMATIC EMBRYO RECEPTOR KINASE1) gene expression were used to investigate SE in 2HA. ROS are involved in cell cycle activation in cultured plant cells (Fehér et al., 2008), while MtSERK1 expresses in 2HA cultures both when callusing and embryogenesis are initiated (Nolan et al., 2009). SERK1 gene expression is associated with SE in most species examined and marks embryogenic competence in some species, while its overexpression can enhance SE in arabidopsis (Hecht et al., 2001; Karami et al., 2009; Schmidt et al., 1997). However, it is now apparent that SERK1 expression is more generally associated with developmental change (Nolan et al., 2009).
Using M. truncatula embryogenic callus it is shown here that the pluripotent procambial cells associated with the leaf veins undergo cell proliferation and differentiate into vasculature. However, most somatic embryos derive from dedifferentiated mesophyll cells that proliferate and acquire totipotency.
MATERIALS AND METHODS
Culture of leaf explants
Cultures were established from explants obtained from leaves of glasshouse-grown Medicago truncatula ‘Jemalong’ and its highly embryogenic descendent, Jemalong 2HA (2HA; Rose et al., 1999). The standard SE protocol used the P4 medium of Thomas et al. (1990) supplemented with 10 µm naphthalene acetic acid (NAA) and 4 µm 6-benzylamino purine (BAP; P4 10:4), followed by transfer to the same medium containing 1 µm abscisic acid (ABA; P4 10:4:1) at 3 weeks (Nolan et al., 2003), unless indicated otherwise. The development of embryogenic calli (grown in darkness) was studied up to 40 d from the beginning of culture. For root development, P4 medium with 10 µm NAA was used (Nolan et al., 2003).
Protoplast isolation, purification and culture
Plants for protoplast isolation were grown in a controlled-environment at low light intensity as described by Tian and Rose (1999). Protoplast isolation was based on Rose and Nolan (1995) and Tian and Rose (1999). The youngest fully expanded leaves from 2- to 4-month-old plants were surface sterilized and cut into 2-mm squares. Approximately 0·4 g of the tissue was plasmolysed in 5 mL of P1 medium (Thomas et al., 1990) in a 5-cm Petri dish for 30–60 min, before being vacuum-infiltrated (700 mm Hg) for 2 min. The P1 medium was removed and replaced with 8 mL of filter-sterilized enzyme medium [0·5 % (w/v) cellulase RS (Yakult Pharmaceutical, Tokyo, Japan), 0·5 % (w/v) macerozyme R10 (Yakult Pharmaceutical), 0·025 % (w/v) pectolyase Y23 (Kikkoman, Tokyo, Japan), 0·45 m mannitol, 7·0 mm CaCl2·2H20, 3·0 mm MES buffer and 0·5 % (w/v) bovine serum albumen, at pH 5·5]. The osmotic pressure of the enzyme medium was 560 mOsm kg−1. The Petri dishes were incubated at 27 °C on a rotary shaker (60 rpm) for 3 h. The protoplast suspension was stored at 4 °C for 60 min before purification.
The protoplast suspension was filtered through a 40-μm nylon mesh filter into a 15-mL centrifuge tube and a 1/3 volume of 80 % (v/v) Percoll containing 0·45 m mannitol added. After mixing, 1 mL of P1 medium (with 0·45 m mannitol) was layered on top of the protoplast suspension before centrifuging at 80 g for 20 min. The protoplast band that formed at the interface of the Percoll-enzyme mixture and P1 medium was transferred to a fresh 15-mL tube, resuspended to 10 mL with P1 medium and pelleted at 100 g for 8 min. Resuspension and pelleting was repeated twice. The final pellet was resuspended in 1–2 mL of incubation medium (P1 medium containing 15 µm NAA, 4 µm BAP and 0·45 m glucose) and the protoplast concentration determined using a haemocytometer.
Protoplasts at a concentration of 8–10 × 105 mL−1 were prepared in incubation medium containing 1 % (w/v) Sea Plaque low-gelling point agarose (Cambrex, Rockland, ME, USA). Five 100-μL droplets were incubated in 2·5 mL of incubation medium in 5 mL Petri dishes as described (Rose and Nolan, 1995). The medium was replaced at 7-d intervals. The osmoticum and NAA concentrations were gradually reduced by adding increasing amounts of P4 10:4 to the incubation medium (incubation medium : P4 10:4 ratios were 2 : 1, 1 : 2, 1 : 5 and 0 : 1). At week 5, the agarose droplets were dispersed with a Pasteur pipette and the microcalli suspension distributed over P4 10:4 medium [containing 0·8 % (w/v) agar] to continue calli development.
Histology of thin sections
Tissue was fixed in 4 % (v/v) glutaraldehyde and 2 % (v/v) paraformaldehyde in 100 mm phosphate buffer (pH 7·2), before dehydration and infiltration in LR White resin as previously described (Rose et al., 2006). The LR White sectioning, staining of 1-μm sections in toluidine blue and azur II (pH 9·0), and light microscopy has also been described (Rose et al., 2006).
Histology of cleared whole-mounts
Tissue pieces were incubated in 15 % NaOH at 60 °C until cleared and then stained in basic fuchsin [1 % (w/v) in 7 % (w/v) NaOH] prior to whole mount microscopy. Basic fuchsin has an affinity for lignin (Kraus et al., 1998), and so stains xylem elements strongly (Carlsbecker et al., 2010). Fuchsin-stained lignin was viewed by bright-field microscopy, except where indicated, fluorescence imaging was performed using a 50-W Hg lamp and a rhodamine filter.
