Abstract
Background and Aims
Transfer cells are plant cells specialized in apoplast/symplast transport and characterized by a distinctive wall labyrinth apparatus. The molecular architecture and biochemistry of the labyrinth apparatus are poorly known. The leaf lamina in the aquatic angiosperm Elodea canadensis consists of only two cell layers, with the abaxial cells developing as transfer cells. The present study investigated biochemical properties of wall ingrowths and associated plasmalemma in these cells.
Methods
Leaves of Elodea were examined by light and electron microscopy and ATPase activity was localized cytochemically. Immunogold electron microscopy was employed to localize carbohydrate epitopes associated with major cell wall polysaccharides and glycoproteins.
Key Results
The plasmalemma associated with the wall labyrinth is strongly enriched in light-dependent ATPase activity. The wall ingrowths and an underlying wall layer share an LM11 epitope probably associated with glucuronoarabinoxylan and a CCRC-M7 epitope typically associated with rhamnogalacturonan I. No labelling was observed with LM10, an antibody that recognizes low-substituted and unsubstituted xylan, a polysaccharide consistently associated with secondary cell walls. The JIM5 and JIM7 epitopes, associated with homogalacturonan with different degrees of methylation, appear to be absent in the wall labyrinth but present in the rest of cell walls.
Conclusions
The wall labyrinth apparatus of leaf transfer cells in Elodea is a specialized structure with distinctive biochemical properties. The high level of light-dependent ATPase activity in the plasmalemma lining the wall labyrinth is consistent with a formerly suggested role of leaf transfer cells in enhancing inorganic carbon inflow. The wall labyrinth is a part of the primary cell wall. The discovery that the wall ingrowths in Elodea have an antibody-binding pattern divergent, in part, from that of the rest of cell wall suggests that their carbohydrate composition is modulated in relation to transfer cell functioning.
Keywords: Aquatic plants, ATPase activity, cell walls, Elodea, immunogold labelling, transfer cells
INTRODUCTION
Transfer cells are specialized plant cells functioning in solute transport across the apoplast/symplast interface (Pate and Gunning, 1972; Gunning and Pate, 1974; Offler et al., 2002). Ubiquitous from liverworts to angiosperms, transfer cells probably evolved ancestrally in land plants as a means to enhance nutrient translocation at the interface between the sporophyte and parental gametophyte (Ligrone et al., 1993); in angiosperms, transfer cells have been recruited in numerous other histological locations and participate in a diversity of processes including phloem loading, secretion and nutrient uptake besides inter-generational nutrient relationships (Offler et al., 2002).
Transfer cells are not a specific cell type but rather a pattern of structural and physiological specialization that is expressed in different tissues either constitutively or in response to external signals. The most distinctive feature of transfer cells is a wall labyrinth apparatus, consisting of cell wall ingrowths and the outlining plasmalemma. It is generally maintained that this apparatus enhances polarized solute transport because of local amplification of active plasmalemma surface areas, but the details of the underlying processes, notably the nature and distribution of membrane transporters and the possible role of the wall matrix, are still largely unknown (Offler et al., 2002).
The wall labyrinth of transfer cells is referred to as a secondary cell wall, essentially because it develops as an addition to the primary wall following the completion of cell enlargement (Offler et al., 2002). Current knowledge of the chemical composition of the wall labyrinth in transfer cells is still extremely scanty. Early histo- and cytochemical studies show that the wall ingrowths contain cellulose and acid polysaccharides (Dashek et al., 1971; Gunning and Pate, 1974). No quantitative difference, however, was detected in cell-wall composition between a maize endosperm cell line displaying transfer-cell morphology and a control cell line (DeWitt et al., 1999). Immunogold cytochemistry, a technique potentially capable of providing detailed information on cell wall topochemistry (Knox, 2008), has so far been applied to vascular transfer cells of Pisum sativum root nodules (Dahiya and Brewin, 2000) and epidermal transfer cells of Vicia faba cotyledons (Vaughn et al., 2007). In both studies the wall ingrowths displayed much the same reactivity as the associated primary cell wall except for the presence of areas positive for antibodies against callose.
