Abstract
Background and Aims
Mechanical stimulation (MS) often induces plants to undergo thigmomorphogenesis and to synthesize an array of signalling substances. In clonal plants, connected ramets often share resources and hormones. However, little is known about whether and how clonal integration influences the ability of clonal plants to withstand MS. We hypothesized that the effects of MS may be modulated by clonal integration.
Methods
We conducted an experiment in which ramet pairs of Leymus secalinus were subjected to three treatments: (1) connected ramet pairs under a homogeneous condition [i.e. the proximal (relatively old) and distal (relatively young) ramets were not mechanically stressed]; (2) connected ramet pairs under a heterogeneous condition (i.e. the proximal ramet was mechanically stressed but the distal ramet was not); and (3) disconnected ramet pairs under the same condition as in treatment 2. At the end of the experiment, we harvested all plants and determined their biomass and allocation.
Key Results
Clonal integration had no significant influence on measured traits of distal L. secalinus ramets without MS. However, under MS, plants with distal ramets that were connected to a mother ramet produced more total plant biomass, below-ground biomass, ramets and total rhizome length than those that were not connected. Partial MS exerted local effects on stimulated ramets and remote effects on connected unstimulated ramets. Partial MS increased total biomass, root/shoot ratio, number of ramets and total rhizome length of stimulated proximal ramets, and increased total biomass, root weight ratio, number of ramets and total rhizome length of connected unstimulated ramets due to clonal integration.
Conclusions
These findings suggest that thigmomorphogenesis may protect plants from the stresses caused by high winds or trampling and that thigmomorphogenesis can be strongly modulated by the degree of clonal integration.
Keywords: Clonal plants, heterogeneous habitats, resource translocation, thigmomorphogenesis
INTRODUCTION
Most plants can propagate asexually to form genetically identical individuals (ramets) by clonal growth (de Kroon et al., 1998). This feature has been long considered to be adaptive in many habitats, particularly in environments where resources or stressful factors are patchily distributed in both time and space (Hartnett and Bazzaz, 1983). When clonal plants encounter heterogeneous habitats, physical connections such as stolons and rhizomes enable ramets to respond collectively to environmental conditions through translocation of resources and/or signals (i.e. clonal integration; Hartnett and Bazzaz, 1983; Slade and Hutchings, 1987; Wijesinghe and Handel, 1994; de Kroon et al., 1998; Gómez et al., 2008; Yu et al., 2009). Daughter ramets are physiologically dependent upon resources translocated from their connected parental ramets, and clonal integration among interconnected ramets reduces the risks associated with the establishment of daughter ramets (Hartnett and Bazzaz, 1983).
In nature, plants can be influenced in terms of morphology, growth and reproduction by mechanical stress (MS) caused by wind (Liu et al., 2007b; Wang et al., 2008) and animal trampling in grazed areas (Pellerin et al., 2006). Wind can affect plant growth, morphology and reproduction directly by MS (Puijalon et al., 2008) and indirectly by changing temperature, water vapour and CO2 concentration around the plants (Grace and Russell, 1977; Retuerto and Woodward, 1993; Anten et al., 2010). Animal trampling may influence herbaceous cover and community diversity (Taddese et al., 2002). When plants are exposed to MS, they produce shorter and thicker stems and allocate more mass to the root system (Liu et al., 2007b). These thigmomorphogenetic responses can enhance the ability of plants to tolerate mechanical damage (Anten et al., 2005, 2010). MS may further lead to a decrease in specific leaf area and/or a decrease in leaflet number per leaf (Puijalon et al., 2008), resulting in a more streamlined plant shape that allows plants to be more resistant to breakage (Puijalon et al., 2007).
In inland semi-arid ecosystems such as the Mu Us sandland in northern China, high wind (Zhang, 1994) and animal trampling (Wu and Ci, 2002) are common and significantly affect the growth and distribution of plants. Rhizomatous clonal plants frequently colonize this inland dry area (Yu et al., 2008; Li et al., 2010a, b). Due to a large network of below-ground rhizomes, ramets may experience partial animal trampling, and ramets on windward slopes may suffer high MS induced by wind, whereas their interconnected ramets on leeward slopes may suffer much less. However, the responsiveness of clonal plants to partial MS has not previously been examined.