Leaf orientation experiments
Histological sections through the calli from leaf explants were outlined and the total area of the explant pseudo-coloured black, while proliferating cells associated with the vasculature were pseudo-coloured grey. A threshold was applied and the area ratio of proliferating to non-proliferating cells in 80 random samples (boxes) in each leaf explant type was measured using ImageJ (http://rsb.info.nih.gov/ij; Abramoff et al., 2004).
ROS detection by DAB
ROS were detected as hydrogen peroxide production by staining leaf explants with DAB (3,3 diaminobenzidine). DAB was prepared fresh as a 1-mg mL−1 solution in water (pH 3·8). Trifoliate leaves were fed DAB solution through their petiole for 16 h before excising explants with a 9-mm tissue punch and culturing on agar-solidified water for the indicated time. After incubation, explants were immersed in boiling 95 % (v/v) ethanol for 10 min to terminate the DAB reaction. Tissue was extracted with 100 % ethanol at 22 °C for 2 h, rinsed twice in distilled water and then cleared with Hoyer's solution (Liu and Meinke, 1998) for 1 h.
Quantification of ROS by luminescence
The luminescence assay was based on the method described by Murphy and Huerta (1990). Briefly, leaf discs (9 mm diameter), cut into four equal sectors, were incubated on water solidified with 0·8 % (w/v) agar for the designated time. The leaf discs were then placed in tubes containing 0·5 mL assay buffer (0·6 m mannitol, 10 mm MES pH 7·0, 1 mm CaCl2, 0·1 mm KCl) and immediately loaded into a luminometer (AutoLumat Plus LB953, Berthold Technologies, Germany) before adding 5 µL of 25-mm luminol and 5 µL of horseradish peroxidase (HRP) to the tube. HRP (Sigma; type VI-A) was prepared as described (Murphy and Huerta, 1990).The assay time was 2 min with measurements taken for 5 s, for a total of nine measurements. Control reactions (assay buffer, luminol and HRP) were run in parallel. Values of the final seven measurements were averaged before subtracting control averages from the leaf disc averages.
Promoter SERK1–GUS expression
Leaf explants were obtained from transgenic plants transformed with a prSERK::GUS reporter construct as described by Nolan et al. (2009). The tissue was placed in fresh GUS staining solution [50 mm sodium phosphate buffer (pH 7·0), 1 mm EDTA, 0·1 % (v/v) Triton X-100, 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, 5 mm potassium ferricyanide and 5 mm potassium ferrocyanide), before vacuum infiltrating for 2–5 min and incubating for 12–48 h at 37 °C. Tissue was cleared with diluted Hoyer's solution.
RESULTS
Development of auxin-induced roots and auxin plus cytokinin-induced embryogenic callus in leaf explants
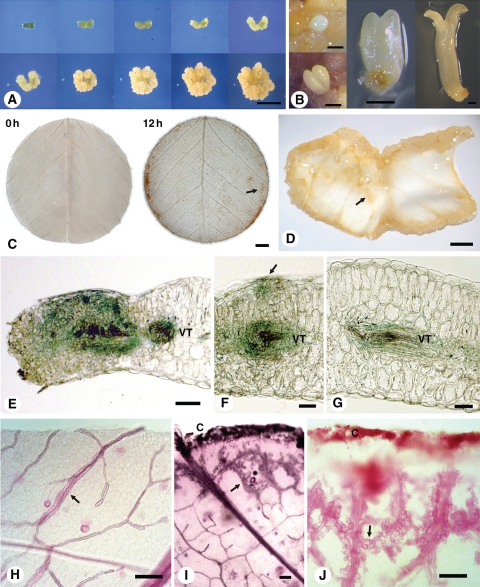
During in vitro auxin-induced root development, root primordia develop from pluripotent procambial cells associated with veins (see Supplementary Data Fig. S1, available online; Rose et al., 2006). However, the fate of procambial cells in embryogenic callus, which forms in the presence of both auxin and cytokinin, remains unclear. The time course of embryogenic callus development is shown in Fig. 1A and different embryo stages in Fig. 1B. Developmental changes during SE in leaf explants are more complex than in auxin-induced root development; nevertheless, there is a consistent pattern of development in the leaf explant. The earliest changes involve enlargement and buckling of the explant as cells expand (Fig. 1A, D) and begin to divide. Callus forms first at the cut surface and the veins (Fig. 1D), but the whole explant eventually calluses and consequently little or no original leaf explant is visible. The first somatic embryos become visible to the naked eye after about week 5 of culture. Normally these embryos progress through globular-, heart-, torpedo- and cotyledonary-stages as shown in Fig. 1B, but some exhibit varying degrees of abnormal development. Some embryos develop quickly to form small plants, while others may develop more slowly, cease development or undergo recurrent SE. However, new embryos form continuously with sustained culturing, resulting in embryogenic calli that exhibit embryos at all stages of development. Approximately 7 % of embryos convert to plants.
Fig. 1.