In the present study, biochemical properties of the wall labyrinth apparatus of epidermal transfer cells in the leaves of the aquatic monocot Elodea canadensis have been investigated using a cytochemical procedure for ATPase localization and a battery of monoclonal antibodies against carbohydrate epitopes, in part coincident with those tested in previous immunocytochemical studies. The differentiation of transfer cells at the water/plant interface is a widespread feature of freshwater angiosperms and is interpreted as an adaptation enhancing nutrient uptake and inorganic carbon inflow in growth-limiting environmental conditions (Rascio et al., 1994, 1999, and references therein). The leaves of Elodea and other aquatic angiosperms constitutively form transfer cells that are directly accessible to experimental manipulation and therefore appear to be a favourable alternative model system for investigating transfer cell properties.
MATERIALS AND METHODS
Samples of Elodea canadensis (Hydrocharitaceae, Liliopsida) were collected in spring from a population growing in a pond in the Botanical Gardens of Padua (Italy).
For standard light and electron microscopy, mature leaves were fixed overnight at 4 °C in 3 % (v/v) glutaraldehyde in 0·1 m Na-cacodylate buffer, pH 6·9, post-fixed with 1 % (w/v) osmium tetroxide in the same buffer for 2 h at room temperature, dehydrated in a graded series of ethanol and propylene oxide, and embedded in Araldite. For electron microscopy, thin sections (80 nm) cut with a diamond knife were collected on 200-mesh uncoated copper grids, stained with uranyl acetate and lead citrate and observed with a TEM300 transmission electron microscope (Hitachi, Japan). For light microscopy, 0·5-μm-thick sections were cut with a diamond histo-knife, mounted on glass slides coated with BioBond (EMS, USA) to enhance section adhesion, stained with 0·5 % (w/v) toluidine blue in 0·5 % (w/v) Na-tetraborate and photographed with a Zeiss Axioskop light microscope equipped with a Sensicam QE (Applied Scientific Instrumentations, USA) digital photocamera.
For ATPase cytochemical localization, the procedure described in detail by Price and Whitecross (1983) was followed. In brief, whole leaves of Elodea were incubated in a reaction medium containing 2 mm ATP, 2 mm Pb(NO3)2, 2 mm Mg(NO3)2 and 5 mm KHCO3 in 0·1 m Tris maleate buffer (pH 7·2), for 2 h at 20 °C under a light source of about 1400 µmol photons m2 s−1 PAR. As for other aquatic plants (Price and Whitecross, 1983; Rascio et al., 1999), the presence of light was essential for ATPase activity. Control samples were incubated in light in a medium lacking ATP or in the dark in complete medium. After incubation, the leaf pieces were briefly rinsed with distilled water and prepared for electron microscopy as described above. The ATPase activity was demonstrated by the formation of electron-dense deposits of leaf phosphate, due to the lead ion (trap ion) reaction with phosphate ions liberated during enzymatic hydrolysis of ATP.
For immunogold electron microscopy, the procedure described in detail in Vaughn et al. (2007) was followed. Briefly, leaves fixed only with glutaraldehyde and embedded in LR White resin were cut with a diamond knife at 100 nm and the sections mounted on 600-mesh uncoated nickel grids. The grids were treated as follows: 1 % (w/v) bovine serum albumin (BSA) in phosphate-buffered saline (PBS), 30 min; primary monoclonal antibodies diluted 1 : 20 (v/v) with PBS-BSA, 4 h; three rinses with PBS–BSA; gold-conjugated goat-anti-rabbit secondary antibody (BB International, UK) diluted 1 : 20 (v/v) in PBS–BSA, 30 min; three rinses with PBS. The grids were then stained for 2 min in 2 % (w/v) aqueous uranyl acetate and 30 s in Reynold's lead citrate. Negative controls were routinely made by omitting the incubation step with the primary antibody. The monoclonal antibodies tested in this study, their specificities and sources are reported in Table 1. For each antibody, three separate tests were run, each on serial sections from a different block, and 10–14 pictures were produced for each test. The number of gold particles per square micrometre was determined using the gravimetrical method described by Vaughn et al. (2007). The length of the plasmalemma and cuticle was evaluated using a manual map reader.
Table 1.
