Abstract
Background and Aims
The three-dimensional distributions of mineral elements in potato tubers provide insight into their mechanisms of transport and deposition. Many of these minerals are essential to a healthy human diet, and characterizing their distribution within the potato tuber will guide the effective utilization of this staple foodstuff.
Methods
The variation in mineral composition within the tuber was determined in three dimensions, after determining the orientation of the harvested tuber in the soil. The freeze-dried tuber samples were analysed for minerals using inductively coupled plasma-mass spectrometry (ICP-MS). Minerals measured included those of nutritional significance to the plant and to human consumers, such as iron, zinc, copper, calcium, magnesium, manganese, phosphorus, potassium and sulphur.
Key Results
The concentrations of most minerals were higher in the skin than in the flesh of tubers. The potato skin contained about 17 % of total tuber zinc, 34 % of calcium and 55 % of iron. On a fresh weight basis, most minerals were higher in tuber flesh at the stem end than the bud end of the tuber. Potassium, however, displayed a gradient in the opposite direction. The concentrations of phosphorus, copper and calcium decreased from the periphery towards the centre of the tuber.
Conclusions
The distribution of minerals varies greatly within the potato tuber. Low concentrations of some minerals relative to those in leaves may be due to their low mobility in phloem, whereas high concentrations in the skin may reflect direct uptake from the soil across the periderm. In tuber flesh, different minerals show distinct patterns of distribution in the tuber, several being consistent with phloem unloading in the tuber and limited onward movement. These findings have implications both for understanding directed transport of minerals in plants to stem-derived storage organs and for the dietary implications of different food preparation methods for potato tubers.
Keywords: Calcium, iron, minerals, potassium, potato, Solanum tuberosum, tuber, zinc
INTRODUCTION
Below-ground storage organs represent a reserve of carbohydrates, vitamins and mineral elements enabling subsequent plant growth, with patterns of their deposition reflecting both transport and storage processes. In major crop plants, such as potato, these organs also contribute significantly to the human diet, supplying many of the minerals required for well-being (White and Broadley, 2005, 2009). Mineral deficiencies are common in both developing and developed nations, particularly due to the consumption of staple crops with inherently low tissue mineral concentrations. Change from a diet consisting of pulses, fruits and vegetables that are rich in bioavailable minerals to a diet predominantly of refined foods with limited nutritional quality is contributing to widespread nutritional deficiencies. Low fertility of some soils where crops are grown and the poor uptake and translocation of some mineral elements to edible portions contribute to low mineral levels in several staple food crops (White and Broadley, 2009; White et al., 2009).
The bioavailability of minerals from plant tissues depends on their chemical form and the presence of promoter substances and anti-nutrients (White and Broadley, 2009). Potato tubers have high concentrations of promoter substances such as ascorbate, β-carotene, protein cysteine and various organic and amino acids that enhance the absorption of essential micronutrients (White et al., 2009). Low concentrations of anti-nutrients such as phytate (0·11–0·27 % of total dry matter; Frossard et al., 2000; Phillippy et al., 2004) and oxalate (0·03 % of total dry matter; Bushway et al., 1984) also improve the bioavailability of mineral nutrients in potato.
Understanding the processes and patterns of the accumulation of minerals in tubers is an important prerequisite to more targeted strategies to enhance the levels of desirable minerals through agronomy or breeding. Several different sampling methods have been used by researchers to investigate the distribution of minerals within potato tubers (Table 1). The concentrations of some minerals were found to be greater in the skin than in the flesh of the tuber (McGuire and Kelman, 1984; Trehan and Sharma, 1996; Wszelaki et al., 2005) and found to vary between the stem end and the bud end of the potato tuber [Macklon and De Kock, 1967 (potassium, iron and phosphorus); LeRiche et al., 2006 (phosphorus, magnesium and calcium); Johnston et al., 1968; Reeve et al., 1969; Bretzloff, 1971 (calcium and magnesium); Bretzloff and McMenamin, 1971 (calcium, magnesium and potassium); Hughes and Swain, 1962; De Kock et al., 1979; Ereifej et al., 1998 (iron and potassium); Heisler et al., 1963 (iron)] (Table 1). Despite this extensive literature, there is still a need for a comprehensive study establishing the detail of the three-dimensional distribution of nutritionally significant minerals within the potato tuber. In particular, in this study we investigated the following: (a) partitioning of minerals between skin and flesh of potato tubers and (b) distribution of minerals in the top, central and bottom portions of a potato having first determined the orientation of the tuber in the soil. The results of this study will be discussed in relation to the processes of mineral accumulation in tuber tissues.
Table 1.
Previous studies describing mineral distribution within the potato tuber
| Reference | Sampling details | Minerals analysed | f. wt/d. wt* |
|---|---|---|---|
| Arteca et al. (1980) | Longitudinal slices into three tissues: stem end, centre section and bud end | Ca, Cl and K | d. wt |
| Bretzloff and McMenamin (1971) | Six different tuber samplings: longitudinal slices, saggital slices, cross-section, paired opposite sectors, concentric zones and sub-samples of blends | Mg, Ca and K | f. wt |
| Bretzloff (1971) | Transversal slice into three tuber tissues: inner pith, outer pith and cortex | Mg and Ca | f. wt |
| Davies and Millard (1985) | Seven tissue types in a transverse section of tuber, from periderm to pith | Ca, N, Mg, P and K | f. wt |
| De Kock et al. (1979) | Core from heel to rose end into 15 pieces, periderm removed at both ends | Ca, K, Mg, phosphate, Fe, Cu and Mn | f. wt |
| Ereifej et al. (1998) | Whole tuber into four parts: bud end, stem end, vascular ring and central core | Ca, Na, Mg, P, Cu, Fe, Mn, Zn and K | d. wt |
| Hughes and Swain (1962) | Central longitudinal cores into ten sections with removal of vascular tissues at both ends | Fe and inorganic P | d. wt |
| Johnston et al. (1968) | Longitudinal central slice into five concentric sections. Each section into four segments from stem to bud end | K, Mg, Ca, Na, Fe, Mn, Zn, Cu, N, P and Cl | f. wt |
| LeRiche et al. (2006) | The central pith strip into eight equal longitudinal segments and numbered from stem to bud end | Mg, Ca and P | d. wt |
| Macklon and De Kock (1967) | Central longitudinal cores divided into 16 equal cylindrical sections | K, P, Fe, Ca, Na, Mg and Cl | f. wt |
| Shekhar and Iritani (1978) | Whole tuber into stem and bud end portions (skin removed from both ends) | Ca, Mg, P and K | d. wt |
| Wurster and Smith (1963) | Longitudinal and cross-sections (radio-iron, Fe59) | Fe | d. wt |
* Sampling on a fresh weight or dry weight basis.