The M. truncatula SE culture system, early signalling and morphological changes. (A) Time course of embryogenic callus development from dark-grown leaf explants over 40 d (0, 5, 10, 15, 20, 24, 28, 32, 36 and 40 d). Explants cultured on P4 10:4 medium (auxin + cytokinin) for 3 weeks followed by transfer to P4 10:4:1 medium (auxin + cytokinin + abscisic acid). (B) Somatic embryo development showing globular, heart, torpedo and cotyledon stages. (C) Detection of ROS production using DAB staining after 0 h and 12 h of explant incubation. The arrow points to DAB staining of fine veins in the explant. (D) Three-week-old callus showing curling of the explant. The arrow shows callus formation starting at the veins. Most extensive callusing is at the cut edges. (E–G) Promoter SERK–GUS expression after culture of explant for 1 week showing GUS expression associated with (E) vascular tissue and mesophyll cells near cut surface, (F) vascular tissue and early cell proliferation (arrow) on upper surface of the explant and (G) vascular tissue. (H–J) Whole mount of cleared leaf (H) showing venation (arrow), and after 1 week of culture showing (I) cell division from vein cells nearest the cut surface (arrow) and (J) cell division associated with veins (arrow). Abbreviations: C, callus from cut edge of explant; VT, vascular tissue. Scale bars: (A) = 1 cm; (B) = 0·5 mm; (C, D) = 1 mm; (E–G) = 50 µm; (H–J) = 100 µm.
Early callus formation and associated signalling in leaf explants
One of the earliest responses in explants is the production of ROS, which occurs within seconds of explant excision (see Supplementary Data Fig. S2). DAB staining highlights that this initial production of ROS is associated with the cut surface and the veins (Fig. 1C). This is consistent with an involvement of ROS in activation of the cell cycle (Pasternak et al., 2007; Fehér et al., 2008).
MtSERK1 expression is indicative of developmental change (Nolan et al., 2009) so MtSERK1 expression was used to assess developmental changes during callus formation (Fig. 1E–G). The most extensive changes occur near the cut surface of the explant. However, changes are also associated with each of the leaf veins. This appears to involve both the onset of cell division and the differentiation of xylem elements (Fig. 1F–G). In addition, protrusions that arise from cell divisions near the uncut explant surface show MtSERK1 expression (Fig. 1F, arrow).
Changes associated with the veins of leaf explants
Changes in veins were also examined with whole-mount techniques. By clearing tissue and staining with basic fuchsin, cell division was observed to occur along the edge of the vasculature (Fig. 1H–J). Cell proliferation also occurred at the margins of the explant, giving rise to callused edges. Then, cell division associated with the veins, starting with the veins nearest the edge of the explant (Fig. 1I), moved inwards toward the explant interior (Fig. 1J), similar to the pattern of ROS production observed in DAB-stained explants (Fig. 1C).
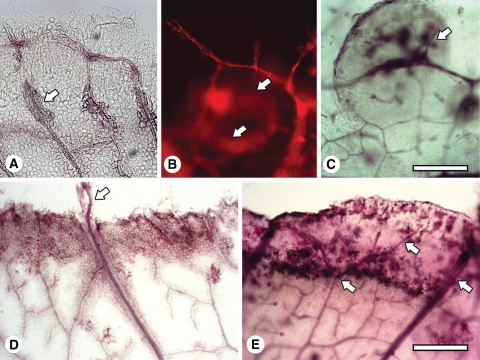
Some of the cells derived from the vascular procambium differentiated into tracheids (Fig. 2A). Such differentiation occurs not only at the termini of veins, but also in tissue lying between veins (Fig. 2B) and where ‘callus islands’ were initiated from veins (Fig. 2C). Frequently, the vascularization process linked new veins to existing veins, forming ring-like structures (Fig. 2B).
Fig. 2.
Whole mounts showing vascularization associated with early callus development. (A) Venation of a leaf explant cultured for 1 -week and the formation of tracheids at the vein (arrow). (B) A newly formed vein associated with cell proliferation after 2 weeks of culture, initiated at an existing vein and reconnecting to it, forming a circular pattern (arrows). Imaged by fluorescence microscopy. (C) Veins forming in a callus island (arrow) after 2 weeks of culture. (D) A major vein that has grown through the callus and into the medium (arrow) after 1 week of culture. (E) Veins (arrows) growing and branching in the newly formed callus at the edge of the explant after 1 week of culture. Scale bars: (A–C) = 200 µm; (D, E) = 400 µm.
Examining the developing edge of the callus revealed that the major veins continued to grow from the leaf into the callus (Fig. 2D, E), similar to the growth of veins that occurs during leaf development in planta. In some cases, major veins grew beyond the edge of the callus (Fig. 2D).
Embryo development
A surprising finding from the present studies was the substantial activity of vein procambial cells in response to auxin and cytokinin that was in addition to the obvious callusing at the explant cut edges (Fig. 1). Procambial cells within the leaf explant exhibited extensive cell division and considerable vascular tissue differentiation (Fig. 2), although there was no obvious development of somatic embryos from these regions of the tissue. Callusing at the cut surface continued concomitant with a curling up of the explant edges (Fig. 1D). It was also observed that embryos form on the surface of the explant (Fig. 3A) and along the cut edge (Fig. 3B).
Fig. 3.
The formation of somatic embryos in embryogenic callus. (A) Globular stage embryos forming on the surface of the explant originally in contact with the medium (arrows) after 26 d of culture. Note the explant has curled at the edges where there is extensive callusing. (B) Somatic embryos (arrows) developing on the top surface of the callus away from the medium after 36 d. (C) Section through 3-week embryogenic callus. Arrows show early embryo development near the surface of the explant. (D) Higher magnification of globular somatic stage embryo (arrow) developing near the surface of a 3-week-old callus. VT = vascular tissue. Scale bars: (A, B) = 1 mm; (C) = 100 µm; (D) = 25 µm.