Monoclonal antibodies utilized for immunocytochemical characterization of cell walls in Elodea leaves
| Antibody | Epitope/target antigen | Reference/source |
|---|---|---|
| LM1 | Undefined/hydroxyproline-rich glycoproteins | Smallwood et al., 1995/J. P. Knox* |
| LM2 | β-Glucuronyl residues/arabinogalactan proteins | Smallwood et al., 1996/J. P. Knox* |
| LM5 | Tetra-(1 → 4)-β-galactan/galactan, rhamnogalacturonan-I | Jones et al., 1997/J. P. Knox* |
| LM6 | Penta-(1 → 5)-α-arabinan/arabinan, rhamnogalacturonan-I | Willats et al., 1998/J. P. Knox* |
| LM8 | Undefined/xylogalacturonan | Willats et al., 2004/J. P. Knox* |
| LM10 | Undefined/unsubstituted and low-substituted (1 → 4)-β-xylan | McCartney et al., 2005/J. P. Knox* |
| LM11 | Undefined/(1 → 4)-β-xylan substituted by arabinose and glucuronic acid | McCartney et al., 2005/J. P. Knox* |
| JIM5 | Undefined/low- or non-methyl-esterified (1 → 4)-α-galacturonan | Willats et al., 2000/J. P. Knox* |
| JIM7 | Undefined/partially methyl-esterified (1 → 4)-α-galacturonan | Willats et al., 2000/J. P. Knox* |
| CCRC-M1 | Fucosylated side group/xyloglucan, rhamnogalacturonan-I | Puhlmann et al., 1994/M. Hahn† |
| CCRC-M2 | Undefined/rhamnogalacturonan-I | Puhlmann et al., 1994/M. Hahn† |
| CCRC-M7 | Arabinosylated (1 → 6)-β-galactan/arabinogalactanproteins, rhamnogalacturonan-I | Puhlmann et al., 1994; Steffan et al., 1995/M. Hahn† |
*Centre for Plant Sciences, University of Leeds, UK (http://www.plantprobes.net/).
† Complex Carbohydrate Research Center, University of Georgia, USA (http://www.ccrc.uga.edu/~carbosource/CSS_home.html).
RESULTS
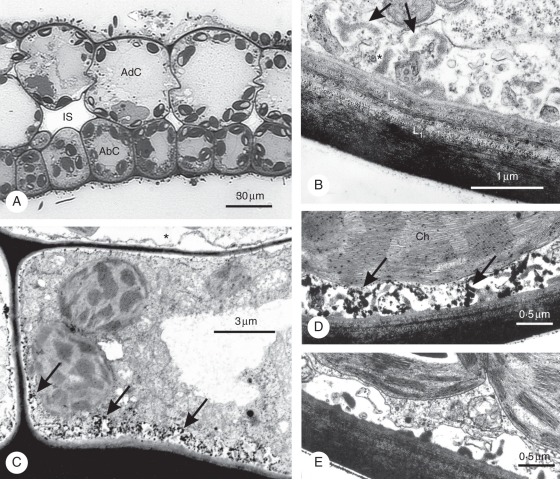
As already described in a former study (Rascio et al., 1994), the leaf lamina in Elodea consists of only an adaxial (upper) and abaxial (lower) cell layer, with small intercellular spaces between them (Fig. 1). Both cell types contain numerous well-differentiated chloroplasts, but the adaxial cells are much larger and more vacuolate than the abaxial ones. The periclinal walls of abaxial cells are particularly thick and, in toluidine-blue-stained sections, they display a bilayered structure, with a densely stained outer layer and a less dense inner layer (Fig. 1A). In sections prepared for electron microscopy, the periclinal walls appear to consist of an outer layer (henceforth referred to as the L1 layer), externally covered by a thin cuticle, and an inner layer (henceforth referred to as the L2 layer) (Fig. 1B). Both cell wall layers display a distinct multilamellar texture but the L1 layer is more heterogeneous and generally more opaque to electrons than the L2 layer. Associated with the inner layer is a a typical labyrinthine apparatus of the reticulate type (Offler et al., 2002), consisting of relatively small wall ingrowths with an amorphous fibrillar core and a electron-transparent outer area outlined by the plasmalemma (Fig. 1B). The L2 layer, but not L1, extends to form the bulk of anticlinal and inner periclinal walls of abaxial cells. The periclinal cell walls in adaxial cells exhibit a similar bilayered structure as in abaxial cells, but they are thinner and never form a wall labyrinth.
Fig. 1.