MATERIALS AND METHODS
Plant material
The potato cultivar Solanum tuberosum ‘Stirling’ was used in this study. The tubers were planted at Balruddery Farm (Dundee, UK, 56 °28′51·6″N; 3 °08′2·76′'W) during April 2007. The crop was grown using standard agronomic practices and was harvested in September 2007. Five tubers of uniform size and shape were selected. The selected tubers were stored at 4 °C for 2 weeks in a cold store after harvest and were subsequently stored in an ambient store (approx. 13 °C) for 4 weeks before they were subjected to mineral analysis.
Tuber position
Inspection of a number of different cultivars growing in the field and glasshouses at SCRI confirmed that the previous orientation of harvested tubers in the field soil can be determined. Some, but not all, cultivars are slightly flattened dorso-ventrally, and most have two additional features which can be used to determine their orientation during growth in the soil. At the apical or bud end of the tuber, more buds are present on the upper (adaxial) surface than the lower surface. At the basal or stolon attachment end of the tuber (herein, the stem end) there is usually a pronounced bulge below the attachment point and none above.
Sample preparation
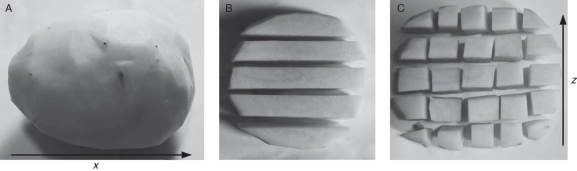
Tubers about 12 cm long were first washed, rinsed in deionized water and briefly air-dried. Five concentric skin (periderm and associated cortex) samples were carefully removed with a vegetable peeler to a depth of 0·3–0·4 mm from the apical to the stolon end of the tuber. The peeled potato tubers were then cut longitudinally into top (A), middle (B) and bottom (C) slices of equal thickness, the orientation of the tuber in the soil having previously been determined. Each of the three slices was further cut into five strips longitudinally and each of the five strips was then cut into five pieces (1–5) of equal length (Fig. 1). Fresh weights of each of the pieces were determined. The samples were then freeze-dried and weighed to determine the dry matter content. Dried samples were then powdered using a clean glass rod and stored in a freezer at –20 °C until mineral analysis.
Fig. 1.
Potato tuber sampling for mineral analysis. (A) Peeled tuber showing the bulge of the abaxial side below the stolon attachment point. (B) The central slice of three longitudinal slices, cut into five apical–basal strips. (C) Each strip divided into five pieces. The x, z co-ordinates refer to the figures that follow.
Mineral analysis
Dried and powdered samples (100 mg) were digested with 3 mL of 69 % HNO3 (Aristar grade, VWR) and 1 mL of 30 % H2O2 (Aristar grade, VWR) in a 1600 W microwave oven (MARS Xpress) with the following program: 2 min at 100 °C, 1 min at 120 °C, 2 min at 160 °C, 20 min at 180 °C and 20 min cooling time. At the end of the procedure, samples were diluted with milliQ high quality sterile water (18·2 MΩ cm conductivity) to 50 mL and kept at 4 °C prior to analysis. Two blank digestions were performed in the same way as for the samples. The concentrations of macro- and micronutrients were estimated using inductively coupled plasma-mass spectrometry (ICP-MS; PerkinElmer ELAN DRCe, Monza, Italy). The analytical technique was standardized using SRM 1573a, a certified reference material from the National Institute of Standards and Technology (NIST), Gaithersburg, USA.
Statistical analysis
GenStat, 12th edition (VSN Ltd, Oxford, UK) was used to analyse the data by analysis of variance (ANOVA) with tuber as a blocking factor. Correlation analysis for average mineral concentrations was performed using the FCORRELATION procedure in GenStat. Three-dimensional surface graphs were drawn using MS Excel 2007.
RESULTS
Dry matter content
Figure 2 and Supplementary Data Table S1a (available online) show the dry matter distribution in each of the three slices sampled for the analysis. Dry weight determination of the tuber revealed that the middle slice and, in particular, the central portion had a much lower dry matter content (around 10 %) compared with the periphery of the tuber (up to 24 % dry matter). In addition to the decrease in dry matter content from the periphery to the inner tissues, an increasing gradient from the bud to the stem end was observed in the upper and lower slices.
Fig. 2.
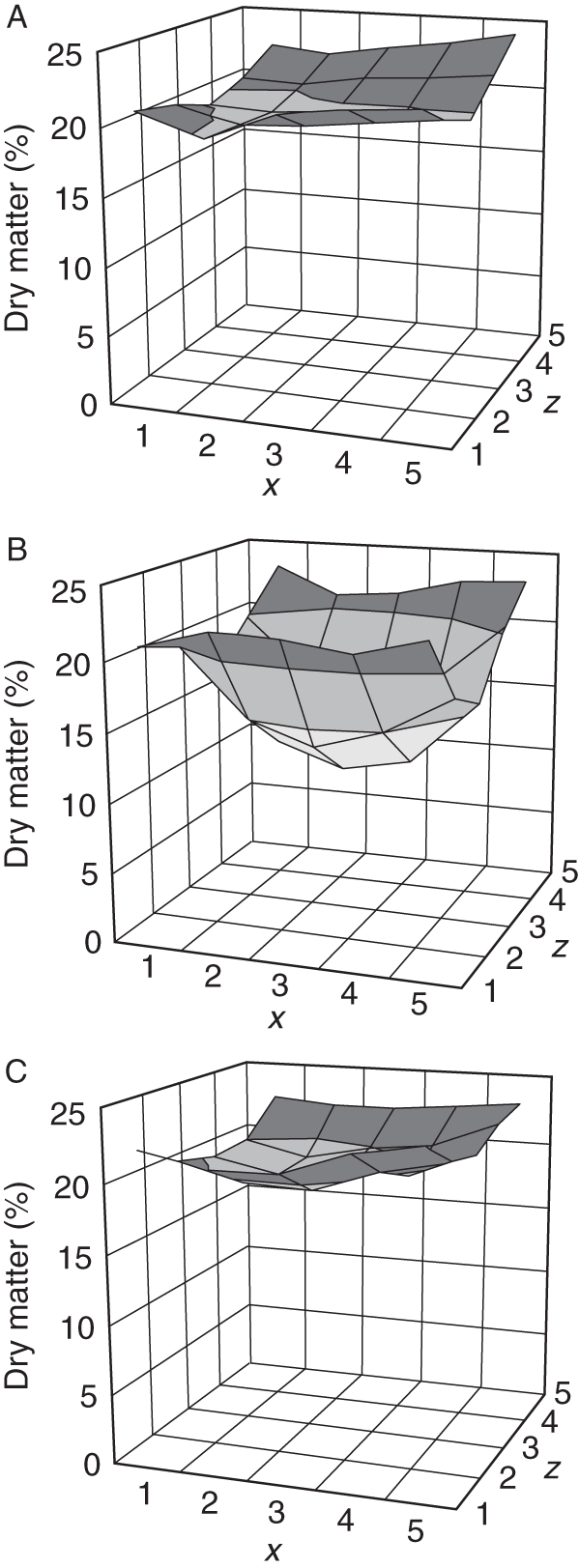
Dry matter profiles (%) of the three longitudinal slices of potato. (A) Top, (B) middle and (C) bottom. The x, z co-ordinates correspond to the pieces shown in Fig. 1.