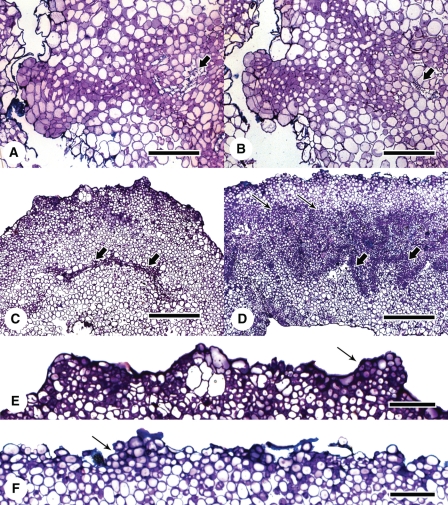
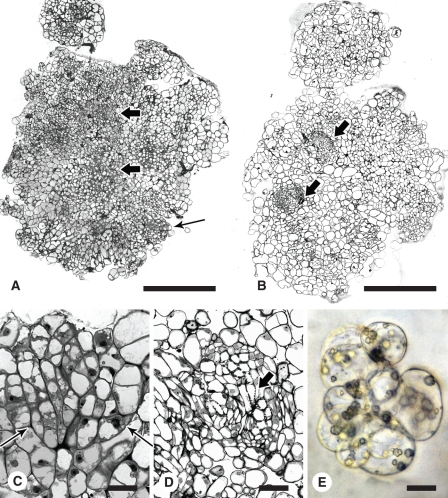
Extensive vascularization hinders investigation into the cellular origins of somatic embryo formation in sectioned material. In the standard protocol, which was developed after substantive hormonal experimentation (Nolan and Rose, 1998), ABA was used in the medium from week 3 onward to improve embryo quality. However, it has subsequently been found that inclusion of ABA in the culture medium, from the beginning of culture, increases embryo number and reduces the amount of callusing without altering the origin of the embryos. In Fig. 3C and D, SE occurs on the upper surface of the callus distal to the vasculature and is therefore likely to require dedifferentiation of mesophyll cells before somatic embryos form. The question arises, however, as to whether cells derived from the division of procambial cells can form embryos? Extensive serial sectioning (1400 1-μm sections) was required to show that only rarely did cell files from the veins reach near the surface to form a somatic embryo (Fig. 4A, B).
Fig. 4.
Investigation into the origin of the cells forming somatic embryos by incubating explants with the adaxial or abaxial surface in contact with the medium. (A, B) Serial sections showing the lineage of cells from the vascular tissue (arrow) to the somatic embryo forming on the surface of a 3-week-old callus. (C) Explant incubated with adaxial surface on the medium for 2 weeks. Arrows show little cellular proliferation or differentiation associated with the veins. (D) Explant incubated with abaxial surface on the medium for 2 weeks. Arrows show massive cell proliferation and vascularization (larger arrows) usually associated with the standard SE procedure. (E) and (F) Respective enlargements of (C) and (D) showing early embryogenesis (arrow) near the explant surface. Scale bars: (A, B) = 400 µm; (C–F) = 100 µm.
Embryo development and vein-derived cells: leaf orientation experiments
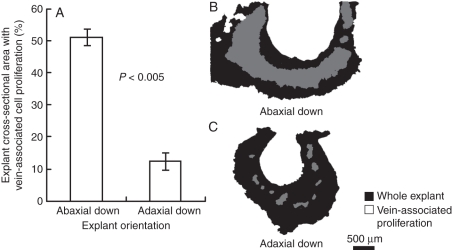
Following the results shown in Fig. 4A and B, the orientation of explants relative to the medium was reversed to obtain more information on the contribution of procambial cells to somatic embryo formation. Numbers of embryos per callus after 7 weeks of culture were similar if the explants were plated adaxial-side down (13·8 ± 1·4; mean ± s.e.), rather than abaxial-side down (15·1 ± 1·7; mean ± s.e.). Histological examination of explants after 2 weeks culture to assess early embryogenesis revealed surprising results. Major differences were found in the amount of cellular- and vein-proliferation associated with the veins originally in the explant, in sections through explants plated adaxial (Fig. 4C) or abaxial side down (Fig. 4D). If the leaf explant was incubated abaxial surface down (Fig. 4D) then, massive cellular and vascular proliferation occurred as shown previously (Figs 1I, J, 2 and 3C). However, if the leaf explant was incubated adaxial surface down (Fig. 4C) there was little cellular and vascular proliferation associated with the leaf veins. Quantifying the amount of proliferation associated with veins in both adaxially and abaxially plated explants (Fig. 5A–C) reinforced the small amount of cell proliferation associated with the vascular tissue when explants are plated adaxial-side down. It is unlikely that enhanced proliferation of procambial cells in explants plated abaxial side down is due to positioning of veins within the explant as they appear equidistant from either surface of the leaf lamina. It also seems unlikely to be due to differential auxin transport through stomata, as the number of stomata in 2HA leaves is actually higher on the adaxial surface (151 ± 0·26 versus 128 ± 0·79 stomata mm−2 in adaxial and abaxial surfaces, respectively; mean ± s.e.; n = 3). In both cases, however, embryo initials formed on the surface away from the medium (Fig. 4E, F). These observations are consistent with most of the embryos developing from dedifferentiated mesophyll cells rather than from cells whose lineage traces back to the procambium. In addition, as callus formed, the explant curled up from the plate such that cells initially in contact with the medium were now on the outside of a semi-circular to horseshoe-shaped explant. It was then embryos were observed forming. The curling of the explant as callusing progresses can be seen in Fig. 1A and D, and in Fig. 3A embryos can be seen forming on the surface of the explant originally in contact with the medium.
Fig. 5.