Leaf anatomy and ATPase cytochemistry in Elodea. (A) Light microscopy of leaf lamina showing adaxial (AdC) and abaxial cells (AbC) and small intercellular spaces (IS). Note the bilayered structure of outer periclinal walls in abaxial cells. (B–E) Electron microscopy of abaxial cells. (B) Detail of outer periclinal wall, showing the L1 and L2 layer and wall ingrowths consisting of a fibrillar core (arrows) and an electron-transparent area (asterisks). (C–E) Cytochemical localization of ATPase. (C and D) Massive lead precipitates mark the plasmalemma in the wall labyrinth area (arrows). Less abundant precipitates are visible on the plasmalemma along the rest of the walls. Note the virtual absence of reaction in the adaxial cell on the top (asterisk). Scattered lead precipitates are also visible along the thylakoid membrane in chloroplasts (Ch). (E) Control from a sample incubated in a medium lacking ATP, showing absence of reaction.
After incubation in a medium containing ATP and lead salts and in the presence of light, a strong reaction was observed at the level of plasmalemma areas associated with the wall labyrinth (Fig. 1C, D). A weaker reaction was also visible on plasmalemma areas facing the inner, non-labyrinthine walls in abaxial cells, whereas virtually no reaction was detected in adaxial cells (Fig. 1C). Some reaction, probably due to CF1-ATPase activity, was observed on thylakoid membranes in chloroplasts (Fig. 1D). No reaction was detected in controls incubated in a medium lacking ATP (Fig. 1E) and a very low reaction in those incubated in complete medium in the dark (not shown).
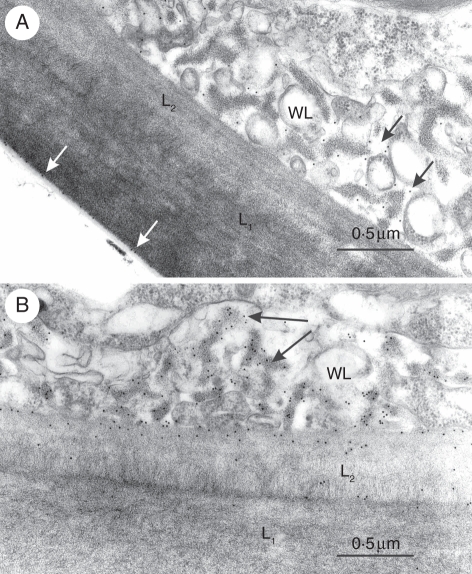
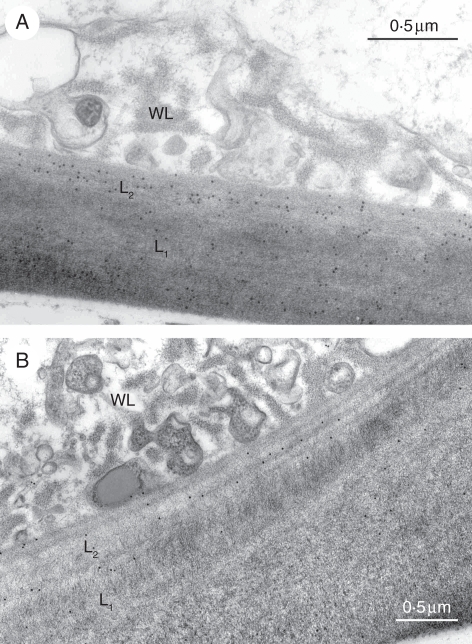
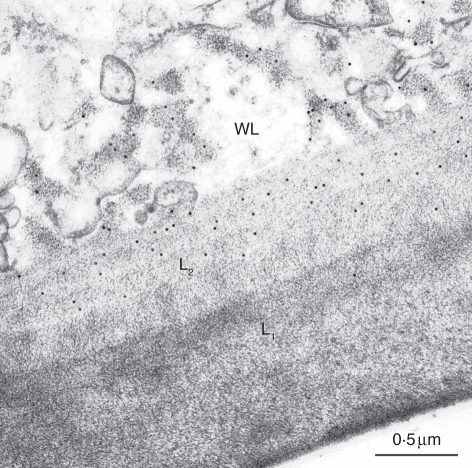
Of the 12 monoclonal antibodies tested, only five gave a positive reaction (Table 2). LM1, an antibody that binds to hydroxyproline-rich glycoproteins, labelled the electron-transparent area of wall ingrowths and the outlining plasmalemma (Fig. 2A). This antibody also specifically labelled the interface between the cuticle and the L1 layer in both abaxial and adaxial cells (Fig. 2A). Of the two antibodies against (1 → 4)-β-xylan, LM10 produced no labelling whereas LM11 strongly labelled the fibrillar core of wall ingrowhts (Fig. 2B). LM11 also labelled the L2 layer in both periclinal and inner cell walls. JIM5 and JIM7, two antibodies against epitopes associated with pectins with diverse degrees of methylation, labelled both the L2 layer and less intensely the L1 layer, but neither of them bound to the wall ingrowths (Fig. 3A, B). CCRC-M7 specifically labelled the fibrillar core of wall ingrowths and the L2 layer (Fig. 4).