Partitioning of minerals between tuber surface layers and flesh
Tuber surface layers were removed by peeling and analysed separately. Prior to peeling, tubers were scrubbed to remove all visible signs of soil and blotted dry, but not subjected to acid treatment to remove all traces of soil minerals bound to the surface. On a fresh weight basis, surface layers contained higher concentrations of most minerals than the flesh portion of the tubers (Table 2). Only phosphorus, sulphur and chlorine have concentrations the same or slightly higher in tuber flesh than in the peel. Three minerals of dietary significance (iron, calcium and zinc) have markedly higher concentrations in surface layers, particularly so for iron. However, the much greater mass of the tuber flesh means that for all minerals other than iron, the tuber flesh contained more reserves of minerals than the surface layers. For example, tuber peel contained 17, 34 and 55 % of the total tuber zinc, calcium and iron, respectively.
Table 2.
Partitioning of tuber minerals to flesh and surface layers
| Surface layer |
Flesh |
|||||||
|---|---|---|---|---|---|---|---|---|
| Concentration |
Amount |
Concentration |
Amount |
|||||
| Mineral | Dry basis (mg g−1) | Wet basis (mg g−1) | Per tuber (mg) | Proportion (%) | Dry basis (mg g−1) | Wet basis (mg g−1) | Per tuber (mg) | Proportion (%) |
| Mg | 1·9 | 0·3 | 5·9 | 10 | 1·0 | 0·2 | 51·0 | 90 |
| P | 3·4 | 0·5 | 10·7 | 7 | 2·8 | 0·6 | 138·5 | 93 |
| S | 1·0 | 0·2 | 3·2 | 5 | 1·1 | 0·2 | 55·5 | 95 |
| Cl | 1·0 | 0·1 | 3·0 | 15 | 0·4 | 0·1 | 17·4 | 85 |
| K | 39·3 | 5·6 | 121·8 | 10 | 22·4 | 4·4 | 1126·1 | 90 |
| Ca | 2·2 | 0·3 | 6·9 | 34 | 0·3 | 0·1 | 13·5 | 66 |
| (μg g−1) | (μg g−1) | (μg) | (%) | (μg g−1) | (μg g−1) | (μg) | (%) | |
| Zn | 32·8 | 4·6 | 101·7 | 17 | 9·8 | 1·9 | 490·0 | 83 |
| Mn | 17·5 | 2·5 | 54·4 | 17 | 5·2 | 1·0 | 258·7 | 83 |
| Fe | 307·6 | 43·4 | 953·9 | 55 | 15·5 | 3·0 | 778·1 | 45 |
| Cu | 10·2 | 1·4 | 31·6 | 13 | 4·3 | 0·8 | 217·1 | 87 |
Mean values of five tubers of S. tuberosum ‘Stirling’.
Minerals in tuber flesh
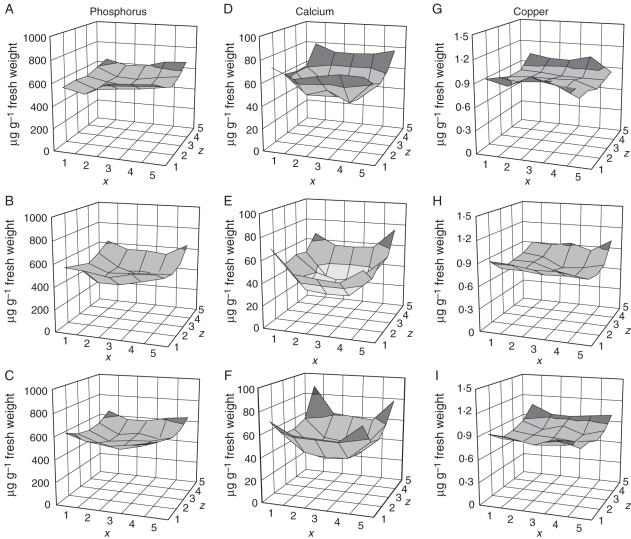
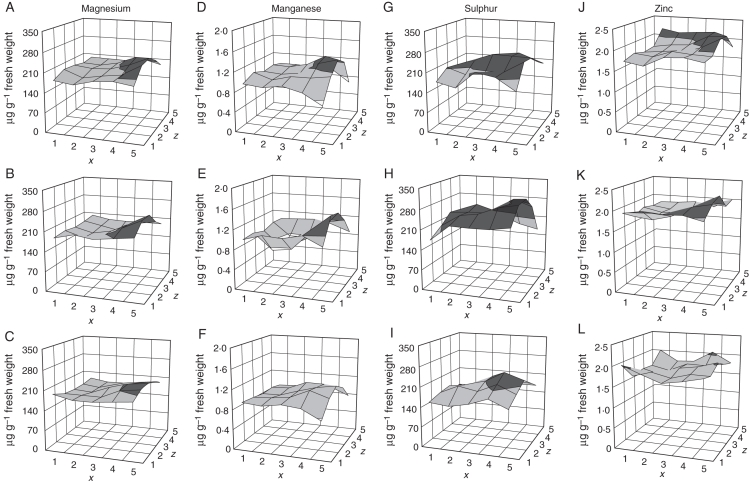
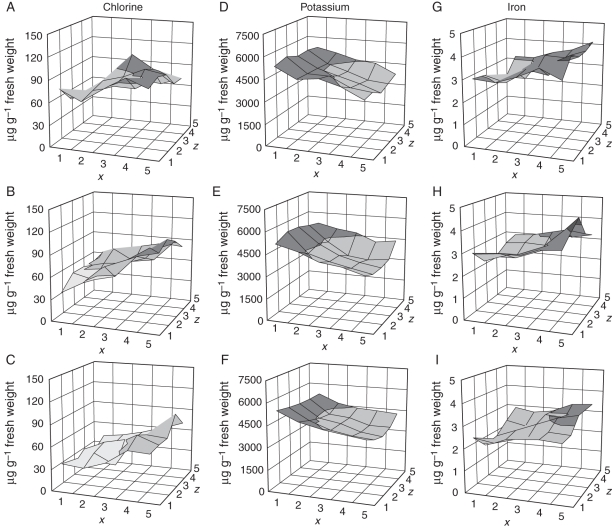
The concentrations of minerals in tuber pieces were expressed on a fresh weight basis and are presented in surface plots to show the distribution in tubers (Figs 3–5). There was an appreciable difference in mineral concentration from tuber to tuber, with a 1·2-fold range for phosphate to a 2·05-fold range for magnesium. The ranking of tubers differed for each mineral, and no one tuber had a low concentration for all minerals. Only mean values for all five tubers are presented here. Calcium, phosphorus and copper followed the same pattern, with the medulla tissue in the centre of the tuber having a much lower concentration than the more peripheral cortex (Fig. 3). However, the decreasing gradient towards the centre of the tuber was much greater for calcium than for phosphorus and copper. These distributions resemble the dry matter distributions and so may be partly explained by them, but show details inconsistent with a very tight linkage with dry matter. Potassium showed a gradual decrease from the bud to the stem end without any difference between peripheral and interior tissue, and chlorine had an opposite trend, increasing towards the stem end (Fig. 4). Like chlorine, iron showed a progressive increase from the bud end to the stem end and also was high near the stolon attachment point. The concentrations of magnesium, manganese, sulphur and zinc were found to be higher at the stem end (Fig. 5). The detail of each element was different. Manganese and zinc, for example, showed a clear dorso-ventral gradient at the stem end of the tuber, with the level rising to the upper side of the tuber. Manganese, though higher near the stolon attachment point, was distinctly low at the stem end of the tuber away from the attachment point.