To examine whether integration of clonal plants affects their responses to MS, we conducted a greenhouse experiment, in which the proximal (relatively old) ramet of the connected or disconnected ramet pairs of Leymus secalinus was either subjected or not subjected to MS and its interconnected distal (relatively young) ramet was left unstimulated. Specifically, we addressed the following questions: (1) Does MS affect the growth of L. secalinus ramets? (2) Does integration of clonal plants modify the MS effects, and consequently influence the plants’ ability to withstand MS?
MATERIALS AND METHODS
Leymus secalinus (Georgi) Tzvelev. is a perennial grass that is distributed widely in the Autonomous Regions of Inner Mongolia and Tibet, and the provinces of Hebei, Gansu, Shanxi and Qinghai, China, as well as in Mongolia and Japan (Ye et al., 2006). It occurs in typical steppe landscapes, sandy grasslands, mountain slopes, farmlands and roadsides (Dong, 1999). Leymus secalinus produces both spreading (guerrilla form) and clumping (phalanx form) ramets (Ye et al., 2006). In the phalanx growth form, connections between ramets have few and short internodes, resulting in closely packed ramets termed ‘clumping ramets’; in the guerrilla growth form, connections among ramets have many long internodes, resulting in widely spaced ramets termed ‘spreading ramets’. Clonal growth by rhizomes plays an important role in the maintenance of populations of this species (Ye et al., 2006).
Experimental design
On 15 July, 2008, 30 similar-sized ramet pairs of L. secalinus were sampled in a sandy grassland near the Ordos Sandland Ecological Station (OSES; 39°29′37″N, 110°11′29″E, 1300 m a.s.l.; Institute of Botany, Chinese Academy of Sciences) located in the Mu Us sandland in northern China. Each ramet pair consisted of a proximal (relatively old) ramet and a distal (relatively young) ramet, interconnected by a rhizome. Each ramet pair was planted in a pair of cylindrical plastic containers (21 cm in diameter and 21 cm in height) filled with 3·5 L topsoil from local habitats. Ten days after transplantation, initial height (the length from shoot base to tip of the longest leaf), basal diameter of each ramet and leaf number were determined. Initial mean ramet height (31·6 ± 0·7 cm, P = 0·528), base diameter (1·9 ± 0·5 cm, P = 0·485) and leaf number (2·5 ± 0·1, P = 0·852) did not differ among the ramets.
After initial measurements, the ramet pairs were randomly assigned to one of three treatments: (1) NS-NS, i.e. both proximal and distal ramets were not subjected to MS and the rhizome connection between proximal and distal ramets remained intact; (2) S-NS, i.e. the proximal ramets were subjected to MS, the distal ramets were not and the rhizome connection remained intact; and (3) S‖NS, i.e. the proximal ramets were subjected to MS, the distal ramets were not and the rhizome connection was severed. There were ten replicates per treatment. The experiment lasted from 25 July to 17 September, 2008, in a greenhouse (mean temperature 23·2 °C) at the OSES.
The MS was accomplished by brushing plants manually with bond typing paper (6·4 mg cm−2) as follows: from 1800 to 1900 h every day during the experiment, the proximal ramets were rubbed 60 times, twice per second. This method allows the swaying of plants to be simulated without significantly changing their microclimate or causing visible damage to the ramets.
Measurements
Before harvesting, measurements were made for each plant on the proximal part (the proximal ramet plus its descendant ramets) and the distal part (the distal ramet and its descendant ramets). Measurements included plant height, base diameter of the leaf sheath (measured by vernier calipers), number of leaves and number of ramets (new ramets plus the original ramet). The experimental plants were harvested and then separated into roots, leaf blades, leaf sheaths and rhizomes. Images of leaves of each ramet were obtained using a scanner (5000S; BenQ Corporation, Taipei, Taiwan), and total leaf area was measured with Image J software (available at http://rsb.info.nih.gov/ij/). Above-ground parts (shoot parts) included leaf blades and leaf sheaths, while below-ground parts included roots and rhizomes. We measured total rhizome length and spacer length (defined as the rhizome length between two adjacent ramets) with a ruler. All plant portions were dried at 80 °C for 48 h and weighed.