Table 2.
Results of immunogold electron microscopy of cell walls in Elodea leaves
| Antibody | Wall ingrowths in abaxial (transfer) cells | Rest of cell walls in abaxial (transfer) cells | Cell walls in adaxial cells |
|---|---|---|---|
| LM1 | Electron-transparent area (14 ± 4) | Wall/cuticle interface in periclinal outer walls (8 ± 3) | Wall/cuticle interface in periclinal outer walls (8 ± 3) |
| Associated plasmalemma (8 ± 3) | |||
| LM2 | − | − | − |
| LM5 | − | − | − |
| LM6 | − | − | − |
| LM8 | − | − | − |
| LM10 | − | − | − |
| LM11 | Electron-opaque area (50 ± 12) | L2 layer (24 ± 9) | L2 layer (22 ± 7) |
| JIM5 | − | L2 layer (28 ± 9) | L2 layer (26 ± 7) |
| L1 layer (26 ± 4) | L1 layer (18 ± 4) | ||
| JIM7 | − | L2 layer (22 ± 6) | L2 layer (20 ± 5) |
| L1 layer (11 ± 3) | L1 layer (12 ± 4) | ||
| CCRC-M1 | − | − | − |
| CCRC-M2 | − | − | − |
| CCRC-M7 | Electron-opaque area(25 ± 8) | L2 layer (28 ± 8) | L2 layer (27 ± 7) |
The average numbers of gold particles per square micrometre are given in parenthesis. The density of labelling of the wall/cuticle interface and plasmalemma/wall ingrowth interface by LM1 is expressed on a linear micrometre basis. The values are means ± s.d. –, no labelling.
Fig. 2.
Immunogold labelling of outer periclinal walls in leaf abaxial cells of Elodea. (A) LM1 labelling of the electron-transparent area of wall labyrinth (black arrows) and the interface between the L1 layer and cuticle (white arrows). (B) LM11 labelling of the electron-opaque area of wall labyrinth (arrows) and of L2 layer. WL, Wall labyrinth.
Fig. 3.
Immunogold labelling of outer periclinal walls in leaf abaxial cells of Elodea: (A) JIM5 and (B) JIM7 labelling of L1 and L2 layers. Note absence of labelling on wall labyrinth (WL). The numbers on bars are micrometers.
Fig. 4.
Immunogold labelling of outer periclinal walls in leaf abaxial cells of Elodea by CCRC-M7, showing antibody binding to the electron-opaque area of the wall labyrinth (WL) and the L2 layer.
DISCUSSION
The results of the cytochemical test performed in this study reveal that the plasmalemma area associated with the wall labyrinth in leaf transfer cells of Elodea is highly enriched with light-dependent ATPase activity. Similar results have been reported for epidermal transfer cells in Ranunculus trichophyllus (Rascio et al., 1999) and charasomes in Chara corallina (Price and Whitecross, 1983). The ATPase activity demostrated in Elodea transfer cells in the present study most likely coincides with the light-dependent proton extrusion activity reported by earlier studies in the same species (Elzenga and Prins, 1989; Marré et al., 1989). It has been suggested that the resulting acidification of the wall labyrinth might enhance CO2 inflow to leaf cells by promoting pH-dependent conversion of HCO3− to CO2 in the periplasmic space (Rascio et al., 1999, and references therein). Known precedents for transporters preferentially associated with the plasmalemma in the wall labyrinth area are of an H+-ATPase, a sucrose-binding protein and a H+-sucrose symporter in cotyledonary transfer cells of Vicia faba (Harrington et al., 1997) and Pisum sativum (Tegeder et al., 1999).