Fig. 3.
Phosphorus, calcium and copper profiles of potato tubers (μg g−1 fresh weight). (A–C) Phosphorus in the top, middle and bottom slices, (D–F) calcium in the top, middle and bottom slices, (G–I) copper in the top, middle and bottom slices. The x, z co-ordinates correspond to the pieces shown in Fig. 1.
Fig. 5.
Magnesium, manganese, sulphur and zinc profiles of potato tubers (μg g−1 fresh weight). (A–C) Magnesium in the top, middle and bottom slices, (D–F) manganese in the top, middle and bottom slices, (G–I) sulphur in the top, middle and bottom slices, (J–L) zinc in the top, middle and bottom slices. The x, z co-ordinates correspond to the pieces shown in Fig. 1.
Fig. 4.
Chlorine, potassium and iron profiles of potato tubers (μg g−1 fresh weight). (A–C) Chlorine in the top, middle and bottom slices, (D–F) potassium in the top, middle and bottom slices, (G–I) iron in the top, middle and bottom slices. The x, z co-ordinates correspond to the pieces shown in Fig. 1.
Statistical analysis showed that all the three slices were significantly different from each other for every element (Supplementary Data Table S2, available online). Interactions between each of the three dimensions in the sampled tubers were particularly high for manganese, reflecting the complexity of its distribution. Significant correlations among different minerals were found (Table 3a, b). Many of the correlations in the data expressed on a dry weight basis are likely to be due simply to the trend in tissue water content. These correlations are reduced when fresh weight concentrations are examined, and the higher correlations represent pairs of elements with similar distributions in tuber flesh tissue such as calcium with phosphorus, copper with phosphorus, and magnesium with manganese.
Table 3.
Correlation coefficients (r) amongst minerals using the five-tuber mean value for each of 75 positions in the tuber
| (a) Dry weight basis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Cl | Cu | Fe | K | Mg | Mn | P | S | Zn | |
| Ca | ||||||||||
| Cl | –0·12NS | |||||||||
| Cu | 0·26* | 0·38*** | ||||||||
| Fe | –0·001NS | 0·85*** | 0·50*** | |||||||
| K | 0·22NS | 0·04NS | 0·78*** | 0·13NS | ||||||
| Mg | –0·07NS | 0·77*** | 0·60*** | 0·87*** | 0·41*** | |||||
| Mn | –0·24* | 0·68*** | 0·32** | 0·75*** | 0·20NS | 0·87*** | ||||
| P | 0·48*** | 0·42*** | 0·81*** | 0·53*** | 0·67*** | 0·69*** | 0·39*** | |||
| S | –0·22NS | 0·77*** | 0·64*** | 0·84*** | 0·46*** | 0·95*** | 0·80*** | 0·60*** | ||
| Zn | 0·02NS | 0·69*** | 0·74*** | 0·85*** | 0·51*** | 0·95*** | 0·78*** | 0·76*** | 0·93*** | |
| (b) Fresh weight basis | ||||||||||
| Ca | Cl | Cu | Fe | K | Mg | Mn | P | S | Zn | |
| Ca | ||||||||||
| Cl | –0·09NS | |||||||||
| Cu | 0·77*** | –0·12NS | ||||||||
| Fe | 0·19NS | 0·72*** | 0·07NS | |||||||
| K | 0·44*** | –0·58*** | 0·54*** | –0·57*** | ||||||
| Mg | –0·02NS | 0·63*** | –0·23* | 0·69*** | –0·50*** | |||||
| Mn | –0·18NS | 0·44*** | –0·20NS | 0·46*** | –0·32** | 0·80*** | ||||
| P | 0·90*** | –0·03NS | 0·80*** | 0·18NS | 0·39*** | 0·04NS | –0·10NS | |||
| S | –0·61*** | 0·58*** | –0·60*** | 0·43*** | –0·54*** | 0·61*** | 0·48*** | –0·59*** | ||
| Zn | 0·21NS | 0·43*** | 0·15NS | 0·68*** | –0·29** | 0·72*** | 0·55*** | 0·22* | 0·39*** | |
NS, not significant. *P < 0·05; **P < 0·01; ***P < 0·001.
DISCUSSION
In unfavourable environments, several plant species form underground carbohydrate-rich structures to enable survival and renewed vigorous growth when suitable conditions return. In the Andes, regular seasonal changes have superimposed upon them the potent periodic effects of the El Niño-Southern Oscillation, adding climate instability and probably further driving the selection of plants with starchy underground storage organs. A number of these Andean roots and tubers from a range of plant families have been domesticated by man (Hermann and Heller, 1997), and amongst them the potato has been particularly widely adopted as a staple food crop. The import of minerals into these storage structures has implications for the subsequent growth of the plant and also for man when he relies on these structures as major foodstuffs. Patterns of accumulation in potato tubers for each mineral will depend on an interacting set of factors, including the developmental anatomy of the tuber, phloem and xylem loading and unloading, movement across the periderm and mechanisms for transport and sequestration within the tuber. The patterns of mineral distribution in tubers are also likely to be dynamic during tuber storage, given that tubers mature, break dormancy and sprout during storage and prior to the next growing season. In this study we investigated the distribution of minerals during the early phase of tuber storage when it would be expected that distribution patterns are dominated by the processes that lay down reserves in the tissues.