Data analysis
A previous study has shown that severing (clonal integration) does not have any effect under homogeneous treatments (Yu et al., 2002). Data for proximal and distal ramets were analysed using one-way ANOVA followed by two planned comparisons, respectively. For the proximal parts, the first planned comparison (S- part of S-NS vs. S‖ part of S‖NS) was used to identify clonal integration effects under MS, and the second one (NS- part of NS-NS vs. S- part of S-NS) was used to examine the local effects of MS. For the distal parts, the first planned comparison (-NS part of NS-NS vs. ‖NS part of S‖NS) was to examine the clonal integration effects without MS, and the second one (-NS part of NS-NS vs. -NS part of S-NS) was carried out to test the remote MS effects due to clonal integration. The tests were considered to be significant at P < 0·05.
RESULTS
In the absence of MS, a physical connection between ramets did not significantly affect the traits of the distal parts investigated (Table 1, CI – MS). Under MS, plants with distal ramets that were connected to a mother ramet produced more total plant biomass, below-ground biomass, ramets and total rhizome length than those that were not connected (Table 1, CI + MS).
Table 1.
P-values of one-way ANOVA with planned comparisons for the effects of clonal integration and MS on the proximal and distal ramets of Leymus secalinus
| Plant trait | CI – MS | CI + MS | Local MS | Remote MS |
|---|---|---|---|---|
| Biomass: | ||||
| Plant biomass | 0·738log | 0·002nor | <0·001nor | 0·064log |
| Shoot biomass | 0·818log | 0·113nor | 0·044nor | 0·106log |
| Root biomass | 0·235log | 0·002log | <0·001log | 0·006log |
| Rhizome biomass | 0·535log | 0·002nor | <0·001nor | 0·163log |
| Biomass allocation: | ||||
| Shoot weight ratio | 0·847nor | 0·005nor | <0·001nor | 0·225nor |
| Root weight ratio | 0·234nor | 0·010log | <0·001log | 0·003nor |
| Rhizome weight ratio | 0·658nor | 0·129nor | 0·022nor | 0·615nor |
| Root/shoot ratio | 0·258nor | 0·003log | <0·001log | 0·176nor |
| Below-/above-ground ratio | 0·687nor | 0·002nor | <0·001nor | 0·223nor |
| Clonal growth: | ||||
| Number of ramets | 0·559nor | 0·004nor | <0·001nor | 0·002nor |
| Clonal expansion: | ||||
| Total rhizome length | 0·629nor | 0·009log | 0·010log | 0·051nor |
| Spacer length | 0·716nor | 0·947nor | 0·364nor | 0·319nor |
| Morphology: | ||||
| Ramet height | 0·582nor | 0·314nor | 0·028nor | 0·537nor |
| Ramet base diameter | 0·836nor | 0·152nor | 0·087nor | 0·016nor |
| Number of leaves | 0·260nor | 0·573nor | 0·747nor | 0·297nor |
| Total leaf area | 0·374log | 0·559nor | 0·385nor | 0·044log |
| Specific leaf area | 0·592nor | 0·998log | 0·448log | 0·980nor |
CI – MS, clonal integration effects without MS: -NS part of the treatment NS-NS versus ‖NS part of the treatment S‖NS; CI + MS, clonal integration effects under MS: S- part of the treatment S-NS versus S‖ part of the treatment S‖NS; Local MS, local MS effects: NS- part of the treatment NS-NS versus S- part of the treatment S-NS; Remote MS, remote MS effects: -NS part of the treatment NS-NS versus -NS part of the treatment S-NS.
Values in bold and italic indicate significant (P < 0·05) and marginally significant (0·05 < P < 0·1) effects, respectively. log: data were logarithmically transformed; nor: untransformed data were used.