In line with cytochemical tests, the immunogold labelling experiments demonstrate that the wall labyrinth in Elodea transfer cells presents distinctive traits relative to the rest of the cell walls. The lack of reactivity of wall ingrowths to both JIM5 and JIM7 suggests that these lack non-methylated and low-methylated homogalacturonan. In contrast, both the L1 and L2 layers bind JIM5 and JIM7. Since JIM5 recognizes both methyl-esterified and un-esterified galacturonan whereas JIM7 requires a certain degree of methylation (Willats et al., 2000), the labelling pattern observed indicates that the homogalacturonan in the L1 and L2 layers is, at least in part, in the methyl-esterified form. In contrast to Elodea transfer cells, the wall ingrowths and associated primary wall in Pisum nodule transfer cells were positive to JIM7 (Dahiya and Brewin, 2000). Likewise, the wall ingrowth inner core and associated cell wall in cotyledonary transfer cells of Vicia were strongly positive to JIM7 (Vaughn et al., 2007). Neither in Pisum nor in Vicia transfer cells were the wall ingrowths labelled by JIM5, suggesting that galacturonan was present here only in the methyl-esterified form.
LM11 strongly labelled the fibrillar core of wall ingrowths in Elodea, indicating the presence of xylan-associated epitopes. LM11 also bound to the L2 layer, though with a lower intensity than in wall ingrowths, whereas no labelling was observed in the L1 layer with this antibody (Table 2). The high level of xylan-associated epitope and the lack of JIM5 and JIM7 epitopes, typically abundant in primary cell walls but absent or scarce in secondary cell walls (O'Neill and York, 2003; McCann and Carpita, 2008; Knox, 2008), might be taken as an indication that the wall ingrowths in Elodea are a secondary cell wall Nevertheless, the absence of labelling with LM10, an antibody that binds to un-substituted or low-substituted xylan (McCartney et al., 2005) and consistently labels secondary cell walls in higher plants (Carafa et al., 2005), suggests that LM11 labelling in Elodea is likely to signal a relatively highly substituted xylan that is not exclusive to secondary cell walls, especially in monocots (Carafa et al., 2005, and references therein). Likewise, the L2 layer is labelled by LM11 but not LM10 and, moreover, it binds both JIM5 and JIM7 and forms the bulk of the inner cell walls that clearly are primary cell walls. We conclude that most likely the L2 layer and the wall labyrinth in epidermal transfer cells of Elodea both belong to a primary cell wall. Recent research on cotton bolls (J. E. Mellon and K. C. Vaughn, unpubl. res.) has revealed that xylan can mask the pectin epitopes recognized by JIM5 and JIM7. This, however, is unlikely to hold for Elodea transfer cells as here the L2 layer binds both LM11 and JIM5/JIM7. A distinct cell wall layer comparable to the ‘uniform layer’ underlying the wall labyrinth in Vicia transfer cells (Vaughn et al., 2007) and possibly functioning as a foundation layer in wall labyrinth development (Farley et al., 2000) appears to be absent in Elodea. The L2 layer is unlikely to be an equivalent of the uniform layer because it has a distinct multilamellar structure, unlike the wall labyrinth it binds JIM5 and JIM7, and it extends to forms the inner cell walls where no wall labyrinth is present.
The positive reaction to CCRC-M7 by the L2 layer and the fibrillar core of wall ingrowths is a major commonality of these two wall areas. CCRC-M7 recognizes an arabinosylated (1 → 6)-β-galactan motif associated with rhamnogalacturonan I (RG-I) and arabino-galactan proteins (AGPs) (Puhlmann et al., 1994; Steffan et al., 1995). RG-I is a major pectic polysaccharide in primary cell walls, whereas AGPs are typically localized at the plasmalemma/cell wall boundary (Carpita and Gibeaut, 1993; Freshour et al., 1996). Interestingly, CCRC-M7 specifically labelled the boundary between the electron-transparent area and plasmalemma in cotyledonary transfer cells of Vicia (Vaughn et al., 2007), whereas this area was not labelled in Elodea. CCRC-M7 binding in Vicia was interpreted as an indication of the presence of arabinogalactan proteins, possibly involved in wall ingrowth development (Vaughn et al., 2007). The localization of CCRC-M7 labelling in the fibrillar core in Elodea transfer cells appears more likely to signal an epitope associated with RG-I. The lack of reaction to LM5 and LM6, two antibodies that recognize galactan and arabinan motifs, suggests that the RG-I present in wall ingrowths and L2 layer lacks galactan and arabinan side branches.