Potato plants acquire mineral elements primarily from the soil solution through their roots (Kärenlampi and White, 2009; Karley and White, 2009; White and Broadley, 2009). The low-transpiring tuber receives minerals and other nutrients primarily through redistribution from above-ground tissues via the phloem (Baker and Moorby, 1969). The most mobile minerals in phloem tissue, found either in ionic form or as part of organic molecules and/or complexes, are magnesium, sulphur, phosphorus, chlorine and potassium, whereas zinc and copper are regarded as having an intermediate mobility in phloem (Marschner, 1995; Westermann, 2005). Marschner (1995) considered calcium, manganese and iron as less mobile, and Westermann (2005) suggested that these elements are essentially immobile in the phloem. Kärenlampi and White (2009) presented data on the leaf and mature tuber concentrations of minerals in potato. As expected for minerals with low mobility in the phloem, compared with diagnostic leaves both calcium and manganese were at very low concentrations (i.e. <3 %) in tubers. Iron did not follow this pattern, and the tuber concentration was >50 % of that of diagnostic leaves; however, in the case of this mineral, soil concentrations are particularly high and other mechanisms may exist to cause this mineral to accumulate in tubers.
Mineral concentrations in potato surface layers
The present results show that the potato surface layer has higher concentrations of minerals than tuber flesh, in agreement with Trehan and Sharma (1996), Wszelaki et al. (2005), Horiguchi and Nishihara (1981) and Sulaiman (2005). Although the peel contained higher concentrations of most minerals than did the flesh, given the relative mass its contribution to the total mineral content of the tuber was small, except for iron of which 55 % of tuber content was in the surface layers (Table 2). For minerals concentrated in the surface layers, the tuber peel to flesh ratios may be impacted by the size and shape of the tuber (Andre et al., 2007). The mineral concentration of potato surface layers may also depend on the geographic and edaphic conditions in which it is grown (Anderson et al., 1999).
Direct uptake of minerals into the mature tuber across the periderm will be limited due to the suberized nature of the periderm. However, in a developing tuber before the process of suberization, the direct uptake of minerals across the living epidermis prior to the full development of the periderm would be possible. Although there could be specific mechanisms that direct the movement of minerals from the phloem to the tuber surface layers, it is clear that the elements regarded as the most phloem mobile (magnesium, phosphorus, sulphur, chlorine and potassium) remain in the tuber flesh and are not directed to the outer layers of the tuber. Calcium and iron in particular are found in the surface layers to a much greater extent than in tuber flesh, and are present at lower concentrations than are found in leaf tissue, and so it is a reasonable assumption that they are delivered at least partially to the tuber by direct movement across the epidermis of the developing tuber, as has been demonstrated by Busse and Palta (2006). Implications of this are that these minerals may not be moved easily via the phloem to the new growing shoots on sprouting of the tuber, and also that including tuber peels in food preparation will retain and maximize the intake of beneficial minerals in humans, particularly iron.
Phosphorus
Phosphorus followed a very similar pattern of distribution to dry matter (Fig. 3), concentrated around the periphery and decreasing towards the centre, as reported by Davies and Millard (1985), and without any polarity as reported by Shekhar and Iritani (1978). In the data presented here, the decrease in phosphorus from the highest value in the periphery to the centre of the tuber flesh is around 1·9-fold, and the decrease in dry matter content is similar at around 2·1-fold. Studies by Johnston et al. (1968) and Macklon and De Kock (1967) using the central longitudinal slice of potato tubers showed an increase in phosphorus concentration from the stem to the bud end. These patterns are not present in the data presented here and may reflect the balance of internal and peripheral tissue in tubers sampled less intensively than in this study. The slight uncoupling of phosphorus and dry matter content in the data presented here gives a parabolic trend for phosphorus along the middle slice when expressed on a dry weight basis (not shown), in agreement with LeRiche et al. (2006).
Phosphorus is an important mineral element in the metabolism of the plant, playing an important role in carbohydrate biosynthesis and energy transfer reactions. It is a part of the structure of DNA, mRNA, ATP and phospholipids in membranes (White and Hammond, 2008). It is also one of the important non-carbohydrate components present in starch, which significantly affects its functional properties (Schoch, 1942). Starch in potato tubers usually comprises about 20 % amylose and 80 % amylopectin (Yook and Robyt, 2002), and the phosphate in starch is confined to the amylopectin fraction in native starch granules (Schoch and Maywald, 1956). Potato starch is more highly phosphorylated than other root and tuber starches (Hizukuri et al., 1970), and this makes potato starch unique with regard to gelatinization temperatures and cross-linking ability (Christensen and Madsen, 1996; Noda et al., 2006). The content of potato starch varies significantly according to cultivar and environmental factors (Hasse and Plate, 1996; Noda et al., 2004a, b). As starch constitutes most of the tuber dry matter, it will have a similar distribution pattern to dry matter and phosphorus. Phosphorylation of starch accounts for around 38 % of the phosphorus in a potato tuber (Quick and Li, 1976).
During plant growth, phosphate which enters the tuber is metabolized to a range of compounds, and the remainder is bound to phytate and starch (Samotus, 1965). The synthesis of phosphostarch is independent of inorganic phosphate level in the tuber, whereas the synthesis of phytin phosphate responds to the inorganic phosphate level in the tuber (Samotus 1965). On a dry weight basis, phytic acid is around 0·1–0·27 % of tuber weight (Phillippy et al., 2004), indicating that the phosphate in tuber phytate has a concentration of around 0·3–0·6 mg g−1, around 20 % of the 2·3 mg g−1 phosphorus measured in tubers in this study. In their study Phillippy et al. (2004) showed that there was a slight increase in phytic acid from the outer to the inner parts of the tuber, a distribution at variance from the phosphorus distribution noted in this study and reinforcing the likelihood that most of the tuber phosphate is associated with starch.
Calcium
Calcium is a mineral required by plants for a wide range of purposes, including signalling pathways within cells and structural roles in both the cell membranes and walls (White and Broadley, 2003). Being a divalent cation, calcium has the ability to bridge two galacturonates of pectin via carboxylate groups and, thereby, provide stability to the cell wall. It also improves membrane stability, and has an influence on resistance of potatoes to environmental stresses including heat (Palta, 1996), and microbial and nematode infection (McGuire and Kelman, 1984).
White and Broadley (2003) suggested that a large amount of calcium in plant tissues is complexed by pectin, lignin and organic acids. In potatoes, pectin constitutes about 0·1 % of the tuber dry weight (von Scheele and Svensson, 1931). Davies and Millard (1985) demonstrated that about 90 % of calcium in potato tubers is present in a water- and salt-soluble form and only a small proportion is present as insoluble components such as calcium oxalate. Bushway et al. (1984) have also shown that potatoes have low concentrations (0·03 % dry matter) of oxalate. Histochemical studies performed by Artschwager (1924) showed that the cortex of the young stolon contained the crystals of calcium oxalate and occasional crystals of basic calcium phosphate. The calcium oxalate crystals were also found in the pith cells of young stolons, but these could no longer be found as tubers matured (Artschwager, 1924).