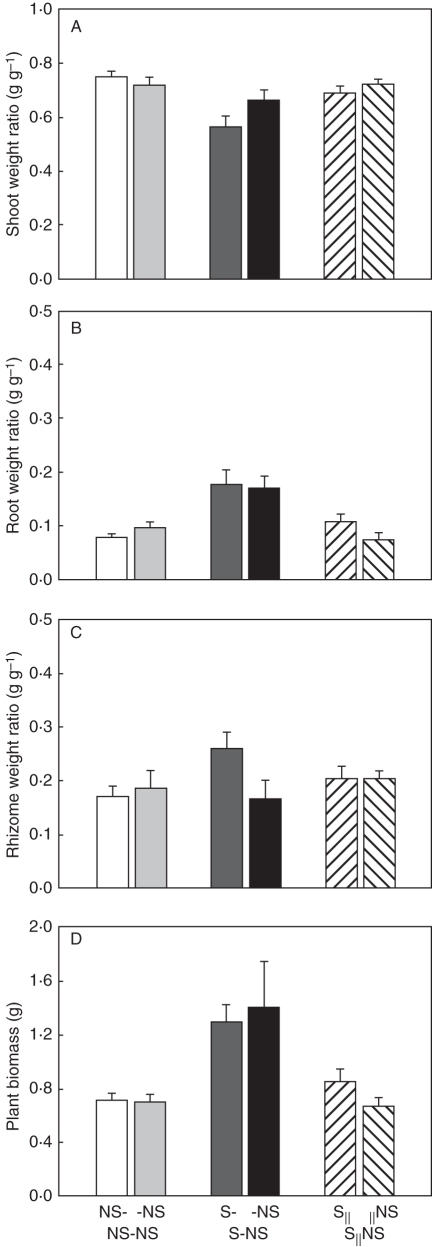
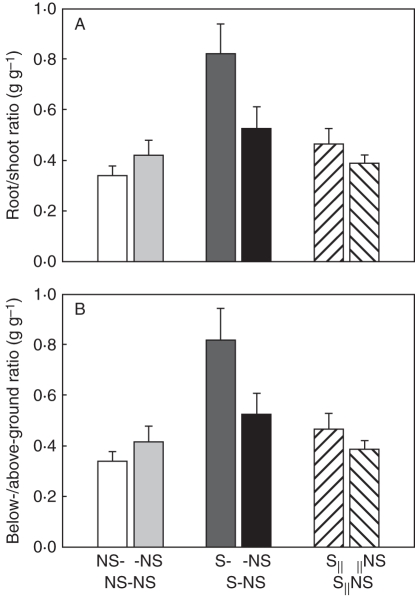
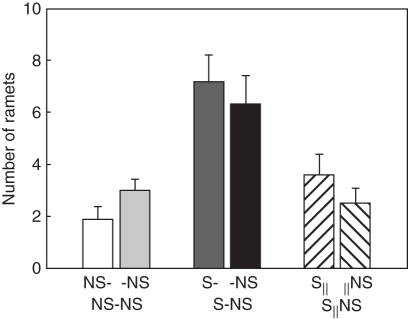
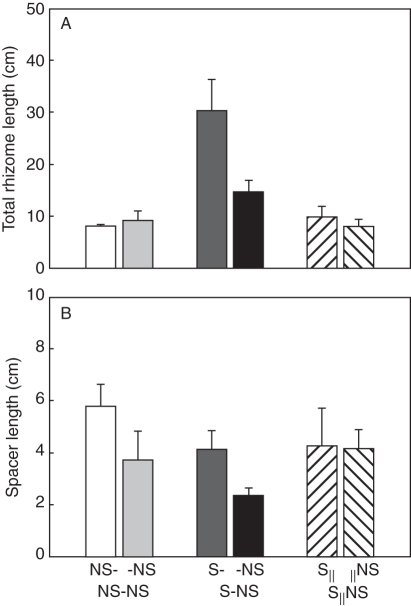
The second planned comparison for proximal parts indicated that local effects of MS on L. secalinus were significant. For example, total biomass increased by about 82 %, biomass allocated to shoot decreased (Fig. 1A) while root (Fig. 1B) and rhizome weight ratio (Fig. 1C) increased. The root/shoot ratio (Fig. 2A) and below-/above-ground ratio (Fig. 2B) increased by about 216 and 140 %, respectively. The number of ramets (Fig. 3) and total rhizome length (Fig. 4A) increased significantly (Table 1).