LM1, an antibody that detects hydroxyproline-rich glycoproteins belonging to the extensin family (Smallwood et al., 1995), produced closely similar results in Elodea and pea nodule transfer cells, in both cases labelling the electron-transparent areas of wall ingrowths and associated plasmalemma (Dahiya and Brewin, 2000). Extensins are thought to be self-assembling amphiphiles that form cross-linking networks or sheets at hydrophobic/hydrophilic interfaces in cell walls (Rapaport, 2006). The observed labelling pattern of LM1 in Elodea transfer cells, including the labelling of the cuticle/wall interface, is in good agreement with this notion.
CONCLUSIONS
The wall-membrane apparatus of leaf transfer cells in Elodea is a specialized structure with distinctive biochemical properties. The high level of light-dependent ATPase activity associated with the plasmalemma in the wall labyrinth area supports the hypothesis that these cells promote photosynthetic activity by enhancing inorganic carbon inflow (Rascio et al., 1994).
The discovery that the wall ingrowths present specific biochemical traits relative to the rest of cell walls, notably the absence or inaccessibility of JIM5 and JIM7 epitopes (associated with homogalacturonan) and the high level of the LM11 epitope (probably associated with glucuronoarabinoxylan), suggests that the composition of the extracellular matrix in the wall-membrane apparatus in Elodea is specifically modulated and may be relevant to transfer cell functioning.
Immunocytochemical analysis is to be extended to other types of transfer cells in order to understand whether the molecular architecture of the wall apparatus varies with specific functional specialization or, more simply, according to the histological location and taxonomy.
ACKNOWLEDGEMENTS
We thank J. Paul Knox (Centre for Plant Sciences, University of Leeds, UK) and Michael G. Hahn (Complex Carbohydrate Research Center, University of Georgia, USA) for generously supplying the antibodies used in this study, and Marco Vigliotti (Dipartimento di Scienze ambientali, SUN, Italy) for technical assistance. The development of CCRC series monoclonals at Complex Carbohydrate Research Center was made possible by National Science Foundation (USA) grants numbers DBI-0421683 and RC0090281.
LITERATURE CITED
- Browning AJ, Gunning BES. An ultrastructural and cytochemical study of the wall-membrane apparatus of transfer cells using freeze-substitution. Protoplasma. 1977;93:7–26. [Google Scholar]
- Carafa A, Duckett JG, Knox JP, Ligrone R. Distribution of cell wall xylans in bryophytes and tracheophytes: new insights into basal interrelationships of land plants. New Phytologist. 2005;168:231–240. doi: 10.1111/j.1469-8137.2005.01483.x. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of the primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the wall during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Dahiya P, Brewin NJ. Immunogold localization of callose and other cell wall components in pea nodule transfer cells. Protoplasma. 2000;214:210–218. [Google Scholar]
- Dashek WV, Thomas HR, Rosen WG. Secretory cells of lily pistils. II. Electron microscope cytochemistry of canal cells. American Journal of Botany. 1971;58:909–920. [Google Scholar]
- DeWitt G, Richards J, Mohnen D, Jones AM. Comparative compositional analysis of walls with two different morphologies: archetypal versus transfer-cell like. Protoplasma. 1999;209:238–245. [Google Scholar]
- Elzenga JTM, Prins HBA. Light-induced polar pH changes in leaves of Elodea canadensis. Plant Physiology. 1989;91:62–67. doi: 10.1104/pp.91.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley SJ, Patrick JW, Offler CE. Functional transfer cells differentiate in cultured cotyledons of Vicia faba L. seeds. Protoplasma. 2000;214:102–117. [Google Scholar]
- Freshour G, Clay RP, Fuller MS, Albersheim P, Darvill AG, Hahn MG. Developmental and tissue-specific structural alterations of the cell-wall polysaccharides of Arabidopsis thaliana root. Plant Physiology. 1996;110:1413–1429. doi: 10.1104/pp.110.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES, Pate JS. Transfer cells. In: Robards AW, editor. Dynamic aspects of plant ultrastructure. London: McGraw-Hill; 1974. pp. 441–480. [Google Scholar]