Quantification of vein-associated cell proliferation in leaf explants with abaxial or adaxial side in contact with the medium. (A) Adaxially plated explants exhibit significantly less cell proliferation associated with the vasculature as compared with abaxially plated explants. (B–C) Representative images of the leaf explant sections used for analysis. Student's t-test was used to assess significance, P < 0·005.
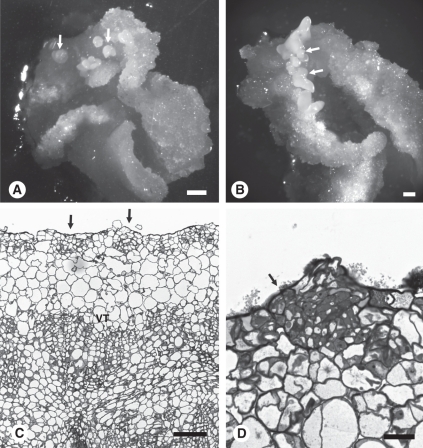
What became apparent from the present observations is the ability of mesophyll cells to dedifferentiate and reprogramme to form embryos near the surface of the explant. Procambial-like cells, on the other hand, have a capacity to differentiate in a number of directions in response to the culture environment, but rarely embryos. The formation of embryogenic callus from isolated mesophyll protoplasts is, however, strikingly different. In the protoplast experimental system (Fig. 6A–E), cell colonies develop many cytoplasmically dense cells that have the capacity to form somatic embryos. Our observations reveal that embryos do not fully develop until they approach the callus surface (Fig. 6A, C). In the meantime, pools of proembryogenic cells form, of which, some cells will initiate embryo development. At this stage, there is no vascular tissue; however, as culture continues, vasculature forms de novo. In protoplasts isolated from wild-type Jemalong (Fig. 6B, D) there is the capacity to differentiate vascular tissue but not embryos, as in leaf explants, clearly indicating the difference between Jemalong and 2HA in hormone responsiveness and the capacity for regeneration.
Fig. 6.
Culture of protoplasts from embryogenic 2HA and wild-type Jemalong. (A) Transverse section of embryogenic callus from 2HA protoplasts. Larger arrows show proembryogenic masses with densely cytoplasmic cells and smaller arrow shows embryos developing near the surface. (B) Transverse section of callus from Jemalong protoplasts showing vascular tissue but no densely cytoplasmic cells or embryos (arrows). (C) Somatic embryo (arrows) developing near the surface of callus derived from 2HA protoplasts. (D) Vascular tissue from Jemalong callus. The arrow indicates a tracheid. (E) Protoplast colony from 2HA. Scale bars: A and B = 400 µm; C = 20 µm; D = 50 µm; E = 25 µm.
DISCUSSION
The nature of callus
Plant biologists typically consider callus as a mass of undifferentiated cells that lacks a defined histological structure. This in some cases is true, as in the early stages of protoplast proliferation (Fig. 6E) before differentiation. Most studies, however, use explants taken from complex tissues of the plant, implying that the subsequently formed callus is likely to be more complex than a simple mass of undifferentiated cells. The present studies reveal that the embryogenic callus induced by auxin plus cytokinin in 2HA, develops a histological structure dependent on cells within the source tissue and explant orientation. The main developmental features from this study are discussed below.
The onset of cell proliferation
While cell proliferation in explants is always readily observed at the cut surface (Figs. 1 and 2), cell division is also initiated along the veins (Figs 1 and 2), which contain a reservoir of procambial cells. This is consistent with the location of expression of MtWUSCHEL (Chen et al., 2009) and MtSERK1 (Nolan et al., 2009), and the production of ROS, reflecting the earliest signalling events associated with the induction of callus. Cell proliferation at the cut surface predominantly arises from dedifferentiating mesophyll cells, similar to that occurring in proliferating mesophyll protoplast cultures (Thomas and Rose, 1983; Sheahan et al., 2004; Fig. 6E). Callus development in 2HA and Jemalong leaf explants is essentially identical along the way to SE (Rose and Nolan, 2006) and both seedlines show the same ROS and SERK responses in the callus before SE, likely reflecting their role in cell division induction during callus formation (Pasternak et al., 2007; Fehér et al., 2008). In terms of gene expression, expression of the ethylene-dependent transcription factor, MtSERF1 in 2HA distinguishes Jemalong from 2HA (Mantiri et al., 2008).
Vascularization of the callus
Formation of vascular tissue is associated with cell division – both at the cut edge of the explant and in proximity to veins. As the explant edge calluses, veins grow into the callus, and in some cases, beyond the callus (Fig. 2D, E). This would appear to be a highly ordered process, with wounding driving vein growth in a manner similar to normal development, via the differentiation of procambial cells (Ye, 2002). In the case of proliferating cells emanating from the procambium, there is also vascularization, albeit much less ordered. Many of the cells differentiate rapidly into tracheids forming circular-type arrangements that connect newly formed veins to the existing vasculature. Again, it appears the newly proliferating cells signal a response to maintain vascularization, though with a novel pattern of vasculature developing (Fig. 2B, C). The vascularization observations are, in principle, similar to classic experiments conducted with Coleus stems, in which the vascular elements re-join after severing (Jacobs, 1952) and with Coleus stem segments where new vascular tissue develops contiguous with pre-existing vascular tissue (Fosket and Roberts, 1964).