Fig. 1.
Biomass allocation (A–C) and plant biomass (D) of Leymus secalinus ramets under NS-, -NS, S-, -NS, S‖ and ‖NS conditions in the NS-NS, S-NS and S‖NS treatments, respectively. Data are means + s.e. (n = 10).
Fig. 2.
Root/shoot ratio (A) and below-/above-ground ratio (B) of Leymus secalinus ramets under the NS-NS, S-NS and S‖NS treatments. Data are means + s.e. (n = 10).
Fig. 3.
Number of ramets of Leymus secalinus ramets under the NS-NS, S-NS and S‖NS treatments. Data are means + s.e. (n = 10).
Fig. 4.
Total rhizome length (A) and spacer length (B) of Leymus secalinus ramets under the NS-NS, S-NS and S‖NS treatments. Data are means + s.e. (n = 10).
The remote MS effects on connected distal ramets, indicated by the second planned comparison for distal parts, showed a 101 % increase in total biomass (Fig. 1D) due in part to the contribution of root biomass to total biomass (Table 1; Fig. 2). The number of ramets (Fig. 3) and total rhizome length (Fig. 4A) of distal ramets were also increased (Table 1).
DISCUSSION
Clonal integration did not significantly affect any of the traits investigated under the homogeneous treatments used here. Conversely, the roles of clonal integration were emphasized in maintaining the growth and morphological characteristics of distal ramets under partial MS. These findings add significantly to our understanding of the effects of clonal integration, MS and their interactions. For example, the effects of MS can be modulated by clonal integration, providing evidence for local and remote MS effects.
Partial MS significantly influenced the growth and morphological characteristics of proximal ramets of L. secalinus. The root weight ratio, rhizome weight ratio and total biomass increased significantly, indicating that more biomass was allocated to below-ground parts. This is consistent with the results by Liu et al. (2007b) with Potentilla reptans. This allocation strategy may be beneficial, because it leads to greater strength against breakage and reduces the risk of uprooting (Puijalon et al., 2007). An increase of ramet numbers and total rhizome length after MS may be linked to the rapid growth of clonal plants to escape from the stress, to the reduction of genet mortality risk and to the increase of plant density to protect the young or leeward ramets from MS (Pai and McCarthy, 2005; Liu et al., 2007b; Gómez et al., 2008). In clonal plants with ramets that are physiologically integrated for a long time, selection will favour rapid growth to enable the plant to keep ramets ahead of stress (Wennström and Ericson, 1992). In other words, when clonal plants grow in stressed conditions, they tend to allocate more biomass to attain higher reproductive yield or to grow more rapidly to escape from the stress.
Distal ramets connected to stimulated proximal ramets can be influenced by MS due to clonal integration. The increases in root and rhizome weight ratios, number of ramets and total rhizome length of distal ramets were consistent with changes in their connected proximal ramets. Stuefer et al. (2004) noted that inducible defences caused by herbivores can be exclusively expressed at the site of damage and they can also be activated in other undamaged parts of the plant by diffusible signals. Here, our study indicates that MS may induce the early warning signals in clonal plants and transmit them to non-stressed ramets. If one or a few ramets of a clonal plant are attacked, the effects induced at the site of damage may be communicated to other uninfected ramets through clonal integration (Wennström and Ericson, 1992). Although the exact mechanisms by which clonal plants withstand mechanical stresses remain unclear, it is likely that daughter ramets are affected by the transmission of specific chemicals or signals through physical connections between proximal and distal ramets.