The results presented here demonstrate that calcium concentrations are higher in the surface layers removed in peel (0·31 mg g−1 fresh weight basis, Table 2), decline to the periphery of the tuber flesh (around 0·07 mg g−1, Fig. 3) and further decrease to the central pith (around 0·02 mg g−1). The detail of this distribution pattern is consistent with earlier work describing a greater concentration in the periphery of the tuber (Johnston et al., 1968; Bretzloff, 1971; Bretzloff and McMenamin, 1971). Calcium moves with water in the xylem, and transpiration is the main driving force for calcium transport in plants (White and Broadley, 2003; Busse and Palta, 2006; Karley and White, 2009). Thus the low-transpiring tuber accumulates less calcium than the above-ground parts of the plants (Palta, 1996; Ozgen et al., 2006; Kärenlampi and White, 2009). Ereifej et al. (1998) suggested that higher concentrations of calcium were present in the vascular ring rather than the central pith, co-localized with xylem, but these authors did not investigate tissue distribution in detail. Busse and Palta (2006) used radiolabelled calcium to demonstrate that calcium does not cross the periderm of mature tubers, but Habib and Donnelly (2002) showed that calcium does diffuse across the periderm of microtubers, indicating a developmental dependency of the peridermal barrier to calcium movement. Busse and Palta (2006) did, however, observe that root uptake can contribute to tuber calcium content, but only in the vicinity of the xylem ring and only from roots attached to the same stolon as the tuber.
The calcium concentration in whole tubers often depends on the surface area to volume ratio of the tuber. A significant association was found between larger tubers and low calcium concentration, and this might be due to either the decrease in the surface area to volume ratio of large tubers, or a low accumulation potential of calcium in cultivated species which typically have large tubers (Bamberg et al., 1993).
Potassium and chlorine
Potassium plays an important role in the translocation and storage of assimilates, osmoregulation, cation–anion balance and enzyme activation (Marschner, 1995; White and Karley, 2010). Potato tubers are considered to be relatively high in potassium (White et al., 2009). Macklon and De Kock (1967) showed a positive correlation between potassium and citric acid within the tuber. In Solanum tuberosum ‘Golden Wonder’, the relative cell membrane potential at the bud end was found to be more negative in immature, mature and sprouting tubers than in the mother tuber (Macklon and De Kock, 1967). At each developmental stage of the tuber, the potassium gradient was correlated with a difference in electrical potential measured between cells at points across the tuber from stem to bud end.
In this study, a uniform decreasing gradient for potassium towards the stem end was found in all the three slices, adding detail to the previous studies of Arteca et al. (1980), Macklon and De Kock (1967), Johnston et al. (1968) and Shekhar and Iritani (1978). Nitsos and Evans (1969) first observed that potassium was required for the activity of the starch synthases and about 1·8 % potassium in tuber dry matter is needed for high starch concentrations in potatoes (Forster and Beringer, 1983; Lindhauer and De Fekete, 1990). However, the essentially planar and equal decline across the three slices suggests that potassium concentrations are not directly related to starch accumulation and that the distribution of potassium may reflect other aspects of polarity in stem-derived tissues, such as energized transport independent of transpirational water flows.
Chlorine has a number of essential biochemical functions in plants (White and Broadley, 2001). In particular, chloride regulates the activities of several cytoplasmic enzymes, provides a major osmoticum in the vacuole and acts as a counterion for cation transport (White and Broadley, 2001; Westermann, 2005). In this study chlorine had a decreasing gradient from the stem end to the bud end, in agreement with Johnston et al. (1968), and this gradient may reflect its functions as an osmotically active anion (White and Broadley, 2001; Westermann, 2005).
Other metals and sulphur
Magnesium has several important roles in plants, including as an essential component of the chlorophyll molecule (White and Broadley, 2009). Comparing magnesium and calcium, the shoot:tuber dry weight ratio of the phloem-immobile calcium is around 45, whereas magnesium has a much smaller ratio, around 5, suggesting that magnesium is translocated effectively in the phloem (Kärenlampi and White, 2009). During senescence magnesium is mobilized from the haulm and transported to the tubers (Marschner, 1995). Tuber magnesium concentration was found to decrease from the stem end to the bud end in our study and some other studies (Johnston et al., 1968; DeKock et al., 1979; Shekhar and Iritani, 1978), but a uniform distribution of magnesium in tubers was found in still other work (Bretzloff, 1971; Bretzloff and McMenamin, 1971). Small amounts of magnesium appear in the starch granules (Brautelcht and Getchell, 1951; Blennow et al., 2005).
In plants, copper, iron, manganese and zinc occur in chemical forms such as inorganic ions, inorganic metal oxides, organic acid salts and organic complexes (Broadley et al., 2007; White and Broadley, 2009). Horiguchi and Nishihara (1981) have shown that in potatoes, the major proportion of these elements was found in the soluble low molecular fraction which comprises free ions and complexes with amino acids and organic acids. High concentrations of copper, iron, manganese and zinc were found in the protein fraction, and only a small amount was found in the starch fraction (Horiguchi and Nishihara, 1981). Levitt and Todd (1952) showed that about 25 % of iron, copper and zinc in potatoes was associated with the protein fraction as metalloprotein complexes.
The distributions of iron, manganese and zinc share similarities with those of magnesium and sulphur, hinting at shared mechanisms of distribution and accumulation. All are high in the central slice of the tuber flesh and have a peak near the stolon attachment point (Fig. 5). Within this group, two minerals (magnesium and zinc) show a dorso-ventral polarity in that the mineral content is higher in the slice of the tuber which is nearer the soil surface while growing on the stolon. Among the other minerals, this higher level in the dorsal slice at the stolon end is less clear, but manganese is present at particularly low levels at the stolon end away from the attachment point. A completely different pattern is shown by copper which rises towards the periphery of the tuber flesh and is low near the stolon attachment point.
Although previous studies have described gradients in iron concentration in potato tubers, the resolution described here reveals unsuspected detail in these patterns. For example, Macklon and De Kock (1967), De Kock et al. (1979), Wurster and Smith (1963) and Heisler et al. (1963) have described a gradient of iron falling towards the apical end of the tuber. Heisler et al. (1963) demonstrated that the percentage of total iron associated with protein is higher at the stem end than the bud end, and that stem-end protein had more iron than the bud-end protein. An explanation for this distribution is that free ionic soluble iron unloads from the phloem in the tuber where the vascular tissue splays out around the swelling tissue, and then becomes bound to protein in that part of the tuber. This may also apply to other minerals moving in the phloem. The slight dorso-ventral polarity seen in the distribution of some minerals may also be a result of the asymmetric distribution of the vascular tissue at the stem end of the tuber. It is possible that the similarity in the pattern of sulphur and heavy metal deposition in tubers is related to the role of sulphur-containing proteins and peptides such as the metallothioneins and phytochelatins which bind metal ions (White and Broadley, 2009).