The development of somatic embryos from pluripotent and totipotent cells
In the 2HA culture system, two types of cells can potentially form embryos – those derived from dedifferentiated mesophyll cells and, rarely, those from procambial cells. Although explant orientation can affect SE (Thibaud- Nissen et al., 2003), in the present experiments where leaf explants were incubated with either the abaxial or adaxial surface on the medium, similar rates of embryogenesis were obtained in either orientation. Unexpectedly however, adaxially plated explants exhibited minimal cell proliferation and vascularization associated with the procambial cells. The greater number of adaxial stomata in 2HA leaves rules out enhanced hormone uptake as the mechanism for superior vascular proliferation in abaxially plated explants. Similarly, since veins appear equidistant from each surface of the leaf lamina, proximity of veins to the medium is also unlikely to influence rates of vascular proliferation in the explant orientation experiments. Auxin plays an important role in specifying adaxial–abaxial cell fate in leaves and thus differential proliferation of the vasculature may therefore reflect established patterns of polar auxin transport within the leaf (Scarpella et al., 2010). In both cases the explant surface in contact with the medium buckled upwards, and many of the initial embryos formed on this surface of the leaf that was now curled away from the medium. We hypothesize that this phenomenon may relate to an ‘auxin pulse’ type of effect, as the part of the explant in contact with the medium experiences the entire 10 µm concentration of auxin. The buckling of the explant, however, exerts a lowering of the auxin concentration, as the explant no longer contacts the source of exogenous auxin. This hypothesis is consistent with the classic paradigm where a high concentration of auxin is necessary for induction of SE, whereas embryo development requires either auxin removal or a lowering of the auxin concentration (Halperin, 1966; Dudits et al., 1991). At any point in callus development, a given somatic embryo will have a complex history; however, it alludes to the significance of cell position in the initiation of SE.
But what of the dividing cells associated with the veins? Using serial sectioning, it was found that only rarely do these cells produce embryos, and only if the veins are positioned so as lineages of these cells can reach the upper surface (Fig. 4A, B). Similarly, in protoplasts, it also appears that embryos only develop fully when they approach the surface, whereas in central regions developing embryos revert to proembryogenic masses (Fig. 6A). Given the need for appropriate auxin gradients in embryogenesis, it may be easier to obtain these near the surface of the callus (Jenik and Barton, 2005).
Callus structure and the dynamic of two cell types
The response of M. truncatula leaf explants to culture in the presence of auxin and cytokinin illustrates a dichotomy of cellular dynamics. The classic and common recognition of dedifferentiation and reprogramming is the visually dominant formation of callus from the wounded surface. This represents the enhancement of a tissue-repair process where signals rapidly established along the wounded surface facilitate a response of mesophyll cells to auxin and cytokinin. The other groups of cells are associated with the vascular procambium. In the presence of auxin alone, these procambial cells differentiate into roots (see Supplementary Data Fig. S1, available online). Division and differentiation of the vasculature from procambial cells usually requires auxin and cytokinin (Ye, 2002), and as shown in this study there is extensive vascularization in response to this hormone combination. Procambial cells also show a ROS signal (Fig. 1C) as well as MtWUSCHEL (Chen et al., 2009) and MtSERK1 (Fig. 1E–G) expression. The present results suggest that cell proliferation indicates to procambial cells that veins should continue growth to serve the expanding cell population (Fig. 2A–C). The common theme is that the proliferating cells of the leaf signal the development of venation. However, it also seems there is a ‘memory’ in that the veins continue to act as leaf veins, especially as seen in the vascularization of callus from the cut edges (Fig. 4D, E). There is some analogy here with recent experimental work on limb regeneration in salamander where many of the existing cell types continue to differentiate into the same cell type, retaining a memory of their previous identity (Kragl, 2009). With 2HA protoplasts, veins only appear after the first embryos, and in this case there can be no ‘memory’ of veins and they therefore appear de novo, possibly in response to other signals relating to the position of the cells in the enlarging callus or again, in response to signals emanating from proliferating tissues.
The capacity to form somatic embryos
Proliferating cells within calli, irrespective of their origin, sometimes form embryos. However, the majority of embryos in M. truncatula derive from the dedifferentiating mesophyll cells as shown by the present explant orientation studies and SE from mesophyll protoplast cultures (Rose and Nolan, 2005). In M. truncatula, if there is not a suitable SE genotype there is only cell division and vascularization – clearly seen in protoplasts of Jemalong (Fig. 6B, D). In the case of M. truncatula, genes associated with embryogenesis such as MtSERK1 and MtWUSCHEL are induced in both Jemalong and 2HA. This response, in the presence of the appropriate hormones (auxin and cytokinin), presumably mimics the propensity in plants for wound-induced regeneration and is particularly apparent with MtWUSCHEL expression (Chen et al., 2009). We argue that it is only those cells that have had embryogenesis de-repressed (embryogenesis has to be switched off in development) that go on to form embryos (Rose et al., 2010) and this has been selected for in the case of 2HA (Rose et al.,1999). There is also a positional effect, as the differentiating embryos need to be near the surface, possibly reflecting an appropriate auxin gradient.
Studies in carrot (Schmidt et al., 1997) and arabidopsis (Kwaaitaal and de Vries, 2007) in particular, provide support for the capacity of procambium cells to produce totipotent cell lineages that give rise to embryos. Other studies show an association of somatic embryo development with vascular tissue, e.g. in oil palm (Schwendiman et al., 1988) and Dactylis (Somleva et al., 2000). However, in M. truncatula leaf explants and mesophyll protoplast cultures, the majority of embryos develop from dedifferentiated mesophyll cells that become totipotent. Studies using chickpea (Sagare et al. 1995), pea (Loiseau et al., 1998) and potato (Sharma and Millam, 2004) explants also show that embryos develop from peripheral cells away from the vasculature and thus implicate dedifferentiation in the genesis of somatic embryos. In studying the mechanism of SE, the cellular origin of somatic embryos and the response of procambial cells are therefore relevant considerations. The key evolutionary responses ultimately linked to the capacity for SE appear to be cellular reprogramming of differentiated cells in response to stress (especially wounding) and the plasticity of procambial cells, with the dominant response dependent on species and explant type.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by an Australian Research Council Centre of Excellence Grant (CEO348212) to R.J.R. and an Australian Research Council Australian Postdoctoral Fellowship (DP0770679) to M.B.S.