- Harrington GN, Franceschi VR, Offler CE, et al. Cell specific expression of three genes involved in plasma membrane sucrose transport in developing Vicia faba seed. Protoplasma. 1997;197:160–173. [Google Scholar]
- Jones L, Seymour GB, Knox JP. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-d-galactan. Plant Physiology. 1997;113:1405–1412. doi: 10.1104/pp.113.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP. Revealing the structural and functional diversity of plant cell walls. Current Opinion in Plant Biology. 2008;11:308–313. doi: 10.1016/j.pbi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. The gametophyte–sporophyte junction in land plants. Advances in Botanical Research. 1993;19:231–317. [Google Scholar]
- McCann MC, Carpita NC. Designing the deconstruction of plant cell walls. Current Opinion in Plant Biology. 2008;11:314–320. doi: 10.1016/j.pbi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. Journal of Histochemistry and Cytochemistry. 2005;53:1–5. doi: 10.1369/jhc.4B6578.2005. [DOI] [PubMed] [Google Scholar]
- Marré MT, Albergoni FG, Moroni A, Pugliarello MC. Evidence that H+ extrusion in Elodea densa leaves is mediated by an ATP-driven H+ pump. Plant Science. 1989;62:21–28. [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, Talbot MJ. Transfer cells: cells specialized for a special purpose. Annual Review of Plant Biology. 2002;54:431–454. doi: 10.1146/annurev.arplant.54.031902.134812. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, York WS. The composition and structure of plant primary cell walls. In: Rose JKC, editor. The plant cell wall. Boca Raton, FL: CRC Press; 2003. pp. 1–54. [Google Scholar]
- Pate JS, Gunning BES. Transfer cells. Annual Review of Plant Physiology. 1972;23:173–196. [Google Scholar]
- Price GD, Whitecross MI. Cytochemical localization of ATPase activity on the plasmalemma of Chara corallina. Protoplasma. 1983;116:65–74. [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, et al. Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1→2)-linked fucosyl-containing epitope. Plant Physiology. 1994;104:699–710. doi: 10.1104/pp.104.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport H. Ordered peptide assemblies at interfaces. Supramolecular Chemistry. 2006;18:445–454. [Google Scholar]
- Rascio N, Mariani P, Dalla Vecchia F, Zanchin A, Pool A, Larcher W. Ultrastructural and photosynthetic features of leaves and stems of Elodea canadensis. Plant Physiology. 1994;144:314–323. [Google Scholar]
- Rascio N, Cuccato F, Dalla Vecchia F, La Rocca N, Larcher W. Structural and functional features of the leaves of Ranunculus trichophyllus Chaix., a freshwater submerged macrophyte. Plant, Cell & Environment. 1999;22:205–212. [Google Scholar]
- Smallwood M, Martin H, Knox JP. An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta. 1995;196:510–522. doi: 10.1007/BF00203651. [DOI] [PubMed] [Google Scholar]
- Smallwood M, Yates EA, Willats WGT, Knox JP. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta. 1996;198:452–459. [Google Scholar]
- Steffan W, Kovàc P, Albersheim P, Darvill AG, Hahn MG. Characterization of a monoclonal antibody that recognizes an arabinosylated (1→6)-β-d-galactan epitope in plant complex carbohydrates. Carbohydrate Research. 1995;275:295–307. doi: 10.1016/0008-6215(95)00174-r. [DOI] [PubMed] [Google Scholar]
- Tegeder M, Wang XD, Frommer WB, Offler CE, Patrick JW. Sucrose transport into developing seeds of Pisum sativum L. The Plant Journal. 1999;18:151–161. doi: 10.1046/j.1365-313x.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Talbot MJ, Offler CE, McCurdy DW. Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of arabinogalactan proteins. Plant Cell Physiology. 2007;48:159–168. doi: 10.1093/pcp/pcl047. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Marcus S, Knox JP. Generation of a monoclonal antibody specific to (1→5)-α-L-arabinan. Carbohydrate Research. 1998;308:149–152. doi: 10.1016/s0008-6215(98)00070-6. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, et al. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydrate Research. 2000;327:309–320. doi: 10.1016/s0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Steele-King CG, et al. A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta. 2004;218:673–681. doi: 10.1007/s00425-003-1147-8. [DOI] [PubMed] [Google Scholar]