Copper contrasts with the distributions of iron, magnesium, manganese, sulphur and zinc in that it was high in the periphery of all the three slices. The distribution of this heavy metal is similar to that seen for phosphate and calcium, suggesting that it may move and deposit in tissues in a manner similar to one of these two elements.
Conclusions
The concentrations of all mineral elements studied, with the exception of phosphorus and sulphur, were greater in the skin than in the flesh of potato tubers of cv. ‘Stirling’. Iron was particularly concentrated in the skin of the tubers, such that 55 % of all tuber iron was found there. Calcium has patterns of distribution that suggest that it largely appears in tubers from transport across the periderm while the tissue is still in a living state and before the periderm becomes suberized. Additional routes, such as weak transpiration streams from stolon roots, are also possible. Copper may also enter tubers across the periderm. Zinc, regarded as being of intermediate phloem mobility along with copper (Marschner, 1995; Westermann, 2005), has a distribution in tubers which suggests that, unlike copper, it is delivered to the tuber by the vascular system and not transported to any extent across the periderm. Two elements, potassium and chlorine, displayed a polar gradient within the tuber, which might be generated by energized polar transport processes. Iron, magnesium, manganese and sulphur all display similar profiles, with high amounts found near the stolon attachment point. These distributions are suggestive of minerals which are unloaded into tuber cortex and become incorporated into proteins near that site. Manipulating the concentrations of these mineral elements and their distribution within the tuber to enhance the dietary value of potatoes will be facilitated by identification of the genetic basis of transport across the periderm before periderm suberization is complete, the activity of stolon roots, and particularly the efficiency of re-mobilization from aerial tissues, and the unloading from phloem and incorporation into proteins.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Christine A. Hackett (BioSS) for statistical advice, and Jacqueline Thompson (SCRI) for assistance with ICP-MS analysis. This work was supported by the Scottish Government under Programme 1: Profitable & Sustainable Agriculture – Plants. N.K.S. gratefully acknowledges the financial support from the International Office, University of Nottingham for a Research Scholarship and SCRI for an SCRI–Universities PhD Scholarship.
LITERATURE CITED
- Anderson KA, Magnuson BA, Tschirgi ML, Smith B. Determining the geographic origin of potatoes with trace metal analysis using statistical and neural network classifiers. Journal of Agricultural and Food Chemistry. 1999;47:1568–1575. doi: 10.1021/jf980677u. [DOI] [PubMed] [Google Scholar]
- Andre CM, Ghislain M, Bertin P, et al. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. Journal of Agricultural and Food Chemistry. 2007;55:366–378. doi: 10.1021/jf062740i. [DOI] [PubMed] [Google Scholar]
- Arteca RN, Poovaiah BW, Hiller LK. Electron microprobe and neutron activation analysis for the determination of elemental distribution in hollow heart potato tubers. American Potato Journal. 1980;57:241–247. [Google Scholar]
- Artschwager E. Studies on the potato tuber. Journal of Agricultural Research. 1924;27:809–835. [Google Scholar]
- Baker DA, Moorby J. The transport of sugar, water, and ions into developing potato tubers. Annals of Botany. 1969;33:729–741. [Google Scholar]
- Bamberg JB, Palta JP, Peterson LA, Martin M, Krueger AR. Screening tuber bearing Solanum (potato) species germplasm for efficient accumulation of tuber calcium. American Potato Journal. 1993;70:219–226. [Google Scholar]
- Blennow A, Sjoland AK, Andersson R, Kristiansson P. The distribution of elements in the native starch granule as studied by particle-induced X-ray emission and complementary methods. Analytical Biochemistry. 2005;347:327–329. doi: 10.1016/j.ab.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Brautelcht CA, Getchell AS. The chemical composition of white potatoes. American Potato Journal. 1951;28:531–550. [Google Scholar]
- Bretzloff CW. Calcium and magnesium distribution in potato tubers. American Potato Journal. 1971;48:97–104. [Google Scholar]
- Bretzloff CW, McMenamin J. Some aspects of potato appearance and texture. III. Sampling tubers for cation analysis. American Potato Journal. 1971;48:246–254. [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytologist. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Bushway RJ, Bureau JL, McGann DF. Determinations of organic acids in potatoes by high performance liquid chromatography. Journal of Food Science. 1984;49:75–81. [Google Scholar]
- Busse JS, Palta JP. Investigating the in vivo calcium transport path to developing potato tuber using 45Ca: a new concept in potato tuber calcium nutrition. Physiologia Plantarum. 2006;128:313–323. [Google Scholar]
- Christensen DH, Madsen MH. Changes in potato starch quality during growth. Potato Research. 1996;39:43–50. [Google Scholar]
- Davies HV, Millard P. Fractionation and distribution of calcium in sprouting and non-sprouting potato tubers. Annals of Botany. 1985;56:745–754. [Google Scholar]
- De Kock PC, Hall A, Inkson RHE. Nutrient distribution in the potato tuber in relation to soil pH. Annals of Botany. 1979;43:299–304. [Google Scholar]
- Ereifej EI, Shibli RA, Ajiouni MM, Hussein A. Mineral contents of whole tubers and selected tissues of ten potato cultivars grown in Jordan. Journal of Food Science and Technology. 1998;35:55–58. [Google Scholar]
- Forster H, Beringer H. Starch content of potato tubers in relation to potassium nutrition and tuber development. Zeitschrift fur Pflanzenernahrung und Bodenkunde. 1983;146:572–582. [Google Scholar]
- Frossard E, Bucher M, Machler F, Mozafar A, Hurrell R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. Journal of the Science of Food and Agriculture. 2000;80:861–879. [Google Scholar]
- Habib A, Donnelly DJ. Calcium translocation and accumulation into potato tubers. Potato Research. 2002;45:17–24. [Google Scholar]
- Hasse NU, Plate J. Properties of potato starch in relation to varieties and environmental factors. Starch. 1996;48:167–171. [Google Scholar]
- Heisler EG, Sicilano J, Treadway RH, Woodward CF. Aftercooking discoloration of potatoes. Iron content in relation to blackening tendency of tissue. Journal of Food Science. 1963;28(453) [Google Scholar]
- Hermann M, Heller J. Andean roots and tubers at the crossroads. In: Hermann M, Heller J, editors. Andean roots and tubers: Ahipa, arracacha, maca and yacon. Promoting the conservation and use of underutilized and neglected crops. Vol. 21. Rome, Italy: Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute (IPGRI); 1997. pp. 5–11. [Google Scholar]