Individual plants are composed of fine-scale tissue mosaics differing in structure and function, whereas clonal plants possess both organismic and clonal modularity (Liu et al., 2007a). Daughter ramets are physiologically dependent upon resources translocated from their parental clone, and clonal integration among intraclonal ramets reduces the establishment risks of daughter ramets (Hartnett and Bazzaz, 1983). Physical connections between ramets permit the internal transport of substances such as water, carbohydrates and mineral nutrients between different parts of the clonal network (Stuefer et al., 2004). As ordinary resources can be transported by the vascular system for long distances within clonal plant networks, non-resource integration induced by MS is likely to follow the same or similar principles (Stuefer et al., 2004).
To our knowledge, this is the first study to demonstrate that local effects of partial MS are modified by clonal integration. L. secalinus can produce performance-enhancing morphologies, which may be beneficial for colonization in high-wind, semi-arid environments. In inland semi-arid ecosystems such as the Mu Us sandland, wind and herbivore trampling are the main causes for MS and rhizome severance. Daughter ramets are physiologically dependent upon resources translocated from their parental ramets and partial MS is avoided as a result of long rhizomes or sand burial (Ye et al., 2006). Our findings indicate that maintaining the physical connections between ramets is an important adaptive strategy for L. secalinus and plays a substantial role in its long-term persistence in windy sandlands.
ACKNOWLEDGEMENTS
We thank Dr Nigel Chaffey, Dr Niels Anten and another anonymous reviewer for their valuable comments on an earlier version of the manuscript. This research was funded by the Chinese Academy of Sciences (KZCX2-YW-431-04), National Natural Science Foundation of China (30521005, 39825106, 30870395) and State Key Laboratory of Vegetation and Environmental Change (Institute of Botany, Chinese Academy of Sciences; VEWALNE – VEgetation-WAter Long-term Networked Experiment in Chinese Steppe zone).
LITERATURE CITED
- Anten NPR, Casado-Garcia R, Nagashima H. Effects of mechanical stress and plant density on mechanical characteristics, growth, and lifetime reproduction of tobacco plants. American Naturalist. 2005;166:650–660. doi: 10.1086/497442. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Alcalá-Herrera R, Schieving F, Onoda Y. Wind and mechanical stimuli differentially affect leaf traits in Plantago major. New Phytologist. 2010;188:554–564. doi: 10.1111/j.1469-8137.2010.03379.x. [DOI] [PubMed] [Google Scholar]
- Dong M. Effects of severing rhizome on clonal growth in rhizomatous grass species Psammochloa villosa and Leymus secalinus. Acta Botanica Sinica. 1999;41:194–198. [Google Scholar]
- Gómez S, Onoda Y, Ossipov V, Stuefer JF. Systemic induced resistance: a risk-spreading strategy in clonal plant networks? New Phytologist. 2008;179:1142–1153. doi: 10.1111/j.1469-8137.2008.02542.x. [DOI] [PubMed] [Google Scholar]
- Grace J, Russell G. The effect of wind on grasses. III. Influence of continuous drought or wind on anatomy and water relations in Festuca arundinacea schreb. Journal of Experimental Botany. 1977;28:268–278. [Google Scholar]
- Hartnett DC, Bazzaz FA. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology. 1983;64:779–788. [Google Scholar]
- de Kroon H, van der Zalm E, van Rheenen JWA, van Dijk A, Kreulen R. The interaction between water and nitrogen translocation in a rhizomatous sedge (Carex flacca) Oecologia. 1998;116:38–49. doi: 10.1007/s004420050561. [DOI] [PubMed] [Google Scholar]
- Li SL, Werger MJA, Zuidema PA, Yu FH, Dong M. Seedlings of the semi-shrub Artemisia ordosica are resistant to moderate wind denudation and sand burial in Mu Us sandland, China. Trees – Structure and Function. 2010a;24:515–521. [Google Scholar]
- Li SL, Zuidema PA, Yu FH, Werger MJA, Dong M. Effects of denudation and burial on growth and reproduction of Artemisia ordoscica in Mu Us sandland. Ecological Research. 2010b;25:655–661. [Google Scholar]