LITERATURE CITED
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Braybrook SA, Harada JJ. LECS go crazy in embryo development. Trends in Plant Science. 2008;13:624–630. doi: 10.1016/j.tplants.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Carlsbecker A, Lee J-Y, Roberts CJ, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465:316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-K, Kurdyukov S, Kereszt A, Wang X-D, Gresshoff PM, Rose RJ. The association of homeobox gene expression with stem cell formation and morphogenesis in cultured. Medicago truncatula. Planta. 2009;230:827–840. doi: 10.1007/s00425-009-0988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudits D, Bögre L, Györgyey J. Molecular and cellular approaches to the analysis of plant embryo development from somatic cells in vitro. Journal of Cell Science. 1991;99:475–484. [Google Scholar]
- Fehér A, Ötvös K, Pasternak TP, Szandtner AP. The involvement of reactive oxygen species (ROS) in the cell cycle activation (G0 to G1 transition) of plant cells. Plant Signaling & Behaviour. 2008;3:823–826. doi: 10.4161/psb.3.10.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket DE, Roberts LW. Induction of wound-vessel differentiation in isolated Coleus stem segments in vitro. American Journal of Botany. 1964;51:19–25. [Google Scholar]
- Fukuda H. Signals that control plant vascular cell differentiation. Nature Reviews. 2004;5:379–391. doi: 10.1038/nrm1364. [DOI] [PubMed] [Google Scholar]
- Guzzo F, Baldan B, Mariani P, Lo Schiavo F, Terzi M. Studies on the origin of totipotent cells in explants of Daucus carota L. Journal of Experimental Botany. 1994;45:1427–1432. [Google Scholar]
- Halperin W. Alternative morphogenetic events in cell suspensions. American Journal of Botany. 1966;53:443–453. [Google Scholar]
- Hecht V, Vielle-Calzada JP, Hartog MV, et al. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiology. 2001;127:803–816. [PMC free article] [PubMed] [Google Scholar]
- Imin N, De Jong F, Mathesius U, et al. Proteome reference maps of Medicago truncatula embryogenic cell cultures generated from single protoplasts. Proteomics. 2004;4:1883–1896. doi: 10.1002/pmic.200300803. [DOI] [PubMed] [Google Scholar]
- Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG. Proteomic analysis of somatic embryogenesis in Medicago truncatula: explant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiology. 2005;137:1250–1260. doi: 10.1104/pp.104.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Goffard N, Nizamidin M, Rolfe BG. Genome-wide transcriptional analysis of super-embryogenic Medicago truncatula explant cultures. BMC Plant Biology. 2008;8:110. doi: 10.1186/1471-2229-8-110. doi:10.1186/1471-2229-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WmP. The role of auxin in differentiation of xylem around a wound. American Journal of Botany. 1952;39:301–309. [Google Scholar]
- Jenik PD, Barton MK. Surge and destroy: the role of auxin in plant embryogenesis. Development. 2005;132:3577–3585. doi: 10.1242/dev.01952. [DOI] [PubMed] [Google Scholar]
- Karami O, Aghavaisi B, Pour AM. Molecular aspects of somatic-embryogenic transition in plants. Journal of Chemical Biology. 2009;2:177–190. doi: 10.1007/s12154-009-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaaitaal MACJ, de Vries SC. The SERK1 gene is expressed in procambium and immature vascular cells. Journal of Experimental Botany. 2007;58:2887–2896. doi: 10.1093/jxb/erm103. [DOI] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Kraus JE, De Sousa HC, Rezende MH, Castro NM, Luque R. Astra blue and basic fuchsin double staining of plant materials. Biotechnic and Histochemistry. 1998;73:235–243. doi: 10.3109/10520299809141117. [DOI] [PubMed] [Google Scholar]
- Liu CM, Meinke DW. The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. The Plant Journal. 1998;16:21–31. doi: 10.1046/j.1365-313x.1998.00268.x. [DOI] [PubMed] [Google Scholar]
- Loiseau J, Michaux-Ferrière N, Le Denunff Y. Histology of somatic embryogenesis in pea. Plant Physiology and Biochemistry. 1998;36:683–687. [Google Scholar]
- Lu C-Y, Vasil IK. Histology of somatic embryogenesis in Panicum maximum (Guinea grass) American Journal of Botany. 1985;72:1908–1913. [Google Scholar]
- Mantiri FR, Kurdyukov S, Lohar DP, et al. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiology. 2008;146:1622–1636. doi: 10.1104/pp.107.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TM, Huerta AJ. Hydrogen peroxide formation in cultured rose cells in response to UV-C radiation. Physiologia Plantarum. 1990;78:247–253. [Google Scholar]
- Nolan KE, Rose RJ. Plant regeneration from cultured Medicago truncatula with particular reference to abscisic acid and light treatments. Australian Journal of Botany. 1998;46:151–160. [Google Scholar]
- Nolan KE, Irwanto RR, Rose RJ. Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiology. 2003;133:218–230. doi: 10.1104/pp.103.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KE, Kurdyukov S, Rose RJ. Expression of the SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE 1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. Journal of Experimental Botany. 2009;60:1759–1771. doi: 10.1093/jxb/erp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak TP, Ötvös K, Domoki M, Fehér A. Linked activation of cell division and oxidative stress defense in alfalfa leaf protoplast-derived cells is dependent on exogenous auxin. Plant Growth Regulation. 2007;51:109–117. [Google Scholar]