- Hizukuri S, Tabata S, Nikuni Z. Studies on starch phosphate: part 1: estimation of glucose 6-phosphate residues in starch and the presence of tuber bound phosphate(s) Starch. 1970;22:338–343. [Google Scholar]
- Horiguchi T, Nishihara T. Heavy metals associated with the major constituents of potato tubers and peanut seeds. Memoirs of the Faculty of Agriculture, Kagoshima University. 1981;17:95–101. [Google Scholar]
- Hughes JC, Swain T. After-cooking blackening in potatoes. II. Core experiments. Journal of the Science of Food and Agriculture. 1962;13:229–236. [Google Scholar]
- Johnston FB, Hoffman I, Petrasovits A. Distribution of mineral constituents and dry matter in the potato tuber. American Potato Journal. 1968;45:287–292. [Google Scholar]
- Kärenlampi S, White PJ. Potato proteins, lipids and minerals. In: Singh J, editor. Advances in potato chemistry and technology. Oxford: Elsevier; 2009. pp. 99–126. [Google Scholar]
- Karley AJ, White PJ. Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Current Opinion in Plant Biology. 2009;12:291–298. doi: 10.1016/j.pbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- LeRiche EL, Wang-Pruski G, Zheljazkov VD. Mineral concentration and distribution in tubers of fertilized and unfertilized potato cultivars Shepody and Russet Burbank as determined by VP-SEM/EDS. Canadian Journal of Plant Science. 2006;86:1349–1353. [Google Scholar]
- Levitt J, Todd GW. Metal–protein complexes in the potato. Physiologia Plantarum. 1952;5:419–429. [Google Scholar]
- Lindhauer MG, De Fekete MAR. Starch synthesis in potato (Solanum tuberosum) tubers: activity of selected enzymes in dependence of potassium content in storage tissue. Plant and Soil. 1990;124:291–295. [Google Scholar]
- Macklon AES, De Kock PC. Physiological gradients in the potato tuber. Physiologia Plantarum. 1967;20:421–429. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- McGuire RG, Kelman A. Reduced severity of Erwinia soft rot in potato tubers with increased calcium content. Phytopathology. 1984;74:1250–1256. [Google Scholar]
- Nitsos RE, Evans HJ. Effects of univalent cations on the activity of particulate starch synthetase. Plant Physiology. 1969;44:1260–1266. doi: 10.1104/pp.44.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Tsuda S, Mori M, et al. Properties of starches from several potato varieties grown in Hokkaido. Journal of Applied Glycoscience (Oyo Toshitsu Kagaku) 2004a;51:241–246. [Google Scholar]
- Noda T, Tsuda S, Mori M, et al. The effect of harvest dates on the starch properties in various potato cultivars. Food Chemistry. 2004b;86:119–125. [Google Scholar]
- Noda T, Tsuda S, Mori M, et al. Determination of the phosphorus content in potato starch using an energy-dispersive X-ray fluorescence method. Food Chemistry. 2006;95:632–637. [Google Scholar]
- Ozgen S, Karlsson BH, Palta JE. Response of potatoes (cv Russet Burbank) to supplemental calcium applications under field conditions: tuber calcium, yield, and incidence of internal brown spot. American Journal of Potato Research. 2006;83:195–204. [Google Scholar]
- Palta JP. Role of calcium in plant responses to stresses: linking basic research to the solution of practical problems. HortScience. 1996;31:51–57. [Google Scholar]
- Phillippy BQ, Lin M, Rasco B. Analysis of phytate in raw and cooked potatoes. Journal of Food Composition and Analysis. 2004;17:217–226. [Google Scholar]
- Quick WA, Li PH. Phosphorus balance in potato tubers. Potato Research. 1976;19:305–312. [Google Scholar]
- Reeve RM, Hautala E, Weaver ML. Anatomy and compositional variation within potatoes. I. Developmental histology of the tuber. American Potato Journal. 1969;46:361–373. [Google Scholar]
- Samotus B. Role of phytic acid in potato tuber. Nature. 1965;206:1372–1373. [Google Scholar]
- von Scheele C, Svensson G. Uber polarimetrische Bestimmung der Kartoffelstarke sowie uber den Zusammenhang zwischen dem Trochkensubstanz und Starkegehalt der Kartoffeln. Lanuw. Versuchs. Sta. 1931;112:1–43. [Google Scholar]
- Schoch TJ. Non-carbohydrate substances in the cereal starches. Journal of the American Chemical Society. 1942;64:2954–2956. [Google Scholar]
- Schoch TJ, Maywald EC. Microscopic examination of modified starches. Analytical Chemistry. 1956;28:382–387. [Google Scholar]
- Shekhar VC, Iritani WM. Starch to sugar interconversion in Solanum tuberosum L. I. Influence of inorganic ions. American Journal of Potato Research. 1978;55:345–350. [Google Scholar]
- Sulaiman MI. Effect of calcium fertilization on the quality of potato tubers (Solanum tuberosum L.) cv. Saturna. Germany: Georg-August-Universitat Gottingen; 2005. Doctoral Thesis. [Google Scholar]
- Trehan SP, Sharma RC. Mineral nutrient composition in peels and flesh of tubers of potato genotypes. Journal of the Indian Potato Association. 1996;23:139–143. [Google Scholar]
- Westermann DT. Nutritional requirements of potatoes. American Journal of Potato Research. 2005;82:301–307. [Google Scholar]
- White PJ, Broadley MR. Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany. 2001;88:967–988. [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Annals of Botany. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortifying crops with essential mineral elements. Trends in Plant Science. 2005;10:586–593. doi: 10.1016/j.tplants.2005.10.001. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- White PJ, Hammond JP. Phosphorus nutrition of terrestrial plants. In: White PJ, Hammond JP, editors. The ecophysiology of plant–phosphorus interactions. Dordrecht: Springer; 2008. pp. 51–81. [Google Scholar]
- White PJ, Karley AJ. Potassium. In: Hell R, Mendel R-R, editors. Plant Cell Monographs 17, Cell biology of metals and nutrients. Dordrecht: Springer; 2010. pp. 199–224. [Google Scholar]
- White PJ, Bradshaw JE, Dale FB, Ramsay G, Hammond JP, Broadley MR. Relationships between yield and mineral concentrations in potato tubers. HortScience. 2009;44:6–11. [Google Scholar]
- Wszelaki AL, Delwiche JF, Walker SD, Liggett RE, Scheerens JC, Kleinhenz MD. Sensory quality and mineral and glycoalkaloid concentrations in organically and conventionally grown redskin potatoes (Solanum tuberosum) Journal of the Science of Food and Agriculture. 2005;85:720–726. [Google Scholar]
- Wurster RT, Smith O. Potato quality XVIII: the distribution of radioiron in the potato tuber and its significance in after-cooking darkening. American Journal of Potato Research. 1963;40:415–420. [Google Scholar]
- Yook C, Robyt JF. Reactions of alpha amylases with starch granules in aqueous suspension giving products in solution and in a minimum amount of water giving products inside the granule. Carbohydrate Research. 2002;337:1113–1117. doi: 10.1016/s0008-6215(02)00107-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.