- Liu FH, Liu J, Yu FH, Dong M. Water integration patterns in two rhizomatous dune perennials of different clonal fragment size. Flora. 2007a;202:106–110. [Google Scholar]
- Liu Y, Schieving F, Stuefer JF, Anten NPR. The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Annals of Botany. 2007b;99:121–130. doi: 10.1093/aob/mcl230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai A, McCarthy BC. Variation in shoot density and rhizome biomass of Acorus calamus L. with respect to environment. Castanea. 2005;70:263–275. [Google Scholar]
- Pellerin S, Huot J, Côté SD. Long-term effects of deer browsing and trampling on the vegetation of peatlands. Biological Conservation. 2006;128:316–326. [Google Scholar]
- Puijalon S, Léna JP, Bornette G. Interactive effects of nutrient and mechanical stresses on plant morphology. Annals of Botany. 2007;100:1297–1305. doi: 10.1093/aob/mcm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puijalon S, Léna JP, Rivière N, Champagne JY, Rostan JC, Bornette G. Phenotypic plasticity in response to mechanical stress: hydrodynamic performance and fitness of four aquatic plant species. New Phytologist. 2008;177:907–917. doi: 10.1111/j.1469-8137.2007.02314.x. [DOI] [PubMed] [Google Scholar]
- Retuerto R, Woodward FI. The influences of increased CO2 and water supply on growth, biomass allocation and water use efficiency of Sinapis alba L. grown under different wind speeds. Oecologia. 1993;94:415–427. doi: 10.1007/BF00317118. [DOI] [PubMed] [Google Scholar]
- Slade AJ, Hutchings MJ. An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea. Oecologia. 1987;73:425–431. doi: 10.1007/BF00385260. [DOI] [PubMed] [Google Scholar]
- Stuefer JF, Gómez S, Mölken TV. Clonal integration beyond resource sharing: implications for defence signalling and disease transmission in clonal plant network. Evolutionary Ecology. 2004;18:647–667. [Google Scholar]
- Taddese G, Saleem MAM, Abyie A, Wagnew A. Impact of grazing on plant species richness, plant biomass, plant attribute, and soil physical and hydrological properties of vertisol in East African highlands. Environmental Management. 2002;29:279–289. doi: 10.1007/s00267-001-0014-2. [DOI] [PubMed] [Google Scholar]
- Wang YH, He WM, Dong M, et al. Effects of shaking on the growth and mechanical properties of Hedysarum laeve may be independent of water regimes. International Journal of Plant Sciences. 2008;169:503–508. [Google Scholar]
- Wennström A, Ericson L. Environmental heterogeneity and disease transmission within clones of Lactuca sibirica. Journal of Ecology. 1992;80:71–77. [Google Scholar]
- Wijesinghe DK, Handel SN. Advantages of clonal growth in heterogeneous habitats: an experiment with Potentilla simplex. Journal of Ecology. 1994;82:495–502. [Google Scholar]
- Wu B, Ci LJ. Landscape change and desertification development in the Mu Us Sandland, northern China. Journal of Arid Environments. 2002;50:429–444. [Google Scholar]
- Ye XH, Yu FH, Dong M. A trade-off between guerrilla and phalanx growth forms in Leymus secalinus under different nutrient supplies. Annals of Botany. 2006;98:187–191. doi: 10.1093/aob/mcl086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Chen YF, Dong M. Clonal integration enhances survival and performance of Potentilla anserina, suffering from partial sand burial on Ordos plateau, China. Evolutionary Ecology. 2002;15:303–318. [Google Scholar]
- Yu FH, Wang N, He WM, Chu Y, Dong M. Adaptation of rhizome connections in drylands: increasing tolerance of clones to wind erosion. Annals of Botany. 2008;102:571–577. doi: 10.1093/aob/mcn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Wang N, Alper P, He WM, Dong M. Physiological integration in an invasive plant increases its spread into experimental communities and modified their structure. American Journal of Botany. 2009;96:1983–1989. doi: 10.3732/ajb.0800426. [DOI] [PubMed] [Google Scholar]
- Zhang XS. Principles and optimal models for development of Maowusu sandy grassland. Acta Phytoecologica Sinica. 1994;18:1–16. [Google Scholar]