- Rose RJ, Nolan KE. Regeneration of Medicago truncatula from protoplasts isolated from kanamycin-sensitive and kanamycin-resistant plants. Plant Cell Reports. 1995;14:349–354. doi: 10.1007/BF00238595. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Nolan KE. Genetic regulation of somatic embryogenesis with particular reference to Arabidopsis thaliana and Medicago truncatula. In vitro Cellular & Developmental Biology – Plant. 2006;42:473–481. [Google Scholar]
- Rose RJ, Nolan E, Bicego L. The development of the highly regenerable seed line Jemalong 2HA for transformation of Medicago truncatula: implications for regenerability via somatic embryogenesis. Journal of Plant Physiology. 1999;155:788–791. [Google Scholar]
- Rose RJ, Wang X-D, Nolan KE, Rolfe BG. Root meristems in Medicago truncatula tissue culture arise from vascular-derived procambial-like cells in a process regulated by ethylene. Journal of Experimental Botany. 2006;57:2227–2235. doi: 10.1093/jxb/erj187. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Mantiri F.R, Kurdyukov S, et al. The developmental biology of somatic embryogenesis. In: Pua EC, Davey MR, editors. Plant developmental biology: biotechnology perspectives. Vol. 2. Berlin: Springer-Verlag; 2010. pp. 3–26. [Google Scholar]
- Sagare AP, Suhasini K, Krishnamurthy KV. Histology of somatic embryo initiation and development in chickpea (Cicer arietinum L.) Plant Science. 1995;109:87–93. [Google Scholar]
- Scarpella E, Barkoulas M, Tsiantis M. Control of leaf and vein development by auxin. Cold Spring Harbor Perspectives in Biology. 2010;2:a001511. doi: 10.1101/cshperspect.a001511. doi:10.1101/cshperspect.a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendiman J, Pannetier C, Michaux-Ferriere N. Histology of somatic embryogenesis from leaf explants of the oil palm Elais guineensis. Annals of Botany. 1988;62:43–52. [Google Scholar]
- Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Millam S. Somatic embryogenesis in Solanum tuberosum L.: a histological examination of key developmental stages. Plant Cell Reports. 2004;23:115–119. doi: 10.1007/s00299-004-0814-y. [DOI] [PubMed] [Google Scholar]
- Sheahan MB, Rose RJ, McCurdy DW. Organelle inheritance in plant cell division: the actin cytoskeleton is required for unbiased inheritance of chloroplasts, mitochondria and endoplasmic reticulum in dividing protoplasts. The Plant Journal. 2004;37:379–390. doi: 10.1046/j.1365-313x.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- Somleva MN, Schmidt EDL, de Vries SC. Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Reports. 2000;19:718–726. doi: 10.1007/s002999900169. [DOI] [PubMed] [Google Scholar]
- Steward FC, Mapes O, Smith J. Growth and organised development of cultured cells. 1. Growth and division of freely suspended cells. American Journal of Botany. 1958;45:693–703. [Google Scholar]
- Steward FC, Mapes MO, Kent AE, Holsten RD. Growth and development of cultured plant cells. Science. 1964;143:20–27. doi: 10.1126/science.143.3601.20. [DOI] [PubMed] [Google Scholar]
- Sussex IM. The scientific roots of modern biotechnology. The Plant Cell. 2008;20:1189–1198. doi: 10.1105/tpc.108.058735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud-Nissen FO, Shealy RT, Khanna A, Vodkin LO. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiology. 2003;132:118–136. doi: 10.1104/pp.103.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MR, Rose RJ. Plastid number and plastid structural changes associated with tobacco mesophyll protoplast culture and plant regeneration. Planta. 1983;158:329–338. doi: 10.1007/BF00397335. [DOI] [PubMed] [Google Scholar]
- Thomas MR, Johnson LB, White FF. Selection of interspecific somatic hybrids of Medicago by using Agrobacterium transformed tissues. Plant Science. 1990;69:189–198. [Google Scholar]
- Tian D, Rose RJ. Asymmetric somatic hybridisation between the annual legumes Medicago truncatula and Medicago scutellata. Plant Cell Reports. 1999;18:989–996. [Google Scholar]
- Vasil IK. A history of plant biotechnology: from the cell theory of Schleiden and Schwann to biotech crops. Plant Cell Reports. 2008;27:1423–1440. doi: 10.1007/s00299-008-0571-4. [DOI] [PubMed] [Google Scholar]
- Verdeil J-L, Alemanno L, Niemenak N, Tranbarger TJ. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends in Plant Science. 2007;12:245–252. doi: 10.1016/j.tplants.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Vogel G. What don't we know? How does a single somatic cell become a whole plant. Science. 2005;309(86) doi: 10.1126/science.309.5731.86. [DOI] [PubMed] [Google Scholar]
- Ye Z-H. Vascular tissue differentiation and pattern formation in plants. Annual Review of Plant Biology. 2002;53:183–202. doi: 10.1146/annurev.arplant.53.100301.135245. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ren N, Wang H, Stromberg AJ, Perry SE. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-like 15. The Plant Cell. 2009;21:2563–2577. doi: 10.1105/tpc.109.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.